Abstract
Although protein kinase A (PKA) activation is known to increase ciliary beat frequency in humans the molecular mechanisms involved are unknown. We demonstrate that PKA is associated with ciliary axonemes where it specifically phosphorylates a 23-kDa protein. Because PKA is often localized to subcellular compartments in proximity to its substrate(s) via interactions with A-kinase–anchoring proteins (AKAPs), we investigated whether an AKAP was also associated with ciliary axonemes. This study has identified a novel 28 kDa AKAP (AKAP28)that is highly enriched in airway axonemes. The mRNA for AKAP28 is up-regulated as primary airway cells differentiate and is specifically expressed in tissues containing cilia and/or flagella. Additionally, both Western blot and immunostaining data show that AKAP28 is enriched in airway cilia. These data demonstrate that we have identified the first human axonemal AKAP, a protein that likely plays a role in the signaling necessary for efficient modulation of ciliary beat frequency.
INTRODUCTION
Mucociliary clearance is an innate host defense mechanism dependent on the coordinated beating of cilia lining the conducting airways. Ciliary beat is achieved by coupling the hydrolysis of ATP by dynein, the molecular motor of the axoneme, to microtubule sliding. To achieve the precise waveform and beat frequency necessary for efficient clearance, a cilium requires the cooperative action of the >200 proteins that compose this organelle. A number of pharmacological studies have demonstrated that ciliary beat frequency (CBF) is a highly regulated process (reviewed in Satir and Sleigh, 1990; Wanner et al., 1996); however, very little is known about the molecular events underlying these regulatory mechanisms.
The influence of cAMP on ciliary motility has been studied in a variety of organisms. Studies of classical nonmammalian systems such as Paramecium, Tetrahymena, and Mytilus gill cilia show a strong correlation between increased cAMP levels, subsequent activation of protein kinase A (PKA), and increased axonemal motility (Stommel and Stephens, 1985; Hamasaki et al., 1989; Christensen et al., 2001). In mammalian airway tissues, a significant increase in CBF results when intracellular cAMP levels are artificially raised in ciliated cells (Tamaoki et al., 1989; Di Benedetto et al., 1991; Wyatt et al., 1998). This increase is blocked by the addition of kinase inhibitors, suggesting a role for both cAMP and PKA in the modulation of CBF.
In human tissue, Morse et al. (2001) recently demonstrated that a sustained receptor-mediated increase in CBF is dependent on the production of cAMP. In these studies, application of adenosine and its nonmetabolizable analog 5′(N-ethylcarboxamido)-adenosine (NECA) to nasal explants generated a concentration-dependent, sustained increase in CBF. Application of an adenylyl cyclase inhibitor greatly diminished the NECA effect. By using additional pharmacological agents, the effects of adenosine and NECA were shown to be mediated through the A2B receptor, which couples to adenylate cyclase through Gs. Similar results were obtained in hamster oviduct where Morales et al. (2000) also demonstrated that adenosine stimulated CBF in an adenylyl cyclase-dependent manner.
These pharmacological data suggest a role for PKA in the regulation of CBF; however, little is known about the molecular events underlying this phenomenon. We are interested in determining whether the compartmentalization of signaling molecules plays a key role in the efficient modulation of human CBF. Specifically, we want to determine whether PKA is anchored in human ciliary axonemes in proximity to a substrate whose phosphorylation state modulates CBF.
A diverse family of functionally related proteins called A-kinase anchoring proteins (AKAPs) targets PKA to discrete locations within a cell (reviewed in Colledge and Scott, 1999). AKAPs bind to the regulatory subunit dimer of the PKA holoenzyme. Most characterized AKAPs bind to type II (RII) regulatory subunits; however, a few AKAPs are known to bind type I (RI) subunits or to bind either subtype of regulatory subunit. In most instances, this interaction is mediated via an amphipathic helix at the surface of the AKAP. Each AKAP also contains unique subcellular-targeting information. Through a protein–protein and/or protein–lipid interaction, AKAP and PKA are specifically localized to a particular organelle or membrane within the cell.
In our study, we have found that a pool of PKA is compartmentalized in human ciliary axonemes. We have also determined the molecular identity of AKAP28, a novel AKAP that is highly enriched in airway cilia. We propose that AKAP28 anchors the PKA holoenzyme in the axoneme in proximity to its substrate(s).
MATERIALS AND METHODS
Cell Culture
Human bronchial epithelial (HBE) cells from normal subjects or cystic fibrosis patients were obtained from excess surgical tissue under protocols approved by the University of North Carolina Institutional Review Board. Passage 1 HBE cells were grown at an air-liquid interface on semipermeable supports as described previously (Gray et al., 1996; Bernacki et al., 1999; Zhang et al., 2002). HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum.
Isolation and Phosphorylation of Axonemes
Axonemes were isolated from well-differentiated HBE (WD-HBE) cells by using a modified calcium shock method (Hastie et al., 1986; Reed et al., 2000; Zhang et al., 2002). To remove membranes, Triton X-100 was added to a final concentration of 0.5%, and samples were incubated on ice for 15–25 min. Cilia were repelleted and the supernatant/membrane fraction was removed. For select experiments, axonemes were resuspended in 1.0% Triton X-100 and incubated on ice for 25 min. All samples were immediately used or frozen on dry ice and stored at −80°C until needed.
Preparations of freshly isolated axonemes were phosphorylated in buffer containing 20 mM HEPES, pH 7.0, 1 mM MgCl2, 10 μM 3-Isobutyl-1-methylxanthine (Sigma-Aldrich, St. Louis, MO), and a 1× dilution of phosphatase inhibitor cocktail 1 (P2850; SigmaAldrich). Axonemes were incubated under five different reaction conditions: 1) γ-32P–labeled ATP (1 μCi, 10 mCi/ml Easytides; PerkinElmer Life Sciences, Boston, MA) alone; 2) ATP + 10 μM cAMP (Sigma-Aldrich); 3) ATP, cAMP + 10 μM heat stable inhibitor of protein kinase A (PKI) (6-22) amide (BIOMIOL Research Laboratories, Plymouth Meeting, PA); 4) ATP + exogenous PKA catalytic subunit (Promega, Madison, WI); and 5) ATP, exogenous PKA catalytic subunit + PKI (6-22) amide. Reactions were incubated at 30°C for 30 min. Laemmeli loading dye was added to a final concentration of 1×, and samples were resolved by SDS-PAGE. Gels were dried and imaged using a STORM-840 PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Radiolabeled RII Overlays
Axonemal proteins (30 μg/lane) were resolved on 15% SDS-PAGE gels, transferred to nitrocellulose, and renatured in block (5% nonfat dry milk, 0.1% bovine serum albumin in 1× Tris-buffered saline [TBS: 50 mM Tris, 75 mM NaCl, pH 7.5] overnight at 4°C). Recombinant murine RIIα was phosphorylated by PKA catalytic subunit in the presence of γ-32P–labeled ATP and unincorporated label was removed using a desalting column. Renatured blots were incubated with 250,000 cpm of 32P-RII/ml in block for 4 h at room temperature, washed in 1× TBS (5 × 15 min), and exposed to a PhosphorImager screen overnight. For competitive peptide experiments, 32P-RII was preincubated with either 1 μM competitive peptide Ht31 (DLIEEAASRIVDAVIEQVKAAGAY), 1 μM negative control peptide Ht31PP (DLIEEAASRPVDAVPEQVKAAGAY), or with no peptide for 30 min before adding probe to the renatured blot. Both Ht31 and Ht31PP were synthesized by the University of North Carolina Custom Peptide Synthesis Facility.
