Abstract
We have been studying the insertion of the seven transmembrane domain (TM) protein opsin to gain insights into how the multiple TMs of polytopic proteins are integrated at the endoplasmic reticulum (ER). We find that the ER components associated with the first and second TMs of the nascent opsin polypeptide chain are clearly distinct. The first TM (TM1) is adjacent to the α and β subunits of the Sec61 complex, and a novel component, a protein associated with the ER translocon of 10 kDa (PAT-10). The most striking characteristic of PAT-10 is that it remains adjacent to TM1 throughout the biogenesis and membrane integration of the full-length opsin polypeptide. TM2 is also found to be adjacent to Sec61α and Sec61β during its membrane integration. However, TM2 does not form any adducts with PAT-10; rather, a transient association with the TRAM protein is observed. We show that the association of PAT-10 with opsin TM1 does not require the N-glycosylation of the nascent chain and occurs irrespective of the amino acid sequence and transmembrane topology of TM1. We conclude that the precise makeup of the ER membrane insertion site can be distinct for the different transmembrane domains of a polytopic protein. We find that the environment of a particular TM can be influenced by both the “stage” of nascent chain biosynthesis reached, and the TM's relative location within the polypeptide.
INTRODUCTION
The majority of membrane proteins synthesized at the endoplasmic reticulum (ER) are delivered via the signal recognition particle (SRP)-dependent targeting pathway and integrated at the ER translocon (High and Laird, 1997; Johnson and van Waes, 1999; Keenan et al., 2001). The structure of the ER translocon has been established, and it can be observed as a “doughnut-like” structure with a central, water-filled pore that spans the entire ER membrane (Matlack et al., 1998; Menetret et al., 2000; but see Beckmann et al., 2001). In its active state the ER translocon is aligned with the large ribosomal subunit, presumably to facilitate the pathway of nascent polypeptide chains toward the site of ER translocation (Beckmann et al., 2001). During the process of membrane protein insertion, small molecules are prevented from freely diffusing across the aqueous ER translocation channel by a complex and coordinated series of “gating” events. This gating is mediated by the ribosome on the cytoplasmic side of the ER membrane (Liao et al., 1997) and by BiP on the luminal side (Haigh and Johnson, 2002).
The ER translocon plays a central role during the cotranslational insertion of a variety of single-spanning and polytopic-integral membrane proteins (Laird and High, 1997; Mothes et al., 1997; Johnson and van Waes, 1999; Heinrich et al., 2000). Recent estimates suggest that the ER translocon is composed from three copies of the Sec61 complex (Matlack et al., 1998; Beckmann et al., 2001). The mammalian Sec61 complex is a heterotrimer composed of the Sec61α, Sec61β, and Sec61γ subunits, and this complex has been shown to be essential for membrane protein integration at the ER (High and Laird, 1997; Matlack et al., 1998; Johnson and van Waes, 1999). It is the polytopic Sec61α subunit that forms the major component of the transmembrane channel via which proteins are integrated into the ER membrane (Do et al., 1996; Laird and High, 1997; Mothes et al., 1997; Heinrich et al., 2000). At a mechanistic level, the Sec61 complex facilitates the partitioning of transmembrane domains (TMs) from the ER translocation channel into the phospholipid bilayer (Heinrich et al., 2000). This lateral exit of TMs is a multistep process, and additional ER components, such as the TRAM protein, may also carry out specific functions during membrane integration (Do et al., 1996; Heinrich et al., 2000).
In the case of polytopic proteins, a single polypeptide contains multiple TMs and these seem to be sequentially inserted into the ER translocon as they emerge from the ribosome (Borel and Simon, 1996b). The way in which each of these multiple TMs are then laterally released from the ER translocon into the phospholipid bilayer is much less clear (High and Laird, 1997). Individual TMs may exit the ER translocon while translation proceeds (Mothes et al., 1997). Alternatively, such lateral exit may only take place upon the completion of protein synthesis and the release of the nascent chain from the ribosome (Borel and Simon, 1996a). In this context, establishing the local environment of the different TMs of a polytopic protein during its membrane integration would be extremely illuminating.
The seven transmembrane domain protein opsin is a well-characterized polytopic protein (Menon et al., 2001) that is well suited to in vitro-based studies (Laird and High, 1997), and we have used a site-specific cross-linking approach to investigate the local environment of different transmembrane domains during its biosynthesis. During this study, we have focused upon the ER proteins that are adjacent to the first and second TMs of the nascent opsin chain during its membrane integration. We find that although both TMs can be cross-linked to subunits of the Sec61 complex, adducts with other components are unique to one or other of the TMs. Thus, we observe a novel component, protein associated with the ER translocon of 10 kDa (PAT-10), is associated with TM1, whereas the translocating chain-associating membrane (TRAM) protein is transiently adjacent to TM2. We conclude that the precise makeup of the ER insertion site can be distinct for the different TMs of a polytopic protein and find that the relative location of each TM within the nascent chain is a key feature in determining its environment.
MATERIALS AND METHODS
The cross-linking reagent bismaleimidohexane (BMH) was purchased from Pierce and Warriner (Chester, United Kingdom). Restriction endonucleases were purchased from PerkinElmer Life Sciences (Herts, United Kingdom). T7 RNA polymerase, transcription reagents, and rabbit reticulocyte lysate were supplied by Promega (Southampton, United Kingdom), and the m7G(5′)ppp(5′)G cap analog was from PerkinElmer Life Sciences (Hitchin, United Kingdom). Easytag l-[35S]methionine was purchased from PerkinElmer Life Sciences (Stevenage, United Kingdom). All reagents for cell culture were obtained from Invitrogen (Paisley, United Kingdom). All other chemicals were purchased from BDH/Merck (Poole, United Kingdom) and Sigma Chemical (Poole, Dorset, United Kingdom). Antisera specific for SRP54 and TRAM were a kind gift from Bernhard Dobberstein (ZMBH, Heidelberg, Germany). Antisera specific for the Sec61α and Sec61β subunits were generously provided by Richard Zimmerman (University of Saarland, Homburg, Germany). The monoclonal antibody specific for the N terminus of bovine opsin was a kind gift from Paul Hargrave (Department of Opthalmology, University of Florida, Gainesville, FL) (Adamus et al., 1991).
Opsin and Neurotensin Receptor-derived Constructs
An EcoRI/HindIII fragment containing the coding region of bovine opsin was subcloned into the plasmid pGEM3z as described previously (Laird and High, 1997), and a duplicated multiple cloning site excised by digestion with PstI. To generate a cys-null form of opsin, all of the native cysteines were changed to glycine by using the QuikChange site-directed mutagenesis kit (Stratagene, Cambridge, United Kingdom). Single cysteines were then introduced by mutation using the same method. The OPΔCHO mutant was generated from OP[cys56] by using the QuikChange site-directed mutagenesis kit (Stratagene) by replacing the Asn residues for Gln. The Opsin TM2 domain duplication mutants were generated from the cys-null opsin by using the Exsite mutagenesis kit (Stratagene). Residues 39–61 inclusive of TM1 were deleted and replaced by residues 75–97 inclusive of TM2 (Table 1). Cysteine residues were subsequently introduced as described above. Syb2-OP was created by splicing the coding region for the N-terminal 94 amino acids of rat synaptobrevin 2 (Syb2) in front of the AUG start codon for opsin by using polymerase chain reaction (PCR)-based overlap extension (Horton et al., 1989). The spliced PCR product was cloned into the pSPUTK in vitro expression vector (Stratagene). The coding region for the rat neurotensin receptor (Tucker and Grisshammer, 1996) was subcloned into pSPUTK and naturally occurring cysteines at positions 142 and 152 were altered to glycines with the Gene Editor site-directed mutagenesis system (Promega, Southampton, United Kingdom).
Table 1.
Partial amino acid sequences of opsin and neurotensin derived polypeptides used in this study
| Name | Amino acid sequence |
|---|---|
| OP (wild type) | MNGTEGPNFYVPFSNKTGVVRSPFEAPQYYLAEPWQFSMLAAYMFLLIMLGFPINFLTLYV |
| TVQHKKLRTPLNYILLNLAVADLFMVFGGFTTTLYTSLHGYFVFGFGPTG | |
| OPTM2×2 (Fwd) | MNGTEGPNFYVPFSNKTGVVRSPFEAPQYYLAEPWQFSILLNLAVADLFMVFGGFTTTLYT |
| TVQHKKLRTPLNYILLNLAVADLFMVFGGFTTTLYTSLHGYFVFGFGPTG | |
| OPTM2×2 (Rev) | MNGTEGPNFYVPFSNKTGVVRSPFEAPQYYLAEPWQFSTYLTTTFGGFVMFLDAVALNLLI |
| TVQHKKLRTPLNYILLNLAVADLFMVFGGFTTTLYTSLHGYFVFGFGPTG | |
| Neurotensin receptor | MHLNSSVPQGTPGEPDAQPFSGPQSEMEATFLALSLSNGSGNTSESDTAGPNSDLDVNTDIYSK |
| VLVTAIYLALFVVGTVGNSVTAFTLARKKSLQSLQSTVHYHLGSLALSDLLILLLAMPVELYNFIW |
The amino acid sequences of the N-terminal regions of wild-type bovine opsin, two opsin derivatives with different TMs, and wild-type rat neurotensin receptor are shown. The presumptive first and second TMs are underlined (Swiss Prot. entries P02699 and P20789), and the various residues altered to generate single cysteine probes indicated in bold.
Templates for the transcription of truncated opsin and neurotensin receptor mRNAs were prepared by PCR (Laird and High, 1997). Forward primers were located 160 bases 5′ of the RNA polymerase promoter, whereas reverse primers were designed to generate truncations encoding the N-terminal 80, 85, 90, 96, 106, 130, 137, 150, 165, 175, and 276 amino acids of opsin. The primer used to truncate opsin at 165 aa incorporated an additional nonnative methionine at the C-terminal end to enhance radiolabeling of the translation product. In addition, a full-length version of opsin was generated where the entire 348 amino acids were translated, but no stop codon was present so that the polypeptide remained attached to the ribosome unless released by treatment with puromycin. The transcription template for the Syb2-OP244 truncation was generated by PCR by using a pSPUTK-specific forward primer and the OP150 reverse primer with Syb2-OP as the template. The transcription template encoding the N-terminal 170 amino acids of the neurotensin receptor was also generated by PCR with the same forward primer and an rNTR170 reverse primer. PCR products were purified directly from the reaction mixture using the Wizard PCR purification kit (Promega).