Cloning of Novel AKAP
We screened a λZAP cDNA expression library (Stratagene, La Jolla, CA) generated from mRNA isolated from WD-HBE cultures for clones containing RII-binding proteins. Recombinant RII was purified from induced bacteria with cAMP agarose beads (Gray et al., 1997). Purified RII was biotinylated using standard methods and used as probe for nonradioactive library screens (Trotter et al., 1999). XL1 Blue MRF' Escherichia coli infected with λZAP phage were plated and incubated at 42°C until plaques began to form, ∼4 h. Nitrocellulose filters wet in 1 mM isopropyl β-d-1-thiogalactopyranoside were laid on plates and incubated for 5 h at 37°C for protein collection. Filters were removed from plates, blocked in 5% nonfat dry milk, probed overnight with RII-biotin prebound to streptavidin-alkaline phosphatase, and developed using standard methods. Positive plaques were cored, tumbled, and rescreened. At least three rounds of plaque purification were performed to isolate pure phage encoding RII-binding domains. Positive clones were excised from phage by in vivo excision, and inserts were sequenced by the University of North Carolina Sequencing Facility. One novel 0.3-kb cDNA sequence was isolated from the library screen. To obtain full-length sequence we performed both 5′ and 3′ rapid amplification of cDNA ends (RACE) using SMART-RACE (CLONTECH, Palo Alto, CA) human trachea cDNA generated from RNA according to the manufacturer's protocol. RACE products were cloned into pTAdv (CLONTECH) and sequenced. Appropriate primers were designed and used to amplify full-length cDNAs of three predicted splice variants from trachea and testis. Polymerase chain reaction (PCR) products were again cloned into pTAdv and sequenced.
Identification of RII-binding Site
cDNAs encoding full-length (aa 1–197) and fragments (1–68 and 64–197) of AKAP28 were generated by PCR with the full-length cDNA of AKAP28 as template. PCR products were subcloned into the pET28c expression vector (Novagen, Madison, WI). After confirming the sequence of each insert, plasmids were transformed into E. coli BL21 Codon Plus (Stratagene, La Jolla, CA) and expressed as His6 fusion proteins. Fusion protein expression was induced overnight at 28°C, and the insoluble fusion proteins were purified according to the Novagen protein purification protocol. After purification, fusion proteins were dialyzed into phosphate-buffered saline (PBS) and subsequently resolubilized by boiling in 1% SDS in PBS. Protein concentration was determined by a bicinchoninic acid protein assay (Pierce Chemical, Rockford, IL). Equal moles of each fusion protein were resolved by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF), overlaid with biotinylated RII, probed with stretavidin-conjugated horseradish peroxidase (HRP), and visualized using enhanced chemiluminescence (ECL) detection.
The predicted RII-binding site was mutated in the pET28c construct encoding amino acids 1–68. Amino acid substitutions were made using the QuikChange XL site-directed mutagenesis kit (Stratagene). After sequence confirmation of the mutagenesis, the plasmid was expressed in bacteria as described above. The soluble mutant fusion protein was purified on a nickel column according to the manufacturer's instructions (Novagen).
Heterologous Expression of AKAP28 and Coimmunoprecipatation with PKA Subunits
Recombinant adenovirus encoding AKAP28 was generated for heterologous expression assays. A cDNA encoding AKAP28 fused to the hemagglutinin (HA) epitope was subcloned into the adenovirus shuttle vector pAdTrack-CMV (pAdTrack-CMV.HAAKAP28). According to the protocol of He et al. (1998), the Vector Core Facility at University of North Carolina at Chapel Hill generated recombinant adenoviruses, AdTrack-CMV (expressing green fluorescent protein [GFP], from pAdTrack-CMV alone) and AdTrack-CMV-HAAKAP28 (expressing GFP and AKAP28-epitope tagged with HA at its carboxy terminus, from pAdTrack-CMV.HAAKAP28).
For heterologous expression, 100-mm dishes of 80% confluent HeLa cells were either mock infected or infected with AdTrack-CMV or AdTrack-CMV-HA-AKAP28. After a 2-h incubation at 37°C, cells were washed and the growth media were replaced. Infected cells were harvested 24 h postinfection.
For immunoprecipation experiments, dishes of infected or mock-infected cells were placed on ice and rinsed two times with ice-cold PBS (50 mM NaH2PO4·H2O, 150 mM NaCl, pH 7.4). Cells were overlaid with 0.4 ml of ice-cold lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% IGEPAL, plus protease inhibitors) and removed by scraping. Lysates were passed through a 27-gauge needle several times and left on ice for 30 min. The resulting homogenate was centrifuged at 14,300 relative centrifugal force (rcf) for 10 min at 4°C to precipitate insoluble material. Protein (300 μg) was diluted to 0.5 ml in dilution buffer (lysis buffer without IGEPAL) and incubated with 5 μg of HA 1.1 monoclonal antibody (Covance, Denver, PA) or isotype-matched IgG (R & D Systems, Minneapolis, MN) while tumbling overnight at 4°C. Immunocomplexes were collected on protein G-agarose and washed extensively with dilution buffer. Bound proteins were analyzed by Western blotting with the appropriate antisera or by RII overlay.
Northern Blot Analysis of AKAP28
Total RNA from differentiating, code-matched human airway cultures was isolated using the RNeasy mini kit (QIAGEN, Valencia, CA) following the manufacturer's instructions. RNA samples were resolved on a 1.5% formaldehyde/agarose gel following standard methods. After electrophoresis, samples were transferred to a Nytran membrane (Schleicher & Schuell, Keene, NH) by capillary blotting and cross-linked using a Stratalinker (Stratagene). Both the time course blot and a multiple tissue expression array (CLONTECH) were probed with 32P-labeled random-primed cDNA corresponding to nucleotides 98–400 of AKAP28 by using standard methods. All blots were analyzed using a STORM-840 PhosphorImager and ImageQuant software.
Antisera Generation and Immunoblot Analysis
Rabbit antisera directed against a GST fusion protein containing amino acids 5–105 of AKAP28 were generated by Covance. Antisera were affinity purified on a column containing His6AKAP28 fusion protein and subsequently used for both Western blotting and immunohistochemistry. Preimmune sera were purified on an affi-gel blue column (Pierce Chemical) to remove complement proteins.
For Western blots, proteins from isolated axonemes or WD-HBE whole cell lysates were resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked for at least 1 h in 10% nonfat dry milk and incubated in 1 μg/ml affinity-purified rabbit anti-AKAP28 IgG, 1 μg/ml preimmune IgG, 0.2 ng/ml affi-gel blue purified anti-EBP50/NHERF IgG, or commercially available antibodies specific for PKA catalytic subunit (0.1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), 0.1 μg/ml PKA type I regulatory subunit (Santa Cruz Biotechnology), 0.1 μg/ml PKA type II regulatory subunit (Santa Cruz Biotechnology), or 1:2000 type IV β tubulin (BioGenex, San Ramon, CA). Blots were incubated overnight at 4°C or at room temperature for 1 h. Blots were washed, incubated with appropriate HRP-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA), and visualized using ECL.
Immunohistochemistry
Histological sections were prepared from paraffin-embedded, 4% paraformaldehyde-fixed blocks of WD-HBE cells. For the analysis of AKAP28 protein, sections were deparaffinzed and rehydrated to water. Sections were rinsed with TBS and then incubated for 30 min at 85°C in preheated, diluted (1.5 ml of concentrated stock in 160 ml of water) antigen unmasking solution (Vector Laboratories, Burlingame, CA). Sections were washed in TBS and permeabilized in TBS with 0.3% Triton X-100 for 30 min. Sections were blocked in 2% normal goat serum, 1% cold water fish gelatin in TBS with 0.3% Triton X-100 for 30 min. Primary antibodies diluted in block (5 μg/ml) were incubated with sections overnight at 4°C. Slides were washed in TBS and processed using the Vectashield Elite ABC kit (Vector Laboratories). After final washes, sections were developed using 3,3′-diaminobenzidine tetrahydrochloride, counterstained with light green SF yellowish (Chroma-Gesellschaft, Schmid Gmb; Köngen, Germany), dehydrated, and mounted.
RESULTS
PKA Copurifies with Human Ciliary Axonemes and Phosphorylates a 23-kDa Substrate
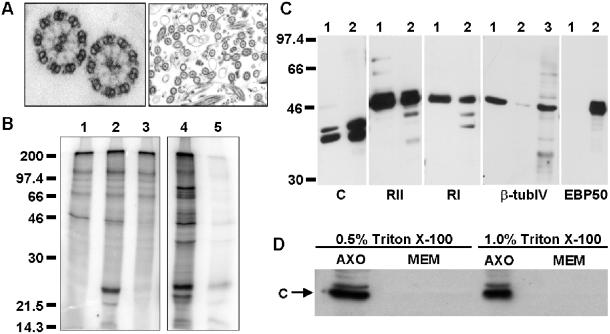
To determine whether PKA is associated with human ciliary axonemes, we used both kinase activity assays and Western blotting techniques. We isolated axonemes from WD-HBE cells by using a modification of the calcium shock method (Hastie et al., 1986; Reed et al., 2000; Zhang et al., 2002). As shown by the electron micrograph (Figure 1A), the 9 + 2 microtubule structure characteristic of the axoneme is preserved during isolation and most axonemes are free of membranes. To determine whether PKA was present in our axonemal preparations, we treated isolated axonemes with [γ-32P]ATP alone or [γ-32P]ATP plus 10 μM cAMP, to specifically activate PKA (Figure 1B). Phosphatase inhibitors were added to each reaction to inhibit known serine/threonine phosphatases and L-isozymes of alkaline phosphatase. A protein with an apparent molecular mass of ∼23 kDa is specifically phosphorylated in the presence of 10 μM cAMP (lane 2), but is not detected under basal conditions (ATP alone, lane 1). The phosphorylation of the 23-kDa band is significantly reduced by the addition of PKI, a PKA-specific inhibitor (lane 3) (Scott et al., 1986). Interestingly, the addition of exogenous PKA catalytic subunit results in the phosphorylation of many axonemal proteins, whereas endogenous PKA specifically phosphorylates the 23-kDa protein (compare lanes 2 and 4).
Figure 1.
Detection of PKA in human airway cilia. (A) Representative electronmicrograph of isolated human airway axonemes. (B) Freshly isolated axonemes were incubated under the following conditions: lane 1, [γ-32P]-ATP alone; lane 2, [γ-32P]-ATP + 10 μM cAMP; lane 3, [γ-32P]-ATP + 10 μM cAMP + 10 μM PKI; lane 4, [γ-32P]-ATP + catalytic subunit of PKA; and lane 5, [γ-32P]-ATP + catalytic subunit of PKA + 10 μM PKI. Treated samples were resolved by SDS-PAGE and phosphorimaged. (C) Isolated axonemes (5 μg, lane 1) and WD-HBE cell lysates (5 μg, lane 2 or 40 μg, lane 3) were resolved by 10% SDS-PAGE and transferred to a PVDF membrane. Membranes were probed with antibodies specific for either the catalytic subunit of PKA (C); the type II regulatory subunit of PKA (RII); the type I regulatory subunit of PKA (RI); β-tubulin type IV, the axonemal specific isoform of β-tubulin; or EBP50/NHERF, a nonaxonemal, cytosolic protein. Membranes were washed and incubated with appropriate secondary antibodies conjugated to HRP. Westerns were developed by enhanced chemiluminescence. All data are representative of multiple independent experiments. (D) Isolated axonemes were incubated with either 0.5 or 1.0% Triton X-100 for 25 min on ice. Detergent extracted axonemes (AXO) and membrane fractions (MEM) were resolved by SDS-PAGE, transferred to Immobilon and probed with an antibody specific for the catalytic subunit of PKA. The membrane fraction is two-thirds the amount of axoneme fraction loaded.
To determine the isoform(s) of PKA present in the axoneme, we used Western blotting techniques to detect specific PKA subunits in isolated human axonemes (Figure 1C). Axonemal preparations (5 μg, lane 1) were compared with whole cell lysates from WD-HBE cultures (5 μg, lane 2). As controls to confirm the purity of the axonemes, antibodies for type IV β tubulin (β-tubIV), the axonemal specific isoform of β tubulin (Renthal et al., 1993), and EBP50/NHERF, a nonaxonemal, cytosolic protein (Short et al., 1998; Mohler et al., 1999), were also used. As expected, type IV β tubulin is highly enriched in the axonemal fraction and EBP50/NHERF is not detectable in isolated axonemes, but is present in whole cell lysates. The catalytic subunit of PKA (C), as well as both type II (RII) and type I (RI) subunits, are detectable by Western blot in ciliary axonemes.