Transcription, Translation, and Cross-Linking
Transcriptions were carried out using T7 or SP6 RNA polymerase (Promega) as appropriate, and the RNA obtained was purified and used for translation reactions. Cultured HT1080 fibroblasts (ATCC-CCL121; American Type Culture Collection, Manassas, VA) were permeabilized with the detergent digitonin (Calbiochem, Nottingham, United Kingdom) as described previously (Wilson et al., 1995) and used to provide a source of ER-derived membranes for most experiments. Where appropriate, standard canine pancreatic microsomes were used as described previously (Laird and High, 1997), whereas canine pancreatic microsomes depleted of their lumenal content by a pH 9.1 or 9.5 wash were made as described by Paver et al. (1989). RNA was translated in a rabbit reticulocyte lysate system (Promega) for 15 min at 30°C in the presence of [35S]methionine and semipermeabilized HT1080 cells or canine pancreatic microsomes. Subsequently, 0.1 mM aurintricarboxylic acid was added to inhibit translation initiation, and 10 min later translation was terminated by the addition of 2 mM cycloheximide. Where nascent chains were released from the ribosome before cross-linking, samples were treated with 2 mM puromycin and 50 mM EDTA for 10 min at 30°C in place of the cycloheximide treatment.
In the case of canine pancreatic micromes, the membrane-associated integration intermediates were isolated as described previously (Laird and High, 1997). Where semi-intact cells were used, the membrane-associated integration intermediates or the membrane-associated polypeptides resulting from puromycin/EDTA treatment were purified from the translation mix by centrifugation for 10 s at 16,000 × g and washed twice by resuspension in KHM buffer (110 mM KOAc, 2 mM MgOAc, 20 mM HEPES, pH 7.2). The resulting membrane pellet was resuspended in KHM and the cross-linking reagent BMH was added to 1 mM. BMH cross-links adjacent proteins via the -SH groups of free cysteines. Samples were incubated at 30°C for 10 min and the cross-linking reaction quenched by addition of 0.1 volumes of 100 mM 2-mercaptoethanol and incubation on ice for 10 min. As observed previously (Laird and High, 1997), the truncated integration intermediates were correctly membrane inserted, and efficient glycosylation of the N-terminal asparagine residues at positions 2 and 15 of ospin chains (Figures 3 and 5) and positions 4, 38, and 42 of the neurotensin receptor chain (Figure 9) was clearly visible.
Figure 3.
Components adjacent to TM1 are dependent upon nascent chain length. BMH-dependent cross-linking from [cys56] of opsin TM1 was carried out using membrane integration intermediates of increasing length as indicated (OP80–OP348). An open diamond indicates an unidentified cross-linking partner of 25 kDa (lanes 2 and 9), whereas an asterisk indicates a cross-linking partner of 21 kDa (lane 23). The position of an additional Sec61β adduct is indicated by an open arrowhead (lanes 27, 34, 41, and 48). We believe this represents cross-linking to a truncated opsin fragment that results from a strong ribosomal pause site present in the mRNA encoding opsin. The position of this opsin fragment is indicated by a white triangle (lanes 22, 29, 36, 43, and 50). Other symbols are as defined in the legend to Figure 1.
Figure 5.
Components adjacent to TM2 are dependent upon nascent chain length. BMH-dependent cross-linking from [cys87] of opsin TM2 was carried out using membrane integration intermediates of increasing length as indicated (OP96–OP348). An open diamond indicates an unidentified cross-linking partner of ∼25 kDa (lanes 2, 9, and 16), and an arrow indicates the location of adducts with the TRAM protein (lanes 35 and 42). Other symbols are as previously defined.
Figure 9.
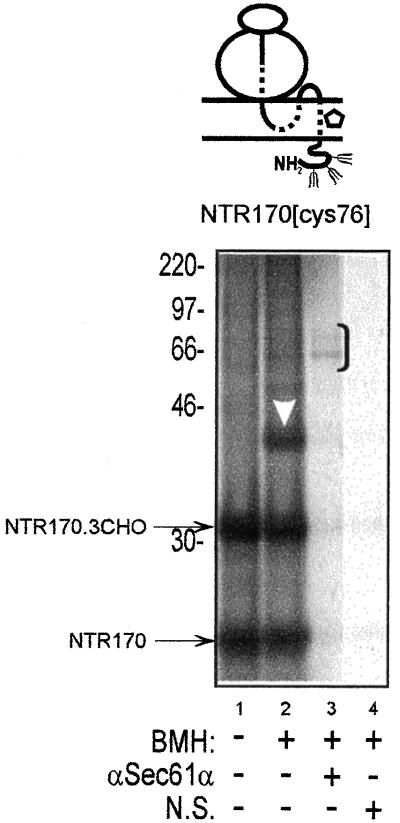
PAT-10 is adjacent to TM1 of the neurotensin receptor. A 170-residue integration intermediate of the neurotensin receptor (NTR170) with a single cysteine probe at residue 76 within TM1 (NTR170[cys76]) was treated with dimethyl sulfoxide or BMH, and the total membrane associated fraction was then analyzed (cf. lanes 1 and 2). The integration intermediate was efficiently N-glycosylated at all three acceptor sites (cf. NTR170 and NTR 170.3CHO) and after BMH treatment a prominent adduct with PAT-10 was observed (lane 2, white arrowhead). Specific BMH-dependent adducts with Sec61α could be immunoprecipitated (lane 3, bracket). Other symbols are as previously defined.
Immunoprecipitation
Denaturing immunoprecipitations were performed by heating the quenched cross-linked samples for 10 min at 95°C in the presence of 1% SDS. Four volumes of Triton immunoprecipitation (IP) buffer (10 mM Tris-HCl, pH 7.6, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100) was then added and the samples were incubated on ice for ∼30 min, followed by centrifugation at 16,000 × g for 5 min. Aliquots of the resulting supernatant were gently agitated overnight at 4°C with the relevant antisera in the presence of 200 μg/ml phenylmethylsulfonyl fluoride and 1 mM methionine. Protein A-Sepharose that had been preincubated with 20% bovine serum albumin for 30 min and then washed five times with IP buffer was added and the incubation continued for 2 h. Protein A-Sepharose–bound material was isolated by centrifugation at 16,000 × g for 1 min, washed four times with IP buffer, and then heated to 95°C for 5 min in SDS-PAGE sample buffer.
Sample Analysis
All samples were analyzed on 12% SDS-polyacrylamide gels and exposed for 3 d to a phosphorimaging plate for visualization on a Fuji BAS 2000 PhosphorImager system (Fuji Photo Film Co. Ltd., Tokyo, Japan).
RESULTS
TM1 of Short Opsin Integration Intermediate OP90 Engages ER Translocon
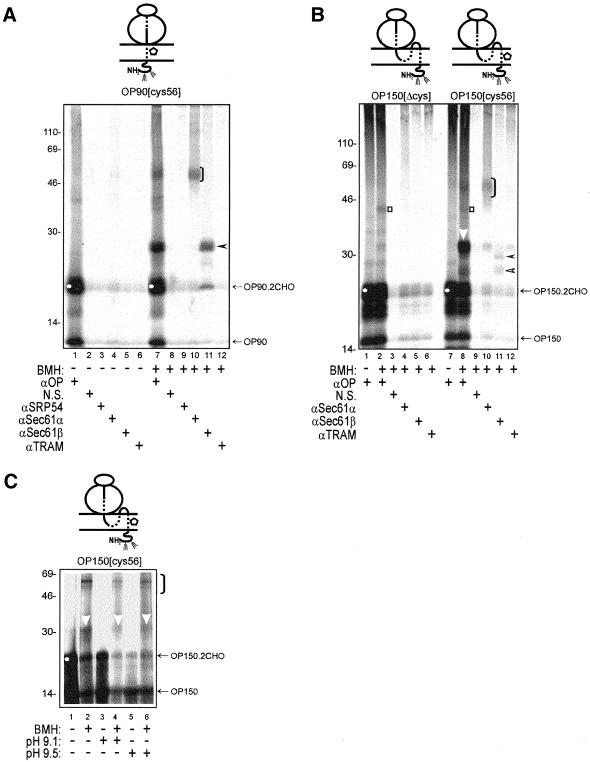
We have previously shown that short opsin chains can be efficiently inserted in the correct transmembrane orientation and used to study the ER components that mediate the membrane integration process (Laird and High, 1997). In this study, we have extended this work to focus specifically upon the ER components that are adjacent to the TMs of opsin during its membrane insertion. We initially generated a 90-amino acid-long integration intermediate of opsin with a single cysteine at residue 56 of TM1. This construct was named OP90[cys56], and we analyzed the molecular details of its membrane integration by cross-linking. The sulfydryl-specific reagent BMH was used to allow adduct formation only between the single cysteine present in the nascent chain and available cysteines of adjacent ER components (cf. Laird and High, 1997). Under these conditions, the addition of BMH generates specific cross-linking products with Sec61α (Figure 1A, lane 10, bracket) and Sec61β (Figure 1A, lane 11, filled arrowhead), demonstrating that in this short opsin integration intermediate TM1 engages two well-characterized subunits of the core ER translocon. In contrast, no cross-linking of TM1 to the TRAM protein, a well-characterized ER translocon-associated component, was observed (Figure 1A, lane 12). Adducts with subunits of the Sec61 complex were not observed in the absence of BMH (Figure 1A, lanes 4 and 5), confirming the specificity of the cross-linking reaction. By using semi-intact mammalian cells as a source of ER-derived membranes we observed a very efficient release of SRP from the membrane-targeted ospin nascent chain. Thus, no residual cross-linking of the membrane-associated chains to SRP54 was observed (Figure 1A, lane 9). This is in contrast to our previous studies of opsin biosynthesis with canine pancreatic microsomes, where a significant fraction of the membrane-targeted chains remained associated with the SRP54 subunit and were not released to interact with the ER translocon (Laird and High, 1997).
Figure 1.
(A) A short opsin integration intermediate is cross-linked to ER translocon components. A representation of the various ribosome bound integration intermediates analyzed is presented above each gel (Laird and High, 1997). The solid line represents the hydrophilic regions of the nascent chain, the dashed line signifies the transmembrane domains, and the open pentagon indicates the location of a single cysteine probe. In this case, a 90-residue-long integration intermediate of opsin with a single cysteine at residue 56 (OP90[cys56]) was synthesized in a rabbit reticulocyte lysate translation system in the presence of semipermeabilized HT-1080 tissue culture cells. The membrane-associated integration intermediates were isolated and treated with either BMH (lanes 7–12) or a solvent control (lanes 1–6). Specific, BMH-dependent, cross-linking products were observed (cf. lanes 1 and 7) and major adducts identified by immunoprecipitation with antibodies specific for Sec61α (lane 10, bracket) and Sec61β (lane 11, filled arrowhead). The doubly N-glycosylated form of the truncated opsin chain is indicated by a white circle (lanes 1 and 7). All opsin-derived products were immunoprecipitated with a mouse monoclonal antibody specific for the N-terminal region of the polypeptide (αOP, lanes 1 and 7). Other antisera recognizing known ER-targeting (SRP54) and translocon-associated components (TRAM) were also tested, in addition to a nonrelated serum (N.S.) to control the specificity of immunoprecipitation. (B) BMH-dependent cross-linking from residue 56C of the nascent chain is site specific. A 150-residue integration intermediate of an opsin-cys null polypeptide (OP150[Δcys]) was treated with BMH (lanes 2–6) and compared with the same integration intermediate with a single cysteine probe located at residue 56 within TM1 (lanes 8–12). In the absence of a cysteine residue, only a very weak adduct is detected upon the addition of BMH (lane 2, open square). In contrast, the OP150[cys56] nascent chain shows distinct BMH-dependent adducts with Sec61α (lane 10, black bracket), Sec61β (lane 11, filled and open arrowheads), and an unidentified protein of 10 kDa (lane 8, white arrowhead). (C) PAT-10 is present in canine pancreatic microsomes before and after a high pH wash. Integration intermediates of OP150[cys56] were generated using standard canine pancreatic microsomes (lanes 1 and 2) or microsomes washed at pH 9.1 (lanes 3 and 4) and 9.5 (lanes 5 and 6). Samples were treated with BMH (lanes 2, 4 and 6) or a solvent control (lanes 1, 3, and 5), and the total products of the reactions were analyzed by gel electrophoresis. BMH-dependent adducts with PAT-10 (lanes 2, 4, and 6, white arrowhead) were clearly visible. The relative mobility of the other major adduct (lanes 2, 4, and 6, black bracket) indicates that it is Sec61α (cf. Figure 1B).
TM1 of Longer Opsin Integration Intermediate OP150 Is Adjacent to a 10-kDa Protein
Previous studies of membrane protein integration have made use of different length integration intermediates to try and mimic different stages of the membrane insertion process and provide a “snapshot” of the process at distinct points (Martoglio and Dobberstein, 1996; Laird and High, 1997). We therefore studied a longer opsin integration intermediate with a single cysteine probe in the same position within TM1. When a membrane integration intermediate of OP150[cys56] was treated with BMH, cross-linking to Sec61α and Sec61β was again detected. The efficiency of cross-linking to Sec61β is significantly reduced in comparison with the adduct detected with OP90[cys56], and a doublet of products is now seen (Figure 1B, lanes 10 and 11; see below). The most striking difference from the shorter OP90 intermediate is the appearance of a particularly strong adduct with an ∼10-kDa component that is clearly distinct from Sec61β (Figure 1B, lane 8, white arrowhead; cf. Figure 1A, lane 7). We named this component PAT-10. As observed with OP90[cys56], the OP150[cys56] integration intermediate was not cross-linked to the TRAM protein (Figure 1B, lane 12) and no nascent chains remained associated with the SRP54 subunit (Figure 1B, lane 8).
During the course of our analysis of OP150[cys56], we carried out an additional control for cross-linking specificity by using a cysteine-free opsin integration intermediate, OP150[Δcys] (Figure 1B, lanes 1–6). When the nascent chain contained no cysteine residues, no detectable adducts with Sec61α, Sec61β, or PAT-10 were detected (Figure 1B, lanes 2, 4, and 5). A very weak BMH-dependent adduct of ∼44 kDa was observed with both OP150[cys56] and OP150[Δcys] (Figure 1B, cf. lanes 1, 2, 7, and 8, open square). This is probably a low-efficiency adduct formed via a free amino group of the opsin chain, and it was not immunoprecipitated by any of the ER component-specific antisera tested.
PAT-10 Is Also Detected in Canine Pancreatic Microsomes and Is Not Removed by Depletion of Luminal Content
The PAT-10 component was not detected during our previous study of opsin biogenesis (Laird and High, 1997). We reasoned that this may be due to the different location of the single cysteine probe in our current study, or because we were now using semi-intact mammalian cells as a source of ER membranes rather than the canine pancreatic microsomes that we had used previously. To address this issue, we analyzed the BMH-dependent cross-linking profile of the OP150[cys56] integration intermediate synthesized in the presence of canine pancreatic microsomes. We found that the nascent chain was cross-linked to PAT-10 (Figure 1C, lane 2, white arrowhead). We therefore concluded that cross-linking to PAT-10 is not dependent upon the source of ER membrane used for study. We noted that the efficiency of the membrane integration of our opsin intermediates was consistently higher in semi-intact cells than canine pancreatic microsomes, as judged by the proportion of fully N-glycosylated nascent polypeptides present (Figure 1, B and C; cf. amounts of OP150.2CHO [white circle] vs. OP150). Likewise, the relative intensity of the PAT-10 adduct was significantly stronger in semi-intact mammalian cells than in canine pancreatic microsomes (cf. Figure 1B, lane 8, and C, lane 2). We therefore decided to use semi-intact cells to carry out a detailed analysis of opsin integration.
We were able to exploit canine pancreatic microsomes to investigate one additional feature of the PAT-10 component, namely, whether PAT-10 could be depleted by prior washing of the microsomal membranes with an alkaline buffer (>pH 9.0). Such treatment can remove soluble components of the ER lumen such as protein disulfide isomerase (Paver et al., 1989). It may also remove peripheral proteins that are loosely associated with the cytoplasmic surface of microsomes, although some components are only removed at a much higher pH (Miller et al., 1995) that can also result in inefficient translocation (Nicchitta and Blobel, 1993). We found that the use of alkaline buffers was ineffective at depleting protein disulfide isomerase from the ER of semi-intact mammalian cells (our unpublished data; Paver et al., 1989). In contrast, >80% of the protein disulfide isomerase (our unpublished data) could be removed from canine pancreatic microsomes by a single pH 9.5 wash (Paver et al., 1989; cf. Nicchitta and Blobel, 1993). BMH-dependent adducts of OP150[cys56] with PAT-10 and Sec61α were clearly visible with both pH 9.1- and pH 9.5-washed microsomes (Figure 1C, lanes 4 and 6, white arrowhead). Although the N-glycosylation of OP-150 was clearly less efficient after washing the microsomes at pH 9.1 and 9.5, quantification confirmed that the amount of the PAT-10 adduct relative to the level of the OP150.2CHO product was not diminished in either case (Figure 1C, lanes 2, 4, and 6; our unpublished data). We conclude that the pretreatment of microsomes with a pH 9.5 wash does not remove the PAT-10 component under conditions where the luminal content has been significantly depleted (cf. Nicchitta and Blobel, 1993).
Opsin TM1 Is Surrounded by a Common Set of ER Components
To analyze the proteinaceous environment of opsin TM1 in greater detail, we chose a nascent chain length of 130 aa as a point between the 90 and 150 aa integration intermediates already described (Figure 1). We then created a series of opsin mutants, each with a single cysteine at one of five sequential positions within TM1 (residues 47–51 inclusive) that spanned its presumptive central region (Table 1). This approach was designed to establish to what extent the location and/or orientation of a cysteine probe could influence its ability to form BMH dependent adducts with adjacent ER components (cf. High et al., 1993; Mothes et al., 1994). This detailed analysis showed that some degree of cross-linking to Sec61α, Sec61β, and PAT-10 could occur from each of the five locations analyzed (Figure 2, lanes 16–20, bracket; 21–25, arrowhead; and 6–10, white arrowhead).
Figure 2.
Site-specific cross-linking from sequential locations with opsin TM1. A set of 130-residue membrane integration intermediates of opsin (OP130) with a single cysteine probe located at residue 47–51 of TM1 were treated with BMH as described for Figure 1. Each cysteine produced an adduct with Sec61α (lanes 16–20, black bracket), albeit with varying efficiency, whereas adducts with Sec61β were barely detectable (lanes 21–25, filled arrowhead). Cysteine probes at residues 47–49 produced weak adducts with PAT-10 (lanes 6–8, white arrowhead), whereas the probes at residues 50 and 51 generated very strong adducts with the same component (lanes 9 and 10).
Although the overall translation efficiency was similar for the five OP130 constructs (Figure 2, lanes 1–10, cf. OP130.2CHO product), there is a clear variation in the intensity of the cross-linking products formed. Most striking is the apparently inverse relationship in the efficiency of cross-linking to Sec61α and PAT-10 such that the Sec61α adduct is strongest when PAT-10 is weakest, and vice versa (Figure 2, cf. lanes 6–10 and 16–20). In the case of Sec61β, the cross-linking efficiency is relatively weak (Figure 2, lanes 21–25) as observed previously with the OP150 integration intermediate (cf. Figure 1B). The data presented in Figure 2 support the view that the Sec61 complex forms the core of the ER membrane insertion site (Heinrich et al., 2000; Beckmann et al., 2001). They also suggest that during the integration of opsin TM1 the components of the ER membrane insertion site may exhibit some degree of asymmetry in their positioning around this TM.