To demonstrate that the signal for PKA was due to the presence of the axonemes and not residual ciliary membranes, we extracted our axonemal preparation with either 0.5 or 1.0% Triton X-100 (Figure 1D). Pelleted fractions (AXO), presumably completely demembranated axonemes, and detergent-extracted membranes (MEM) were compared by Western blot with the PKA catalytic subunit antibody. The catalytic subunit remains tightly associated with the axoneme pellet in the presence of detergent and is not detectable in the membrane fraction. Because the regulatory subunits of PKA are generally thought to target the holoenzyme, it is likely that all PKA subunits remain with detergent-extracted axonemes.
These experiments demonstrate that PKA copurifies with demembranated human ciliary axonemes and preferentially phosphorylates a single substrate. Therefore, PKA is associated with the axoneme as either a structural component or as a constituent that strongly adheres to the axoneme through the purification process. Additionally, PKA is likely anchored in proximity to its preferred 23-kDa substrate.
An Axonemal RII-Binding Protein Is Detectable by Overlay
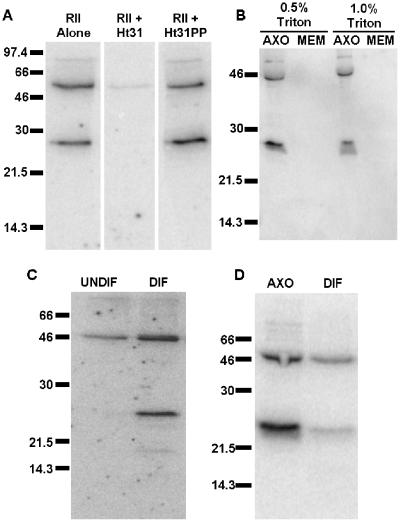
To determine how PKA is anchored within the axoneme, we assessed whether PKA-binding proteins were also contained within axonemes. In particular, we assayed for RII-binding proteins by performing overlays with recombinant RII as probe (Hausken et al., 1998). In radiolabeled RII overlay assays of isolated cilia from WD-HBE cells, we repeatedly detect two prominent RII-binding proteins (Figure 2A). Competitive inhibitor peptide Ht31, but not Ht31PP, a negative control peptide, specifically blocked RII-binding to these proteins. One of the detected proteins has an apparent molecular mass of 50 kDa. This protein may be RII dimerizing with itself because RII is detectable at this molecular mass by Western blot of cilia preparations (Figure 1C). The other detected RII-binding protein is 28 kDa.
Figure 2.
Detection of RII-binding proteins in airway axonemes. (A) Isolated axonemes (30 μg/lane) were resolved by 15% SDS-PAGE, transferred to nitrocellulose, and blocked overnight in 5% nonfat dry milk, 0.1% bovine serum albumin in 1× TBS. Membranes were incubated with 32P-labeled RII probe either preblocked with no peptide, 1 μM competitive Ht31, or 1 μM negative control Ht31PP. (B) Isolated axonemes were incubated with either 0.5 or 1.0% Triton X-100 for 25 min on ice. Detergent-extracted axonemes (AXO) and membrane fractions (MEM) were resolved by SDS-PAGE, transferred to PVDF, blocked, and incubated with 32P-labeled RII probe. The membrane fraction is two-thirds the amount of axoneme fraction loaded. (C) Equal protein from whole cell lysates of undifferentiated (UNDIF) and well-differentiated (DIF) human primary airway cells were overlaid with radiolabeled RII. (D) Equal protein from isolated axonemes and differentiated airway cell lysates were also overlaid with radiolabeled RII. All data are representative of multiple independent experiments.
As seen with PKA (Figure 1D), the RII-binding protein adheres tightly to the axoneme and is not extractable with detergent (Figure 2B). In RII overlays comparing poorly differentiated human airway cells lacking cilia with WD-HBE cells, we find that the 28-kDa RII-binding protein is not detectable in undifferentiated cells (Figure 2C) but is easily detected in differentiated cells. At longer exposures, other RII-binding proteins are faintly detected in whole cell lysates (our unpublished data); however, the predominant proteins detected by RII overlay in WD-HBE cells are 50 and 28 kDa. Additionally, the putative AKAP is significantly enriched in the axonemal fraction of the cell compared with whole cell lysate (Figure 2D). These data suggest that the putative AKAP specifically targets the axoneme. Because no AKAPs of 28 kDa have been previously characterized, we designed experiments to determine the molecular identity of this protein.
Determination of Axonemal AKAP Identity
To determine the identity of the axonemal AKAP, we screened a cDNA expression library generated from mRNA isolated from WD-HBE cultures by using recombinant, biotinylated RII as probe. Three known AKAPs were identified in our screen: AKAP350, a multiply spliced AKAP targeted to the centrosome or Golgi complex of cells (Schmidt et al., 1999; Takahashi et al., 1999); S-AKAP84, which colocalizes with mitochondria in sperm flagella (Lin et al., 1995); and AKAP220, an AKAP that is targeted to testicular peroxisomes (Lester et al., 1996). We also isolated one novel sequence from the library based on its ability to bind RII. Sequence analysis revealed the insert contained ∼0.3 kb of a novel cDNA with homology to a rat cDNA encoding TAKAP80, a sperm fibrous sheath protein (Mei et al., 1997).
5′ and 3′ RACE and analyses of available expressed sequence tag and genomic databases were used to identify the full-length sequence for the novel AKAP. This gene, which maps to a region of chromosome Xq24 when searched against the human genome database, seems to encode at least three splice variants (submitted to National Center for Biotechnology Information as AF514780, AF514781, and AF514782). Full-length transcripts of all three splice variants are present in both trachea and testis as determined by reverse transcription (RT)-PCR. The full-length cDNA we chose to characterize further (accession no. AF514780) encodes a protein of 197 amino acids that migrates at 28 kDa by SDS-PAGE (see below). Following the tradition of the AKAP community, we have named this protein AKAP28 for AKAP of 28 kDa.