Environment of Opsin TM1 Alters with Increasing Chain Length
The data presented in Figure 2 indicate that the exact location of a cysteine probe within TM1 influences the intensity of adduct formation rather than the pattern of components that can be detected adjacent to the nascent chain. We further analyzed the proteinaceous environment of TM1 during the biogenesis of opsin by analyzing a variety of integration intermediates and using a single fixed location for the cysteine probe present in each case. We chose residue 56 as the location for the cysteine residue because it had consistently yielded efficient adducts with all of the cross-linking partners of the shorter opsin chain lengths analyzed (Figure 1, A and B; our unpublished data). By cross-linking from cys56 of opsin TM1 and using increasingly longer integration intermediates we hoped to examine whether the proteinaceous environment of TM1 changed significantly at any point during the biogenesis of the complete opsin polypeptide.
The shortest chain length we analyzed was an 80-amino acid integration intermediate of opsin (OP80). At this chain length, the polypeptide was efficiently N-glycosylated, confirming that its amino terminus was fully translocated across the ER membrane and that the integration intermediate was spanning the ER membrane insertion site as indicated (Figure 3, lanes 1 and 2, white circle; Laird and High, 1997). The OP80[cys56] intermediate generated only one major BMH-dependent cross-linking product (Figure 3, lane 2, open diamond). At this stage of ospin biosynthesis, cys 56 of TM1 is almost certainly located within the ribosome and this ∼25-kDa protein is probably ribosomal. The addition of only five amino acids to the nascent chain length (OP85[cys56]) resulted in specific adducts with the ER translocon components Sec61α and Sec61β (Figure 3, lanes 12 and 13), in addition to the 25-kDa component observed with OP80[cys56] (Figure 3, lane 9, open diamond). At this chain length, the adducts with Sec61β are stronger than those seen with Sec61α, consistent with the idea that Sec61β is encountered by the nascent chain at an early stage of its membrane insertion (cf. Laird and High, 1997). When OP96[cys56] was analyzed it was no longer cross-linked to the 25-kDa putative ribosomal protein (Figure 3, lane 16) but now formed strong adducts with both Sec61α and Sec61β (Figure 3, lanes 19 and 20).
When the integrating nascent chain is 130 aa (OP130[cys56]) a substantial change in the profile of cross-linking partners is observed (Figure 3, cf. lanes 16 and 23). Adducts with Sec61α and Sec61β are still observed (Figure 3, lanes 26 and 27), however, particularly efficient adducts with PAT-10 (Figure 3, lane 23, white arrow) and an ∼21-kDa component (Figure 3, lane 23, asterisk) were also seen. These adducts are so strong that, like the nascent chain, background levels are apparent even after immunoprecipitation in the presence of SDS when using a nonrelated serum (Figure 3, lane 24). The cross-linking of PAT-10 to TM1 of the OP130[cys56] integration intermediate confirms the cross-linking of OP130 to PAT-10 from cysteines located at residues 47–51 of TM1 (cf. Figure 2, lanes 6–10). A comparison of these various adducts indicates that PAT-10 is cross-linked more efficiently when the single cysteine probe is located toward the cytosolic side of TM1 (cf. Figures 2, lane 10, and 3, lane 23). In contrast to PAT-10, the 21-kDa adduct observed with OP130[cys56] (Figure 3, lane 23, asterisk) was not readily apparent from more distal locations within TM1 (i.e., residues 47–51; Figure 2, lanes 6–10). As previously observed with the OP150[cys56] intermediate (Figure 1B, lane 11), the OP130[cys56]-Sec61β adduct appears as a doublet (Figure 3, lane 27). The exact basis for this behavior is unclear, but it may reflect the presence of a strong ribosomal pause site in the mRNA encoding the OP130 integration intermediate (see below).
For OP150[cys56], strong adducts with PAT-10 and Sec61α, plus a weaker doublet of Sec61β products, are observed (Figure 3, lanes 30, 33 and 34). In contrast, cross-linking to the ∼21-kDa protein no longer occurs (cf. Figure 3, lanes 23 and 30). With OP165[cys56] the most notable features are strong cross-linking to PAT-10 and Sec61α (Figure 3, lanes 37 and 40), whereas with OP276[cys56], cross-linking to PAT-10 remains pronounced but the adduct with Sec61α becomes noticeably weaker and more diffuse (Figure 3, lanes 44 and 47, respectively). In an attempt to represent a very late stage of opsin integration, we generated a full-length polypeptide of 348 aa that remained bound to the ribosome because the encoding mRNA lacked a stop codon (OP348). In this case, very weak cross-linking of TM1 to Sec61α and no adducts with Sec61β were seen (Figure 3, lanes 54 and 55). Most striking was the observation that strong cross-linking of OP348[cys56] to PAT-10 could still be detected (Figure 3, lane 51).
When the pattern of the Sec61α and PAT-10 adducts presented in Figure 3 is examined, the size of these adducts increases in proportion to the size of the nascent opsin chain (Figure 3, lanes 12, 19, 26, 33, and 40 and lanes 23, 30, 37, 44, and 51). This is distinct from the Sec61β adducts where from OP130[cys56] to OP276[cys56] two adducts were obtained. For each opsin integration intermediate examined, the relative mobility of the larger adduct was roughly proportional to the size of the nascent chain (Figure 3, lanes 13, 20, 27, 34, 41, and 48, filled arrowheads). In contrast, the relative mobility of the smaller adduct remained roughly constant and was independent of nascent chain length (Figure 3, lanes 27, 34, 41, and 48, open arrowheads). This smaller adduct is most likely generated by a common truncated opsin fragment (position indicated by white triangles in Figure 3, lanes 22, 29, 36, 43, and 50) that may result from a strong ribosomal pause site present in the mRNA encoding opsin (cf. Wolin and Walter, 1988).
It should be noted that at no point during our analysis of opsin integration intermediates ranging from 80 to 348 aa was any evidence for the cross-linking of opsin TM1 to the TRAM protein observed (Figure 3, lanes 7, 14, 21, 28, 35, 42, 49, and 56). Additional experiments were carried out to show that the cross-linking of opsin integration intermediates to Sec61α, Sec61β, and PAT-10 was dependent upon the presence of a ribosome at the C terminus of the nascent polypeptide. When the ribosome was removed from the integration intermediates before the addition of BMH, no adducts with these components were observed (our unpublished data). Thus, like the subunits of the Sec61 complex, PAT-10 is adjacent to a membrane-inserting opsin chain when it is in the context of an active ER translocon.
TM2 of an Opsin Integration Intermediate (OP150) Engages ER Translocon
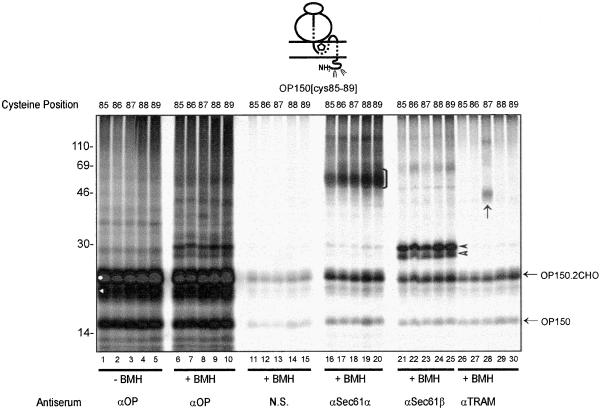
A simple model for the integration of multiple TMs at the ER would be one where the make up of the ER insertion site would be identical for each of the individual TMs present in a polytopic protein. To investigate whether such a model has any factual basis, we extended our site-specific cross-linking analysis to encompass TM2 of the opsin nascent chain. Building on our findings with TM1, we began this analysis with a fixed length of integration intermediate and varied the specific position of the cysteine probe within TM2. Given that the membrane integration of TM2 occurs after that of TM1, we used a chain length of 150 aa (OP150) to generate suitable integration intermediates and varied the location of the cysteine probe from residue 85–89 within TM2 (Table 1). When these OP150 integration intermediates were treated with BMH, a number of cross-linking products were observed (Figure 4, lanes 6–10). Among these, clear adducts with both Sec61α and Sec61β could be detected from each of the five locations analyzed (Figure 4, lanes 16–20 and 21–25). As with OP150[cys56], the OP150[cys85–89]-Sec61β adducts appeared as dimers (Figure 4, lanes 21–25, filled and open arrowheads). The strong cross-linking of opsin TM2 to two subunits of the Sec61 complex indicates that this complex is a principal component of the ER insertion site that is used by TM2.
Figure 4.
Site-specific cross-linking from sequential locations with opsin TM2. A set of 150-residue membrane integration intermediates of opsin (OP150) with a single cysteine probe located at residues 85–89 of TM2 were treated with BMH. Each cysteine produced similar adducts with Sec61α (lanes 16–20, black bracket) and Sec61β (lanes 21–25, filled and open arrowheads). Only the cysteine at residue 87 formed a detectable adduct with the TRAM protein (lane 28, black arrow). Other symbols are as previously defined.
This detailed analysis of TM2 yielded two other striking results. First, we could detect cross-linking of OP150 to the TRAM protein (Figure 4, lane 28, black arrow). In contrast to the Sec61 complex, the association of the TRAM protein with the nascent opsin chain can be detected from only one of the five cysteine probes utilized during the analysis. Thus, TRAM could only be efficiently cross-linked from OP150[cys87] (Figure 4, cf. lanes 26–30). Second, no evidence for the cross-linking of PAT-10 to TM2 was obtained with the five OP150 integration intermediates analyzed.
Environment of Opsin TM2 Alters with Increasing Chain Length
We extended our analysis of TM2 to establish whether its proteinaceous environment was influenced by nascent chain length. We chose a cysteine probe within TM2 that had yielded detectable adducts with all of the cross-linking partners observed with the OP150 integration intermediate, i.e., residue 87 (cf. Figure 4, lanes 18, 23 and 28). Like opsin TM1 (cf. Figure 3), the shorter integration intermediates (OP96[cys87], OP106[cys87], and OP130[cys87]) formed adducts with an unidentified protein of ∼25 kDa that is probably ribosomal (Figure 5, lanes 2, 9, and 16; Laird and High, 1997). This adduct was not readily apparent with OP137 (Figure 5, lane 23), whereas at longer chain lengths several unidentified adducts were apparent (Figure 5, lanes 30 and 37).
Weak cross-linking to Sec61β could be detected with OP96[cys87] where the cysteine probe should be located deep within the ribosome (Figure 5, lane 6, filled arrowhead). As the length of the nascent chain was increased, the efficiency of cross-linking to Sec61β increased, reaching a maximum intensity with OP137[cys87] (Figure 5, lanes 13, 20, and 27, filled arrowhead). With OP150[cys87], two Sec61β adducts were visible with the size of the upper adduct being proportionate to the increase in nascent chain length (Figure 5, lane 34, cf. filled arrowhead and open arrowhead). The larger Sec61β adduct was not detected with OP175[cys87] or longer integration intermediates (Figure 5, lanes 41, 48, and 55). The smaller Sec61β adduct was apparent with the remainder of the opsin nascent chains analyzed (OP137[cys87] to OP348[cys87]; Figure 5, lanes 27, 34, 41, 48, and 55, open arrowhead). The relative mobility of this adduct remained constant, independent of the nascent chain length, and as discussed above, is probably caused by the cross-linking of Sec61β to a population of short opsin chains that are generated by a natural ribosomal pause site present in the encoding mRNA. In the case of OP276 and 348, the resulting Sec61β adducts are actually smaller than the fully N-glycosylated opsin integration intermediate (Figure 5, cf. lanes 43 and 50, white circle, and lanes 48 and 55, open arrowhead). We therefore conclude that cross-linking of Sec61β to authentic opsin nascent chains only occurs up to a chain length of ∼150 aa.
Cross-linking to Sec61α was apparent with OP130[cys87], a longer chain length than that required to give Sec61β adducts (Figure 5, lane 19, bracket), whereas with OP137[cys87] the cross-linking of TM2 to Sec61α was at its strongest (Figure 5, lane 26, bracket). Significant cross-linking to Sec61α was still detected with OP150[cys87] (Figure 5, lane 33, bracket; see also Figure 4, lanes 16–20), whereas at longer chain lengths the Sec61α adduct were noticeably weaker and less distinct (Figure 5, lanes 40, 47, and 54, brackets). In the case of the TRAM protein, specific adducts with TM2 could be detected with OP150[cys87] and OP175[cys87] (Figure 5, lanes 35 and 42, black arrow; cf. Figure 4, lane 28), but not with either shorter or longer integration intermediates. At no point during this analysis did we obtain any evidence for the cross-linking of opsin TM2 to PAT-10. The only “small” cross-linking partner of TM2 was found to be Sec61β (Figure 5, lanes 2, 6, 9, 13, 16, 20, 23, 27, 30, and 34, filled and open arrowheads). As indicated for the TM1 adducts, the cross-linking of opsin TM2 to the components of the ER insertion site also required the presence of a ribosome bound integration intermediate (our unpublished data).
On the basis of the data outlined above, we conclude that the Sec61 complex mediates the membrane insertion of both TM1 and TM2 of opsin. This is fully consistent with its role as a core component of the ER translocon. It is striking that the precise makeup of the ER insertion site is different for TM1 and TM2. Hence, TM1 is consistently found adjacent to PAT-10, whereas TM2 is transiently associated with the TRAM protein. PAT-10 is a small protein that is associated with the ER translocon, and because it can be cross-linked using BMH, we can assume that it contains one or more cysteine residues. One obvious candidate for such a protein was Sec61γ, the smallest subunit of the Sec61 complex (Hartmann et al., 1994). However, immunoprecipitation analysis of the PAT-10 adducts using a serum recognizing Sec61γ indicated that PAT-10 is distinct from this component (our unpublished data). Since PAT-10 behaved as a novel ER translocon-associated protein, we further characterized its association with nascent opsin chains.
PAT-10 Cross-Linking Is Independent of N-Glycosylation
The N terminus of opsin contains two sites for the attachment of N-linked glycans and these are efficiently used in vitro (cf. Figures 1–5). A previous study had established that a small ER protein, RAMP4, can bind to nascent polypeptides and may act to regulate the efficiency with which particular precursors were N-glycosylated (Schröder et al., 1999). We therefore obtained well characterized antisera recognizing RAMP4 and tested them for their ability to immunoprecipitate the PAT-10 adduct (our unpublished data). No immunoprecipitation was obtained and we therefore conclude that PAT-10 is not RAMP4.
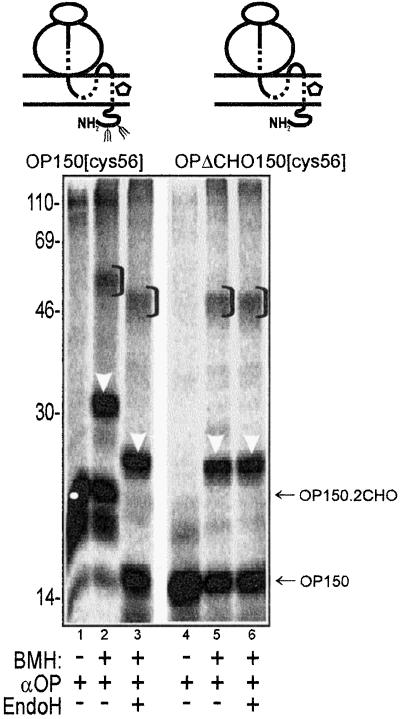
Although PAT-10 is distinct from RAMP4, it remained possible that the recruitment of PAT-10 by TM1 was dependent upon the N-glycosylation of the opsin nascent chain. To address this question we generated a version of opsin that lacked the two consensus N-glycosylation sites of the wild-type protein (OPΔCHO). We then compared the cross-linking profiles obtained with the OP150[cys56] and OP150ΔCHO[cys56] integration intermediates. This analysis clearly showed that both OP150[cys56] and OP150ΔCHO[cys56] are cross-linked to PAT-10 (Figure 6, lanes 2 and 5, white arrowheads). As expected, if the bulk of the N-linked glycans are removed from the OP150 nascent chain by endoglycosidase H (EndoH) treatment, the resulting PAT-10 adduct has the same mobility as the OP150ΔCHO adduct (Figure 6, cf. lanes 3 and 6). Furthermore, the mobility of the OP150ΔCHO[cys56]-PAT-10 adduct is unaffected by EndoH treatment (Figure 6, cf. lanes 5 and 6, white arrows) showing that the PAT-10 protein is not N-glycosylated.
Figure 6.
Association of TM1 with PAT-10 does not require N-glycosylation of the nascent opsin chain. The BMH-dependent cross-linking partners of OP150[cys56] and OP150ΔCHO[cys56], a derivative lacking the two N-glycosylation sites of the wild-type protein, were compared. In both cases, adducts with Sec61α and PAT-10 were clearly apparent (lanes 2, 3, 5, and 6). EndoH treatment confirmed that the mobility of the deglycosylated adducts was identical to that of the cross-linking products obtained with the OP150ΔCHO nascent chain (cf. lanes 2, 3, 5, and 6). All symbols are as previously defined.
PAT-10 Cross-Linking Is Independent of TM1 Amino Acid Sequence
In the case of RAMP4, a specific motif within the nascent polypeptide acts to recruit the RAMP4 interaction (Schröder et al., 1999). Hence, it was feasible that a specific sequence element within TM1 may be required to observe an association with PAT-10. We therefore set out to radically alter the sequence of TM1 to determine the effect that this had upon PAT-10 cross-linking. We had already established that TM2 of opsin did not show significant cross-linking to PAT-10 (Figures 4 and 5), and we therefore used this stretch of amino acids to replace that present in the original opsin TM1 (Table 1 and Figure 7). The transmembrane orientation of TM1 is the opposite of TM2, and we inserted the amino acid sequence of TM2 in a “forward” orientation with the first amino acid of TM2 on the luminal side of the ER membrane [Figure 7, TM2x2 (Fwd)]. We also created a second version of opsin where the amino acid sequence of TM2 was artificially inserted in “reverse” order across the transmembrane-spanning region (Table 1 and Figure 7).
Figure 7.
(A) Membrane topology of truncated opsin polypeptides before and after the substitution of TM1. TM1 of the original opsin integration intermediates (OP) was replaced by inserting the amino acid sequence of opsin TM2 in place of TM1. Two such derivatives were prepared, one with the amino acid sequence of TM2 inserted in its original form, OPTM2x2(Fwd), and a second version where the order of the amino acids was reversed, OPTM2x2(Rev) (Table 1). TMs are shown as shaded regions with the approximate location of the single cysteine probe indicated by an open pentagon and sites of N-linked glycosylation indicated by branched structures. Five versions of each of the two derivatives were generated, each with a cysteine at a different location within the first transmembrane span (Table 1). (B and C) PAT-10 remains associated with TM1 when its amino acid composition is altered. In B, a set of 130 residue membrane integration intermediates of OPTM2x2(Fwd) with a single cysteine probe located at residue 49–53 within the first TM, were treated with BMH (B, lanes 6–10) or solvent control (B, lanes 1–5). Adducts with PAT-10 (lanes 6–10, white arrowhead) and Sec61α (lanes 16–20, black bracket) were apparent in all cases, whereas cross-linking to Sec61β was weak and variable, in part reflecting differences in translation efficiency (lanes 21–25, filled arrowhead). Cross-linking to an unidentified protein of ∼25 kDa was also observed (lanes 6–10, open diamond), but no adducts with the TRAM protein could be seen (lanes 26–30). In C, a set of 150-residue membrane integration intermediates of OPTM2x2(Rev), with a single cysteine probe located at residues 47–51 within the first TM, was treated with BMH (C, lanes 6–10) or solvent control (C, lanes 1–5). Antisera used to immunoprecipitate the resulting products are as indicated for B. Adducts with PAT-10 (lanes 6–10, white arrowhead), Sec61α (lanes 16–20, black bracket), and Sec61β (lanes 21–25, filled and open arrowheads) were detected in all cases. Cross-linking to an unidentified protein of ∼21 kDa (lanes 6–10, asterisk) was also observed, but no adducts with the TRAM protein could be seen (lanes 26–30). Other symbols are as previously defined.