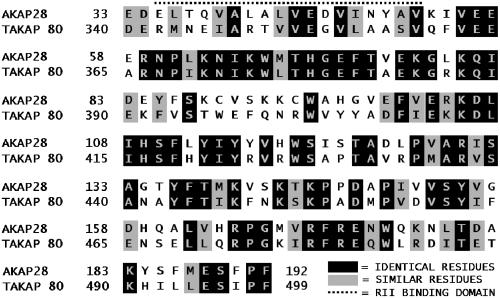
The novel clone found in the screening of the well-differentiated airway cell cDNA expression library has no obvious sequence homology with any previously characterized human proteins. The protein encoded by the open reading frame of the AKAP cDNA does, however, share 50% identity and 67% conserved homology to a 159-amino acid segment of TAKAP80 (amino acids 340–499), a rat testis-specific, developmentally regulated AKAP present on the fibrous sheath of sperm (Figure 3) (Mei et al., 1997). The predicted RII-binding region of TAKAP80 is contained in the 159-amino acid stretch that shares significant homology to AKAP28.
Figure 3.
Alignment of AKAP28 with TAKAP-80. The amino acid sequences of AKAP28 and TAKAP-80 were aligned by Clustal W. Identical residues are shaded in black, whereas similar charged residues are shaded gray. The predicted RII binding site is marked with a dotted line.
AKAP28 Contains a Single RII-binding Site
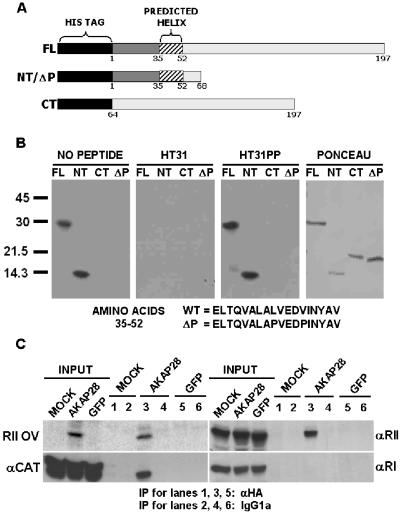
To map the location of the RII-binding site of AKAP28, we performed RII overlay assays on purified, recombinant His6 fusion proteins containing amino acids 1–197 (FL), amino acids 1–68 (NT), and amino acids 64–197 (CT) of AKAP28 (Figure 4A). Both FL and NT fusion proteins bind to biotinylated RII by overlay, whereas the CT fusion protein does not (Figure 4B). Moreover, preincubation of probe with the competitive peptide HT31 blocks RII binding to His6-FL and NT proteins, whereas the negative control peptide HT31PP does not. Hence, RII binding to AKAP28 is specific and the binding motif is contained within the first 68 amino acids of AKAP28.
Figure 4.
Analysis of PKA binding to AKAP28. (A) Schematic representation of full-length and truncation His6-AKAP28 proteins. All proteins are drawn to scale and the amino acid numbers are shown. (B) Equal moles of His6 fusion proteins were resolved by SDS-PAGE, transferred to PVDF membrane, probed with biotinylated RII, subsequently incubated with streptavidin-HRP, and developed with ECL. Biotinylated RII was either preblocked with no peptide, 1 μM competitive Ht31 or 1 μM negative control Ht31PP. A representative Ponceau-stained membrane is also shown. (C) HeLa cells were either mock infected (MOCK) or infected with a control adenovirus expressing GFP, or an adenovirus expressing both GFP and HA-tagged AKAP28 (AKAP28). Cells lysates (300 μg) were incubated with monoclonal HA.11 or mouse IgG1a. Immunocomplexes were collected on protein G and subsequently analyzed by Western blot or RII overlay as indicated. Inputs (one-fifth of total) for all cell lysates are shown. Lanes 1, 3, and 5 were incubated with HA antisera, whereas 2, 4, and 6 were incubated with mouse IgG1a. All data are representative of multiple independent experiments.
In all but one previously characterized AKAP (Diviani et al., 2000), the RII-binding motif is an amphipathic helix that binds to the RII subunit dimer. An amphipathic helix is predicted to form between amino acids 35 and 52 of AKAP28. On further analysis, we found that amino acids 35–52 can be aligned with other AKAP RII-binding motifs and shares all of the eight semiconserved positions predicted by Vijayaraghavan et al. (1999). The binding motif is defined by: X{L,I,V}X3{A,S}X2{L,I,V}{L,I,V}X2{L,I,V}{L,I,V}X2{A,S}{L,I,V}, where X is any amino acid and single letters in brackets represent the conserved amino acids that are favored at a particular position.
To provide experimental evidence that amino acids 35–52 define the RII-binding motif of AKAP28 we mutated residues 43L and 47V of the His6-NT fusion protein to prolines (His6-ΔP). The double mutant is similar to the substitutions made in AKAP-Lbc to completely abolish RII-binding (Diviani et al., 2001). We predicted that these mutations would disrupt the amphipathic helix and abolish RII binding in AKAP28. The mutated protein runs aberrantly large by SDS-PAGE and is unable to bind to RII as His6-NT does (Figure 4B). These data provide evidence that AKAP28 is an AKAP that contains a single RII-binding region located between residues 35–52.
AKAP28 Associates with PKA in HeLa Cells
To determine whether AKAP28 associates with PKA in human cells as well as binding to RII by overlay, we expressed HA-tagged AKAP28 in HeLa cells by using recombinant adenovirus. Infected cells were lysed and coimmunoprepitation experiments were performed with either HA antisera or control IgG. Immune complexes from mock-infected (mock), control-infected (expressing GFP), and infected (expressing GFP and HA-AKAP28) cells were either overlaid with 32P-RII to detect AKAP28 or blotted to detect the presence of PKA subunits (C, RI, and RII) (Figure 4C). PKA subunits were not detectable by Western in control IgG precipitants (Figure 4C, lanes 2, 4, and 6) or from mock and control-infected lysates treated with HA antisera (Figure 4C, lanes 1 and 5). In contrast, both RII and C subunits of PKA associate with HA-AKAP28 as determined by Western blot (Figure 4C, lane 3). RI, however, does not seem to bind HA-AKAP28 under these conditions (Figure 4C, lane 3). AKAP28 preferentially binds type II PKA when heterologously expressed in a human cervical adenocarcinoma cell line and is able to copurify with PKA catalytic subunit. This suggests that AKAP28 functions as a type II PKA anchor within cells.