Both mutant versions of opsin [OPTM2x2(Fwd) and OPTM2x2(Rev)] were efficiently membrane inserted and N-glycosylated confirming that they had the same transmembrane topology as wild-type opsin with their N-termini translocated into the ER lumen (Figure 7, B and C; our unpublished data). As with our previous studies we also engineered five individual versions of both opsin-derived mutants, each with a cysteine residue at a different location within the first TM. Most strikingly, when ribosome bound integration intermediates of the OP130TM2x2(Fwd) and OP150TM2x2(Rev) polypeptides were analyzed by BMH-dependent cross-linking, adducts with PAT-10 were observed in each case and from all of the locations analyzed (Figures 7, B and C, lanes 6–10, white arrowheads). Hence, the association of PAT-10 with TM1 is independent of its precise amino acid composition, because this can be replaced with two alternative TM2-derived sequences without disrupting PAT-10 cross-linking.
The first TMs of both OP130TM2x2(Fwd) and OP150TM2x2(Rev) could be cross-linked to Sec61α and Sec61β from most of the cysteine locations tested, although with varying efficiency (Figure 7, B and C, lanes 16–25, brackets and filled and open arrowheads). Likewise, adducts with either an ∼25-kDa component (Figures 7B, lanes 6–10, open diamond) or an ∼21-kDa component (Figures 7C, lanes 6–10, asterisk) could be detected from each of the variations tested. In contrast, no cross-linking of the opsin TM2 amino acid sequence to the TRAM protein could be detected when it was present in the position of the first TM span (Figures 7, B and C, lanes 26–30). When TM2 is in its native location, cross-linking to the TRAM protein is chain length dependent (Figure 5). We therefore studied two additional integration intermediates: OP165TM2x2(Fwd) and OP130TM2x2(Rev) but again found no cross-linking to the TRAM protein (our unpublished data).
Taken together, these data indicated that the most crucial factor in the association of opsin TM1 with PAT-10 was its relative location within the nascent chain, and not any sequence specific properties of this TM. Likewise, the specific cross-linking of TM2 to the TRAM protein also seems to be a consequence of the location of the TM and not of its amino acid sequence.
PAT-10 Cross-Linking Is Independent of TM1 Orientation
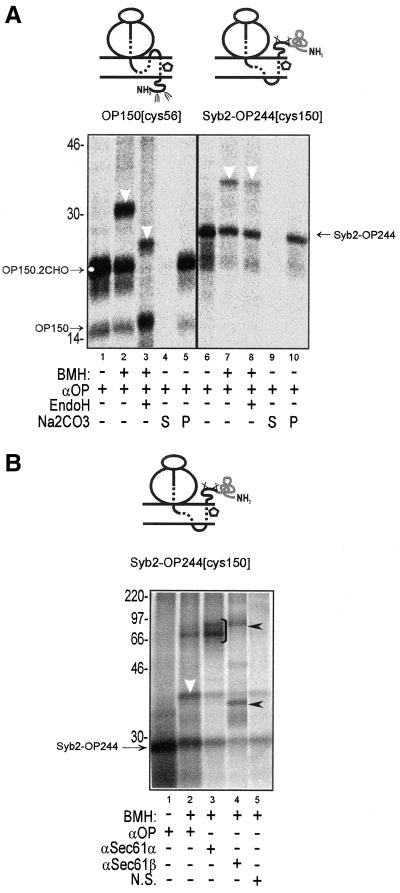
Having established that there was no absolute amino acid sequence requirement for the recruitment of PAT-10 by opsin TM1, we next investigated whether it was the transmembrane orientation of opsin TM1 that was responsible for its pronounced PAT-10 association. It has been established that the addition of a large tightly folded protein domain can prevent the translocation of the N-terminal domain of a polypeptide across the ER membrane (Denzer et al., 1995). We therefore attached the N-terminal domain of rat synaptobrevin 2 to the N terminus of the opsin coding region (Figure 8A, Syb2-OP244) to prevent the translocation of its N terminus. The resulting protein was efficiently membrane inserted (Figure 8A, lane 6). Like the correctly oriented OP150 integration intermediate, Syb2-OP244 was completely resistant to extraction with alkaline sodium carbonate buffer and hence efficiently integrated into the lipid bilayer (Figure 8A, lanes 4, 5, 9, and 10). However, in contrast to an authentically integrated opsin intermediate (OP150), the N-terminal domain of an Syb2-OP244 integration intermediate was not N-glycosylated (Figure 8A, cf. nascent chains in lanes 1–3 and 6–8). BMH-dependent cross-linking occurred from a single cysteine located at an equivalent position within TM1 of each construct (residue 56 for OP150 and residue 150 For Syb2-OP244), and cross-linking to PAT-10 was observed in both cases (Figure 8A, lanes 2, 3, 7, and 8, white arrowhead). The authenticity of the inverted Syb2-OP244 integration intermediate was further confirmed by showing that the nascent chain could be cross-linked to the Sec61α and Sec61β subunits of the Sec 61 complex (Figure 8B, lanes 3 and 4). We also detected the nascent chain present in adducts that contained both Sec61α and Sec61β (Figure 8B, lane 4, upper filled arrowhead). Because the Sec61α subunit contains multiple cysteine residues, this presumably reflects its ability to be cross-linked to both the nascent opsin chain and the β subunit of the Sec61 complex at the same time (cf. Laird and High, 1997). On the basis of our analysis of Syb2-OP244, we conclude that the association of PAT-10 with opsin TM1 is independent of its transmembrane orientation.
Figure 8.
(A) Association of PAT-10 with ospin TM1 is not dependent upon its transmembrane orientation. The cytosolic domain of synaptobrevin 2 was added to the N terminus of opsin (Syb2-OP244) to prevent its translocation. Single cysteine probes were included at equivalent locations in TM1 of OP150 and Syb2-OP244 to give OP150[cys56] and Syb2-OP244[cys150]. Membrane integration intermediates of these two nascent chains were then treated with BMH or a solvent control as indicated, and the resulting adducts were compared. In each case, BMH-dependent cross-linking to PAT-10 was observed (lanes 2 and 7). EndoH treatment confirmed that TM1 had adopted a different transmembrane topology in the two nascent chains (cf. lanes 2, 3, 7, and 8). If the membrane associated material (cf. lanes 1 and 6) was treated with alkaline sodium carbonate solution (Na2CO3) and reisolated by centrifugation, all of the OP150 and Syb2-OP244 polypeptide chains were found in the resulting membrane pellets (P, lanes 5 and 10). No material was recovered in the supernatant fractions (S, lanes 4 and 9). The resistance of the membrane-associated material to extraction with alkaline sodium carbonate solution indicates that both OP150 and Syb2-OP244 were efficiently integrated into the lipid bilayer (cf. Laird and High, 1997). (B) Syb2-OP244 nascent chain is cross-linked to subunits of the Sec61 complex. The Syb2-OP244[cys150] integration intermediate was cross-linked to adjacent ER components by using BMH, and the resulting products were analyzed by immunoprecipitation. Specific BMH-dependent adducts with Sec61α (lane 3, bracket) and Sec61β (lane 4, lower filled arrowhead) were observed in addition to a high-molecular-weight adduct containing the radiolabeled nascent chain, Sec61α, and Sec61β (lane 4, upper filled arrow head). Other symbols are as previously defined.
Association of PAT-10 Is Not Restricted to Opsin Nascent Chains
Previous studies in Drosophila have identified specialized ER components that interact with specific isoforms of opsin (Colley et al., 1991), and we therefore wished to establish the association of PAT-10 that we observed was restricted solely to nascent opsin chains. We carried out a site-specific cross-linking analysis from the first TM of the neurotensin receptor, a distinct seven TM protein (Table 1; Tucker and Griss-hammer, 1996, and references therein). In this case, a truncated chain of 170 amino acids was used and we found that the N terminus was efficiently glycosylated at all three acceptor sites, confirming that we had generated an authentic integration intermediate (Figure 9, lane 1). The neurotensin polypeptide used for his analysis was engineered to contain a single cystine located near the center of the presumptive first transmembrane domain (residue 76, Table 1). When cysteine dependent cross-linking was initiated by adding BMH to the trapped integration intermediate, we observed strong cross-linking to a component of 10 kDa that seems identical to the PAT-10 component cross-linked to TM1 of opsin nascent chains (Figure 9, cf. lanes 1 and 2). The 170-amino acid integration intermediate of the neurotensin receptor was also cross-linked to the Sec61α subunit (Figure 9, lane 3). Hence, as we found with opsin, the neurotensin receptor is adjacent to PAT-10 in the context of a membrane-integrating nascent polypeptide chain. We conclude that the association of PAT-10 with nascent polypeptide chains is not restricted to TM1 of opsin and that TM1 of the neurotensin receptor is also adjacent to this component during the membrane integration of this polypeptide.
DISCUSSION
We have carried out a detailed cross-linking analysis to establish the nearest neighbors of TM1 and TM2 during the integration of the seven transmembrane-spanning protein ospin. We find that both TMs use the Sec61 complex during membrane insertion, but that the accessory components that are associated with the two TMs are distinct with respect to both their identity and behavior.
Sec61 Complex Plays a Central Role during Membrane Insertion of Multiple TMs
As the single cysteine probes emerge from the ribosome, we find both TM1 and TM2 are first cross-linked to putative ∼25-kDa ribosomal protein(s) and the Sec61β subunit. We saw a similar behavior with a single cysteine probe located in the hydrophilic loop region located between TM2 and TM3 (Laird and High, 1997). Hence, as the nascent opsin chain emerges from the ribosome, different regions of the polypeptide are adjacent to a similar set of ribosomal and ER proteins. This behavior suggests that the entire nascent polypeptide chain follows a defined route through the ribosome and into the ER translocon (Beckmann et al., 2001).
At specific nascent chain lengths, both TM1 and TM2 are efficiently cross-linked to the α and β subunits of the Sec61 complex. This is in accord with previous studies of polytopic membrane protein insertion (Laird and High, 1997; Mothes et al., 1997), and we conclude that the Sec61 complex plays a central role during the membrane insertion of both TM1 and TM2. This proposal fits well with our current understanding of the structure and function of the Sec61 complex, which lies at the heart of the ER translocon (Johnson and van Waes, 1999; Menetret et al., 2000; Beckmann et al., 2001).
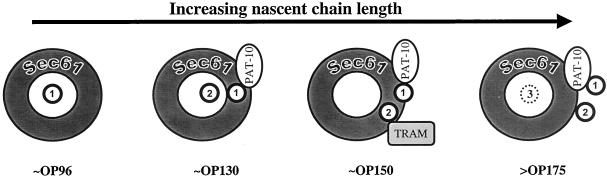
By probing the environment of the opsin TMs from several adjacent locations, we could analyze the spatial relationship between the nascent, membrane-inserting, polypeptide chain and the ER translocon (cf. Figure 10.) In the case of TM2, we find that the Sec61α and Sec61β subunits of the ER translocon can be efficiently cross-linked from any of the five positions analyzed. These data support a model where at the stage of opsin synthesis we have analyzed (OP-150), TM2 is located inside a Sec61-lined channel that spans the ER membrane (Johnson and van Waes, 1999; Menetret et al., 2000; Beckmann et al., 2001). In the case of TM1, where an OP-130 integration intermediate was analyzed in detail, a significant degree of asymmetry was observed. Hence, when cross-linking to Sec61α was at its strongest, cross-linking to the novel component PAT-10 was weak, and vice versa (cf. Figure 10). Thus, the specific environment of a particular TM may depend on both the “stage” of biosynthesis that is analyzed and its relative location within the nascent polypeptide (see below).
Figure 10.
Model outlining the proteins adjacent to opsin TM1 and TM2 during membrane insertion. We find that the local environment of TM1 and TM2 alters during membrane insertion of the nascent opsin chain. TM1 is represented by circle number 1 and TM2 by circle number 2, the hypothetical ER membrane insertion site is viewed from above, and each cartoon represents a different stage of opsin integration as represented by particular integration intermediates (Johnson and van Waes, 1999). The ribosome and regions of nascent chains connecting the TMs have been omitted for simplicity. With the short integration intermediates (OP96), TM1 is adjacent to subunits of the Sec61 complex. As the nascent chain length is increased (OP130), TM1 can be cross-linked to PAT-10 and Sec61, and TM2 is also now found adjacent to Sec61. As more of the nascent chain is synthesized (OP150), TM1 remains adjacent to PAT-10 and Sec61, whereas TM2 is next to the TRAM protein in addition to Sec61. When the nascent chain length is >175 residues (>OP175), the association of TM2 with TRAM is lost, and there is a substantial reduction in the efficiency of cross-linking to Sec61 from both TM1 and TM2. In contrast, we find that TM1 remains adjacent to PAT-10 until the completion of opsin synthesis. We propose that at these longer chain lengths (>OP175), TM1 and TM2 have moved to the periphery of the ER membrane insertion site. At this stage of synthesis, TM3 (dashed circle number 3) is predicted to have entered the ER membrane insertion site and this event may precipitate the lateral “exit” of TM1 and TM2.
Lateral Exit of TMs
A key feature of membrane protein synthesis is that one or more TMs must exit laterally from the ER translocon into the lipid bilayer (High and Laird, 1997; Heinrich et al., 2000). By analyzing different lengths of ribosome-bound nascent opsin chains, we hoped to reflect different stages of the membrane integration process. Furthermore, we could directly compare the environments of TM1 and TM2 at comparable stages of the membrane insertion process. These experiments revealed clear alterations in the cross-linking profiles as the length of the nascent chain being analyzed was altered. Efficient cross-linking of TM1 and TM2 to subunits of the Sec61 complex can then be detected until a chain length of between 150 and 165 amino acids is reached (cf. Figure 10). When longer chain lengths are studied, a significant reduction in the efficiency of cross-linking to the Sec61 complex is observed from single cysteine probes located in TM1 and TM2 (Figures 3 and 5). These data are consistent with both TM1 and TM2 moving to a more peripheral location with respect to the Sec61 complex at a similar stage during opsin biosynthesis. The simplest model to describe these observations is one where TM1 and TM2 laterally exit the ER translocon together (Heymann and Subramaniam, 1997; High and Laird, 1997). Such a model would also account for the observation that TM2 is required to stabilize TM1 in the correct transmembrane orientation when truncated forms of opsin are expressed in vivo (Heymann and Subramaniam, 1997). In more general terms, our cross-linking data strongly support the proposal that the transmembrane domains of a polytopic protein are sequentially integrated into the lipid bilayer during protein biosynthesis at the ER (Figure 10; High and Laird, 1997; Mothes et al., 1997).
TM-specific Accessory Proteins
Our comparison of TM1 and TM2 revealed unexpected, and quite striking, differences in the “accessory” proteins that were found adjacent to these two TMs (summarized in Figure 10). In the case of TM2, it showed a precise and stage specific ability to be cross-linked to the TRAM protein (Figure 10), a well-characterized translocon associated component (Johnson and van Waes, 1999). In contrast, TM1 formed a very strong adduct with PAT-10 that was detected once a chain length of 130 amino acids had been synthesized (Figure 10). This association of TM1 with PAT-10 was sustained until the entire polypeptide chain (348 residues) had been made, and was only lost upon the release of the nascent chain from the ribosome.
TM1 Remains Associated with PAT-10 during Membrane Insertion
The properties of PAT-10 are particularly interesting because they conform to those one might expect of a TM-specific chaperone (High and Laird, 1997). Furthermore, the fact that this component is resistant to extraction with alkaline buffer at pH 9.5, is consistent with PAT-10 being an integral membrane protein although the possibility that it is a peripheral protein that is tightly associated with the ER membrane cannot be excluded (cf. Miller et al., 1995). Strong cross-linking of TM1 to PAT-10 is chain length dependent and occurs at a point after strong adducts to Sec61α and Sec61β are seen (Figure 10). Most notably, once detected, PAT-10 remains adjacent to TM1 throughout the synthesis of the rest of the molecule and is only absent when translation is complete and the ribosome has been released from the nascent chain. Indeed, the behavior of PAT-10 suggests that it may act to shield TM1 of ospin from the Sec61 complex during or after its lateral exit from the ER translocon (Figure 10). This would enable TM1 to disengage the ER translocon while still maintaining a link to the translation machinery via the association of PAT-10 with the ribosome.
PAT-10 seems to function at a stage after the Sec61 complex and to remain associated with TM1 for the remainder of the membrane integration process (cf. Figure 10). It was clear from our initial analysis that PAT-10 is distinct from the Sec61β subunit. A number of other small ER proteins were potential candidates for PAT-10 on the basis of their size, the presence of one or more cysteine residues capable of mediating BMH-dependent cross-linking, and evidence of their proximity to the ER translocon (Johnson and van Waes, 1999). Antisera that recognized these components during immunoprecipitation experiments were obtained (RAMP4, Schröder et al., 1999; Sec61γ, SPC12, and Dad1, our unpublished data), but in no case did these recognize the Opsin-PAT-10 adducts. We therefore conclude that PAT-10 is most likely a novel protein that is closely associated with the ER translocon during the membrane insertion of nascent opsin chains.
We further characterized the properties of the PAT-10 adduct and determined a number of features. First, we established that PAT-10 does not associate with opsin TM1 simply because the flanking region of the polypeptide is N-glycosylated. Second, we found that the association of PAT-10 with opsin TM1 is not dependent upon its amino acid sequence, and that TM1 can be altered in a number of ways without affecting its cross-linking to PAT-10. In particular, the amino acid sequence of TM2, which does not associate with PAT-10 in its natural location, can be inserted in place of TM1 and a PAT-10 association observed. Thus, we conclude that it is the relative location of TM1 within the opsin nascent chain that is important for its proximity to PAT-10. A clear feature of TM1 is its topology, and we artificially extended the N terminus of the opsin chain, so as to prevent its translocation across the ER membrane (Denzer et al., 1995) and thereby reverse its transmembrane orientation. Even under these conditions, TM1 was found to be adjacent to the PAT-10 component. We therefore conclude that PAT-10 specifically associates with the first TM of the polytopic membrane protein opsin, irrespective of the amino acid sequence and transmembrane orientation of this TM.
To investigate whether PAT-10 associates specifically with nascent opsin-derived polypeptides, we analyzed a second seven TM protein distinct from opsin, the rat neurotensin receptor (Table 1). We found that a 170-amino acid-long integration intermediate of the neurotensin receptor is also cross-linked to PAT-10 from a single cysteine probe located near the middle of TM1. Our data therefore suggest that the association of PAT-10 with nascent polytopic membrane proteins is not restricted to opsin-derived chains.
On the release of the ribosome from the nascent chain, the association of TM1 with PAT-10 is lost. We therefore propose that PAT-10 may perform some “chaperone-like” function by associating with the particular TMs of nascent polytopic membrane proteins (High et al., 1997). This association may either facilitate membrane insertion per se, or it may modulate the assembly/packing of individual transmembrane domains together. The proximity of PAT-10 with the nascent chain seems to be regulated by the release of the ribosome, a process that occurs naturally upon chain termination, and which can be reproduced experimentally by the use of puromycin. This loss of PAT-10 cross-linking upon the release of the ribosome from the nascent chain may simply reflect the ability of the released polypeptide chain to rapidly diffuse away from the ER membrane insertion site (Heinrich et al., 2000). Alternatively, the ribosome may actively recruit PAT-10 to the ER membrane insertion site (Blobel and Dobberstein, 1975), a role that would be consistent with recent findings that the ribosome regulates various aspects of the gating process during membrane protein insertion at the ER translocon (Liao et al., 1997; Haigh and Johnson, 2002).