Northern Blot Analysis of AKAP28
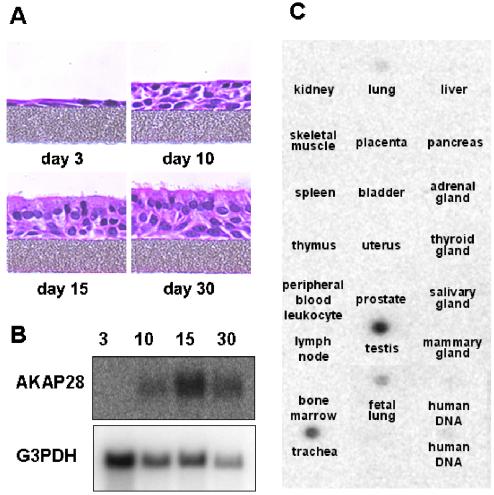
The mRNA expression of axonemal proteins is often up-regulated as ciliated cells differentiate (Andrews et al., 1996; Reed et al., 2000; Zhang et al., 2002). To determine whether AKAP28 mRNA expression also follows this profile, we examined the temporal expression of AKAP28 in differentiating HBE cells. Initially, after plating the HBE cells are a monolayer of undifferentiated cells (Figure 5A). Over time, the cultures differentiate into a pseudostratified epithelium similar to native tissue. Total RNA isolated from differentiating HBE cells at 3, 10, 15, and 30 d postplating was analyzed by Northern blot with 32P-labeled, random primed AKAP28 cDNA (nucleotides 98–400) as probe (Figure 5B). Message for AKAP28 is not readily detected at early stages of differentiation. However, AKAP28 gene expression (∼0.8-kb message) is up-regulated as cells differentiate and begin to display a mucociliary phenotype (days 10 and 15). The absence of AKAP28 message in undifferentiated cells and its up-regulation during differentiation suggest a functional role for AKAP28 in differentiated HBE cells and is consistent with the expression pattern observed for axonemal proteins.
Figure 5.
Northern blot analysis of AKAP28. (A) Code-matched HBE cultures were differentiated on semipermeable supports. Cultures were fixed postplating at day 3, 10, 15, and 30. Cells were sectioned and stained with H&E. (B) Total RNA isolated from differentiating HBE cells at 3, 10, 15, and 30 d postplating (20 μg/lane for samples 3, 10, and 15; 6 μg/lane for 30) was analyzed by Northern blot with 32P-random primed cDNA corresponding to nucleotides 98–400 of AKAP28 cDNA. The same blot was probed with G3PDH as a loading control. Similar results were obtained on two separate blots. (C) A multiple tissue expression array was probed with the same AKAP28 probe used in B. A selected panel is shown; all tissues not shown were negative for the signal.
We also probed a human multiple tissue array to determine the tissue distribution of AKAP28 (Figure 5C). We found that AKAP28 is predominantly expressed in trachea and testis; faint message was also detected in whole adult and fetal lung. No other tissues were positive for the message. Trachea, which contains ciliated columnar cells, and testis, with both developing sperm and ciliated cells of the ductuli efferentes of the epididymis, are the only tissues on the blot that contain axoneme-based organelles. The tightly restricted pattern of AKAP28 expression suggests that this AKAP has a function specific for cells containing axonemes.
Localization and Distribution of AKAP28
To determine the subcellular distribution of AKAP28, we generated rabbit polyclonal antisera directed against a GST fusion protein containing amino acids 5–105. Antisera were affinity purified and subsequently used for Western blotting and tissue staining.
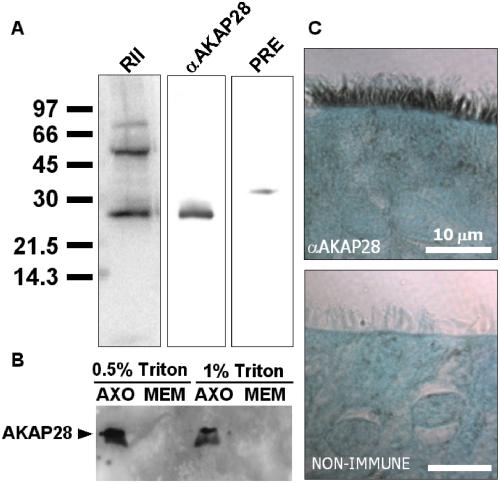
Proteins from isolated axonemes were analyzed with the AKAP28 antisera. Parallel lanes of a single gel transferred to PVDF membrane were either overlaid with RII or blotted with AKAP28 antisera. As seen in Figure 6A, the 28-kDa protein detected by RII overlay and AKAP28 as detected by Western comigrate by SDS-PAGE. An equal amount of purified preimmune IgG does not detect a 28-kDa protein. Thus, it is highly likely that the protein detected by RII overlay is AKAP28. Additionally, as with the RII overlay (Figure 2B) detergent extraction of the ciliary preparation did not remove AKAP28 from the axoneme (Figure 6B).
Figure 6.
Characterization of AKAP28 localization in WD-HBE cells. (A) Isolated axonemes were analyzed with affinity-purified AKAP28 antisera. Parallel lanes of a single gel transferred to PVDF membrane were either overlaid with radiolabeled RII (RII) or Western blotted (αAKAP28). An equal amount of purified preimmune IgG (PRE) was used as a negative control. (B) Isolated axonemes were incubated with either 0.5 or 1.0% Triton X-100 for 25 min on ice. Detergent-extracted axonemes (AXO) and membrane fractions (MEM) were resolved by SDS-PAGE, transferred to PVDF, and probed with αAKAP28 antibody. The membrane fraction is two-thirds the amount of axoneme fraction loaded. (C) Sections of WD-HBE cells were deparaffinzed and rehydrated to water. Sections were treated with an antigen unmasking solution, washed, permeabilized, and blocked. Affinity-purified AKAP28 antisera and nonimmune IgG were incubated with sections overnight at 4°C. Sections were processed using the Vectastain Elite ABC kit, developed using 3,3′-diaminobenzidine tetrahydrochloride, counterstained with light green SF yellowish, dehydrated, and coverslipped.
We used immunohistochemistry to determine the distribution of AKAP28 in WD-HBE cells (Figure 6C). Intense staining for AKAP28 is seen in the cilia of columnar cells, but absent from goblet and basal cells. Thus, AKAP28 localizes to airway cilia where it likely plays a role in anchoring PKA near its axonemal substrate.
DISCUSSION
Several pharmacological studies have identified signaling pathways involved in the regulation of ciliary beat frequency. To better understand human deficiencies in mucociliary clearance, it is necessary to delineate the molecular mechanisms underlying these pathways. Specifically, we are interested in understanding the molecular events linking the production of cAMP, the subsequent activation of PKA, and the associated increase in CBF. Our study provides the first biochemical data showing that a pool of PKA is localized within human ciliary axonemes (Figure 1). Under conditions that should inhibit known serine/threonine phosphatases and L-isozymes of alkaline phosphatase, this pool of enzyme is responsible for the specific cAMP-dependent phosphorylation of a 23-kDa substrate (Figure 1B). Additionally, compartmentalization of type II PKA in the axoneme is likely mediated via AKAP28, a novel axoneme-specific–anchoring protein.