It should be noted that we assume all of the unidentified ∼10-kDa cross-linking partners we can detect are with a single component (PAT-10). We cannot at present exclude the possibility that the adducts we describe are in fact with two or more proteins of strikingly similar properties. Previous cross-linking studies of the ER translocon with specific secretory proteins have also revealed small (9–11 kDa), as yet unidentified ER components that bear some similarities to PAT-10 (Kuroiwa et al., 1993; Hegde and Lingappa, 1996). It remains to be seen whether these components are all one and the same, but if so, PAT-10 may prove to be a generic ER translocon-associated protein.
TM2 Is Transiently Associated with TRAM Protein
In the case of single-spanning membrane proteins, the TRAM protein can be cross-linked to the nascent chain from probes located within the TM region during membrane integration (Do et al., 1996; Heinrich et al., 2000). The association of the TRAM protein with single-spanning membrane proteins occurs at a discrete stage(s) of integration (Do et al., 1996) and can be induced by the introduction of a charged residue into the TM (Heinrich et al., 2000). To date, the limited cross-linking studies of polytopic membrane proteins that have been carried out have failed to reveal any cross-linking of the nascent chains to the TRAM protein (Laird and High, 1997; Mothes et al., 1997).
In this study, we show that although opsin TM1 is not detected in proximity to the TRAM protein, TM2 is. Interestingly, TM2 contains a charged residue (aspartic acid at residue 68; Table 1), whereas TM1 does not, consistent with the proposal that TRAM might contribute to the ER translocon retention of amino acid sequences that are not sufficiently hydrophobic to partition into the lipid bilayer (Heinrich et al., 2000). Any such effect is transient, however, because we observe the loss of TM2 cross-linking to TRAM as the chain length of the integration intermediate is increased. Furthermore, our data show that the presence of a charged amino acid residue alone is not sufficient to stabilize the association of a TM with TRAM. Hence, when the amino acid sequence of TM2 is introduced as the first TM spanning region of opsin, no cross-linking to TRAM is detected.
We conclude that, when a protein contains multiple transmembrane domains, the relative position of each TM plays a central role in dictating the accessory proteins that are associated with it during its membrane insertion. On the basis of the data presented herein, we have created a unifying model to describe the sequence of proteinaceous environments that are experienced by both TM1 and TM2 of opsin during the membrane insertion of the nascent polypeptide (Figure 10).
ACKNOWLEDGMENTS
We thank Reinhard Grisshammer for supplying the rat neurotensin cDNA and Bernhard Dobberstein, Paul Hargrave, Bruno Martoglio, and Richard Zimmerman for providing antisera used during the course of this work. We thank Ben Abell, Viki Allan, and Neil Bulleid for extremely valuable comments during the preparation of this manuscript. This work was supported by funding from the Biotechnology and Biological Sciences Research Council and the European Union, and by the award of a Biotechnology and Biological Sciences Research Council Professorial Fellowship (to S.H.).
Abbreviations used:
- BMH
bismaleimidohexane
- ER
endoplasmic reticulum
- OP
opsin
- OPΔCHO
nonglycosylated opsin
- PAT-10
protein associated with the ER translocon of 10 kDa
- SRP
signal recognition particle
- Syb2
synaptobrevin 2
- TM
transmembrane domain
- TRAM
translocating chain-associating membrane
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0198. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0198.
REFERENCES
- Adamus G, Zam ZS, Arendt A, Palczewski K, McDowell JH, Hargrave PA. Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 1991;31:17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
- Beckmann R, Spahn CM, Eswar N, Helmers J, Penczek PA, Sali A, Frank J, Blobel G. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 2001;107:361–372. doi: 10.1016/s0092-8674(01)00541-4. [DOI] [PubMed] [Google Scholar]
- Blobel G, Dobberstein B. Transfer of proteins across membranes I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975;67:835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel AC, Simon SM. Biogenesis of polytopic membrane proteins: membrane segments assemble within translocation channels prior to membrane assembly. Cell. 1996a;85:379–389. doi: 10.1016/s0092-8674(00)81116-2. [DOI] [PubMed] [Google Scholar]
- Borel AC, Simon SM. Biogenesis of polytopic membrane proteins: membrane segments of P-glycoprotein sequentially translocate to span the ER membrane. Biochemistry. 1996b;35:10587–10594. doi: 10.1021/bi960950q. [DOI] [PubMed] [Google Scholar]
- Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Denzer AJ, Nabholtz CE, Spiess M. Transmembrane orientation of signal-anchor proteins is affected by the folding state but not the size of the N-terminal domain. EMBO J. 1995;14:6311–6317. doi: 10.1002/j.1460-2075.1995.tb00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do H, Falcone D, Lin J, Andrews DW, Johnson AE. The cotranslational integration of membrane proteins into the phospholipid bilayer is a multistep process. Cell. 1996;85:369–378. doi: 10.1016/s0092-8674(00)81115-0. [DOI] [PubMed] [Google Scholar]
- Haigh NG, Johnson AE. A new role for BiP: closing the aqueous translocon pore during protein integration into the ER membrane. J Cell Biol. 2002;156:261–270. doi: 10.1083/jcb.200110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E, Sommer T, Prehn S, Gorlich D, Jentsch S, Rapoport TA. Evolutionary conservation of components of the protein translocation complex. Nature. 1994;367:654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Lingappa VR. Sequence-specific alteration of the ribosome-membrane junction exposes nascent secretory proteins to the cytosol. Cell. 1996;85:217–228. doi: 10.1016/s0092-8674(00)81098-3. [DOI] [PubMed] [Google Scholar]
- Heinrich SU, Mothes W, Brunner J, Rapoport TA. The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell. 2000;102:233–244. doi: 10.1016/s0092-8674(00)00028-3. [DOI] [PubMed] [Google Scholar]
- Heymann JA, Subramaniam S. Expression, stability, and membrane integration of truncation mutants of bovine rhodopsin. Proc Natl Acad Sci USA. 1997;94:4966–4971. doi: 10.1073/pnas.94.10.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S, Laird V. Membrane protein biosynthesis - all sewn up? Trends Cell Biol. 1997;7:206–210. doi: 10.1016/S0962-8924(97)01035-0. [DOI] [PubMed] [Google Scholar]
- High S, Laird V, Oliver JD. The Biosynthesis of Membrane Proteins at the Endoplasmic Reticulum. Austin, TX: R.G. Landes Co; 1997. [Google Scholar]
- High S, et al. Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane inserted signal sequence. J Biol Chem. 1993;268:26745–26751. [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Johnson AE, van Waes MA. The translocon: a dynamic gateway at the ER membrane. Annu Rev Cell Dev Biol. 1999;15:799–842. doi: 10.1146/annurev.cellbio.15.1.799. [DOI] [PubMed] [Google Scholar]
- Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Sakaguchi M, Mihara K, Omura T. Detection of a novel 9-kDa endoplasmic reticulum membrane protein in mammalian cells by chemical cross-linking with translocating nascent peptides. J Biochem. 1993;114:541–546. doi: 10.1093/oxfordjournals.jbchem.a124213. [DOI] [PubMed] [Google Scholar]
- Laird V, High S. Discrete cross-linking products identified during membrane protein biosynthesis. J Biol Chem. 1997;272:1983–1989. doi: 10.1074/jbc.272.3.1983. [DOI] [PubMed] [Google Scholar]
- Liao S, Lin J, Do H, Johnson AE. Both lumenal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell. 1997;90:31–41. doi: 10.1016/s0092-8674(00)80311-6. [DOI] [PubMed] [Google Scholar]
- Martoglio B, Dobberstein B. Snapshots of membrane-translocating proteins. Trends Cell Biol. 1996;6:142–147. doi: 10.1016/0962-8924(96)10001-5. [DOI] [PubMed] [Google Scholar]
- Matlack KE, Mothes W, Rapoport TA. Protein translocation: tunnel vision. Cell. 1998;92:381–390. doi: 10.1016/s0092-8674(00)80930-7. [DOI] [PubMed] [Google Scholar]
- Menetret JF, Neuhof A, Morgan DG, Plath K, Radermacher M, Rapoport TA, Akey CW. The structure of ribosome-channel complexes engaged in protein translocation. Mol Cell. 2000;6:1219–1232. doi: 10.1016/s1097-2765(00)00118-0. [DOI] [PubMed] [Google Scholar]
- Menon ST, Han M, Sakmar TP. Rhodopsin: structural basis of molecular physiology. Physiol Rev. 2001;81:1659–1688. doi: 10.1152/physrev.2001.81.4.1659. [DOI] [PubMed] [Google Scholar]
- Miller JD, Tajima S, Lauffer L, Walter P. The β subunit of the signal recognition particle receptor is a transmembrane GTPase that anchors the α subunit, a peripheral membrane GTPase, to the endoplasmic reticulum membrane. J Cell Biol. 1995;128:273–282. doi: 10.1083/jcb.128.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes W, Heinrich SU, Graf R, Nilsson I, von Heijne G, Brunner J, Rapoport TA. Molecular mechanism of membrane protein integration into the endoplasmic reticulum. Cell. 1997;89:523–533. doi: 10.1016/s0092-8674(00)80234-2. [DOI] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994;13:3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta CV, Blobel G. Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell. 1993;79:989–998. doi: 10.1016/0092-8674(93)90276-v. [DOI] [PubMed] [Google Scholar]
- Paver JL, Hawkins HC, Freedman RB. Preparation and characterization of dog pancreas microsomal membranes specifically depleted of protein disulphide-isomerase. Biochem J. 1989;257:657–663. doi: 10.1042/bj2570657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder K, Martoglio B, Hofmann M, Holscher C, Hartmann E, Prehn S, Rapoport TA, Dobberstein B. Control of glycosylation of MHC class II-associated invariant chain by translocon-associated RAMP4. EMBO J. 1999;18:4804–4815. doi: 10.1093/emboj/18.17.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J, Grisshammer R. Purification of a rat neurotensin receptor expressed in Escherichia coli. Biochem J. 1996;317:891–899. doi: 10.1042/bj3170891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Allen AJ, Oliver J, Brookman JL, High S, Bulleid NJ. The translocation, folding, assembly and redox-dependent degradation of secretory and membrane proteins in semi-permeabilized mammalian cells. Biochem J. 1995;307:679–687. doi: 10.1042/bj3070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin SL, Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988;7:3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]