Changes in ciliary beat frequency are likely mediated via a cascade of phosphorylation/dephosphorylation events that alter the activity of axonemal proteins. In our studies of isolated human ciliary axonemes, we have identified one major 23-kDa protein that is phosphorylated in a cAMP-dependent manner (Figure 1B). Parallels may be drawn to other axonemal systems. For example, Salathe et al. (1993) studied the in vitro cAMP-dependent phosphorylation of ovine axonemes and found that a protein with an apparent molecular mass of 26 kDa was consistently phosphorylated (Salathe et al., 1993). Similar results were obtained in studies of Paramecium (29-kDa), mussel gill (27-kDa), and Tetrahymena (34-kDa) axonemes (Stommel and Stephens, 1985; Hamasaki et al., 1989; Chilcote and Johnson, 1990).
Further studies in Paramecium revealed that p29, the axonemal protein consistently phosphorylated in this species, copurified with the 22S outer arm dynein (Bonini and Nelson, 1990; Hamasaki et al., 1991). Additionally, cAMP-dependent phosphorylation of p29 has been linked to both increased swimming velocity of permeabilized paramecia and increased velocity of microtubules gliding across surfaces coated with dynein isolated from phosphorylated axonemes (Barkalow et al., 1994). In studies of Tetrahymena, it has been demonstrated that its PKA substrate, p34 also copurifies with 22S dynein (Christensen et al., 2001). In a number of biochemical tests, Christensen et al. (2001) elegantly demonstrate that p34 is the functional ortholog of the Paramecium 22S regulatory light chain p29. Functionally it seems the regulation of ciliary motility by PKA involves the phosphorylation of a dynein regulatory light chain. To date, however, the primary sequence of this regulatory light chain has not been determined.
Both type I and type II isoforms of PKA are detectable by Western blot in human ciliary axonemes (Figure 1C). Although the kinetic and enzymatic specificities of the three catalytic subunits (Cα, β, and γ) of PKA are indistinguishable, different regulatory subunits (RI and RII) display different cAMP-binding affinities and are differentially located within the cell. RI (α and β) is mainly cytoplasmic; however, at times it is compartmentalized. For example, RI holoenzyme is tightly bound to plasma membranes of erythrocytes and is enriched at the neuromuscular junction (Rubin et al., 1972; Imaizumi-Scherrer et al., 1996). Additionally, RI is associated with activated B-cell receptors and accumulates at the “cap” site when lymphocytes are stimulated with anti-CD3 antibodies (Skalhegg et al., 1994; Levy et al., 1996). In contrast, RII (α and β) often tightly associates with cellular structures and organelles. No studies determining the specific roles of RI and RII within the axoneme have been conducted. Electron microscopic studies of axonemes with RI- and RII-specific antibodies will be necessary to determine where each PKA isotype is localized within this structure. Moreover, we are uncertain whether type I PKA and type II PKA serve overlapping or distinct roles in the axoneme. Pharmacological experiments using isotype-specific agonists will be necessary to delineate the roles of RI and RII in the regulation of CBF.
In this study, we have focused on identifying the protein responsible for anchoring type II PKA in the axoneme. We have determined the molecular identity of AKAP28, an AKAP highly enriched in airway cilia. Due to the insolubility of the axoneme, we are unable to coimmunoprecipate AKAP28 with its binding partners from primary airway cells. Using a heterologous system, however, we have been able to coimmunoprecipate epitope-tagged AKAP28 with both RII and PKA catalytic subunit from HeLa cells (Figure 4C). We are unable to detect RI in the same immune complexes. This experimental evidence for preferential binding to RII is in agreement with the RI/RII binding predictions made by Miki and Eddy (1999) in Ala- and Val-scanning mutagenesis studies of AKAP-binding domains. These data show that AKAP28 preferentially binds type II PKA and likely serves as its anchor in the axoneme.
Because type I PKA also copurifies with axonemes (Figure 1C), RI-specific–anchoring proteins are also likely to be present. Unlike RII-binding proteins, RI-specific AKAPs are not detectable by overlay (Miki and Eddy, 1999). Identifying RI-binding AKAPs in the axoneme is difficult because the insolubility of this organelle prohibits the coimmunoprecipitation experiments necessary for the detection of RI-binding proteins.
Sequence analysis indicates that AKAP28 is most closely related to a rat testis-specific, developmentally regulated AKAP, TAKAP-80 (Mei et al., 1997). A 159-amino acid stretch (residues 340–499) at the carboxy terminus of TAKAP-80 shares 50% identity and 67% conserved homology with amino acids 33–192 of AKAP28 (Figure 3). In contrast, no similarity is found in the N termini of these proteins. According to Mei et al. (1997), TAKAP-80 is exclusively expressed in testis where mRNA expression is up-regulated during a time interval that corresponds to the initiation of spermiogenesis (Mei et al., 1997). In the study by Mei et al. (1997), total RNA from whole lung was probed, however, no message was detected. Either TAKAP-80 is not present in rat lung or the percentage of RNA from ciliated cells was too low to detect TAKAP-80 mRNA expression on the blot. Because tracheal RNA was not tested, we are uncertain whether TAKAP-80 is specifically expressed in rat testis or whether TAKAP-80 is the rat ortholog of human AKAP28. TAKAP-80 protein was detected by Western blot in fibrous sheath preparations from rat sperm, but no staining of rat tissues has been published. In contrast, our staining of airway sections with AKAP28-specific antibodies indicates that AKAP28 is enriched in airway cilia. It is also detectable by Western blot in axoneme preparations. We have not determined the localization of AKAP28 in testis and sperm. Because many components of ciliary and flagellar axonemes are conserved, it is likely that a protein targeted to ciliary axonemes would also localize to the axoneme of sperm flagella. Another possibility, however, is that AKAP28 contains both fibrous sheath- and axoneme-targeting information. In ciliated airway cells, where fibrous sheaths do not exist, the protein would be compartmentalized in the axoneme. In sperm, AKAP28 could preferentially target the fibrous sheath. Further study of AKAP28 in both sperm and testis is needed to resolve these questions.
In addition to AKAP28, we have identified two smaller splice variants expressed from the same gene (National Center for Biotechnology Information accession no. AF514781 and AF514782). The predicted splice variants were initially detected by 5′ and 3′ RACE of human tracheal cDNA and by the examination of expressed sequence tag databases. Their presence was confirmed in both trachea and testis by RT-PCR and the subsequent sequencing of amplified products. All three splice variants of this gene are detected by RT-PCR in both tissues. To date, we have not detected the predicted AKAP28 isoforms by Western blot of axonemes or in whole cell lysates of WD-HBE cells. Either the predicted splice forms are never translated into protein or are expressed at low levels that currently prohibit detection.
Axonemal AKAPs have also been detected in Chlamydomonas flagella. However, unlike RII overlays of human ciliary axonemes where RII-binding proteins of 50 and 28 kDa are detected (Figure 2A), AKAPs of 240 and 97 kDa are detected in Chlamydomonas flagellar axonemes (Gaillard et al., 2001). Using genetic mutants of Chlamydomonas, Gaillard et al. (2001) found that AKAP240 is a component of the central pair apparatus and that AKAP97 is part of the radial spoke stalk. The molecular identity of AKAP240 has not been determined; however, AKAP97 has been defined molecularly as radial spoke protein 3 (RSP3). To date, the human ortholog of RSP3 has not been characterized. Although both human and Chlamydomonas axoneme systems contain AKAPs, based on size, the AKAPs readily detected in each system do not seem to be the same proteins. One possibility is that human RSP3 may be a lower affinity AKAP than AKAP28 or found at a lower concentration than AKAP28 in human axonemal preparations. Also, this difference could be due to species variations or in differences between flagella and cilia. In Chlamydomonas, agents that reduce cAMP concentrations or inhibit the activity of PKA increase axoneme motility (Hasegawa et al., 1987). The inhibitory effect of cAMP on Chlamydomonas axonemes is opposite of the stimulatory effect seen in other organisms. Moreover, unlike the relatively small phosphoproteins detected in other axoneme-based systems in response to cAMP, two proteins of >270 kDa are phosphorylated in response to cAMP in Chlamydomonas (Hasegawa et al., 1987). Although many axonemal proteins are conserved across species, specific signaling pathways and the compartmentalization of particular components vary.
In summary, we have presented the first biochemical data demonstrating the copurification of PKA with human ciliary axonemes. Additionally, we have identified the first human A-kinase anchoring protein targeted to the axoneme. We propose that AKAP28 localizes PKA to a position in the axoneme where it is able to readily interact with its substrate. This compartmentalization likely plays a role in the regulation of outer dynein arm activity and the control of ciliary beat frequency. Future experiments will be designed to examine the physiological role AKAP28 plays in human cilia.
ACKNOWLEDGMENTS
We thank Kristen Radde-Gallwitz and Jennifer Michaux for cell culture expertise and technical assistance, Colleen O'Dell for assistance with library screening, Dr. R. Jude Samulski and the University of North Carolina Vector Core for preparing adenoviruses, Kim Burns and Tracy Eldred of the University of North Carolina Cystic Fibrosis Histology Core and Dr. Scott Randell and the members of the University of North Carolina Cystic Fibrosis Center Tissue Culture Core. We also thank the members of the Milgram laboratory for helpful suggestions. This work was supported by HL-60280, HL-63103, and HL-34355.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–07–0391. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–07–0391.
REFERENCES
- Andrews KL, Nettesheim P, Asai DJ, Ostrowski LE. Identification of seven rat axonemal dynein heavy chain genes: expression during ciliated cell differentiation. Mol Biol Cell. 1996;7:71–79. doi: 10.1091/mbc.7.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkalow K, Hamasaki T, Satir P. Regulation of 22S dynein by a 29-kD light chain. J Cell Biol. 1994;126:727–735. doi: 10.1083/jcb.126.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, William Davis C, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol. 1999;20:595–604. doi: 10.1165/ajrcmb.20.4.3442. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Nelson DL. Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J Cell Sci. 1990;95:219–230. doi: 10.1242/jcs.95.2.219. [DOI] [PubMed] [Google Scholar]
- Chilcote TJ, Johnson KA. Phosphorylation of Tetrahymena 22 S dynein. J Biol Chem. 1990;265:17257–17266. [PubMed] [Google Scholar]
- Christensen ST, Guerra C, Wada Y, Valentin T, Angeletti RH, Satir P, Hamasaki T. A regulatory light chain of ciliary outer arm dynein in Tetrahymena thermophila. J Biol Chem. 2001;276:20048–20054. doi: 10.1074/jbc.M008412200. [DOI] [PubMed] [Google Scholar]
- Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G, Manara-Shediac FS, Mehta A. Effect of cyclic AMP on ciliary activity of human respiratory epithelium. Eur Respir J. 1991;4:789–795. [PubMed] [Google Scholar]
- Diviani D, Langeberg LK, Doxsey SJ, Scott JD. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr Biol. 2000;10:417–420. doi: 10.1016/s0960-9822(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Gα 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- Gaillard AR, Diener DR, Rosenbaum JL, Sale WS. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP) J Cell Biol. 2001;153:443–448. doi: 10.1083/jcb.153.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Barkalow K, Richmond J, Satir P. cAMP-stimulated phosphorylation of an axonemal polypeptide that copurifies with the 22S dynein arm regulates microtubule translocation velocity and swimming speed in Paramecium. Proc Natl Acad Sci USA. 1991;88:7918–7922. doi: 10.1073/pnas.88.18.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Murtaugh TJ, Satir BH, Satir P. In vitro phosphorylation of Paramecium axonemes and permeabilized cells. Cell Motil Cytoskeleton. 1989;12:1–11. doi: 10.1002/cm.970120102. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Hayashi H, Asakura S, Kamiya R. Stimulation of in vitro motility of Chlamydomonas axonemes by inhibition of cAMP-dependent phosphorylation. Cell Motil Cytoskeleton. 1987;8:302–311. doi: 10.1002/cm.970080403. [DOI] [PubMed] [Google Scholar]
- Hastie AT, Dicker DT, Hingley ST, Kueppers F, Higgins ML, Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil Cytoskeleton. 1986;6:25–34. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- Hausken ZE, Coghlan VM, Scott JD. Overlay, ligand blotting, and band-shift techniques to study kinase anchoring. Methods Mol Biol. 1998;88:47–64. doi: 10.1385/0-89603-487-9:47. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Scherrer T, Faust DM, Benichou JC, Hellio R, Weiss MC. Accumulation in fetal muscle and localization to the neuromuscular junction of cAMP-dependent protein kinase A regulatory and catalytic subunits RIα and Cα. J Cell Biol. 1996;134:1241–1254. doi: 10.1083/jcb.134.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester LB, Coghlan VM, Nauert B, Scott JD. Cloning and characterization of a novel A-kinase anchoring protein. AKAP 220, association with testicular peroxisomes. J Biol Chem. 1996;271:9460–9465. doi: 10.1074/jbc.271.16.9460. [DOI] [PubMed] [Google Scholar]
- Levy FO, Rasmussen AM, Tasken K, Skalhegg BS, Huitfeldt HS, Funderud S, Smeland EB, Hansson V. Cyclic AMP-dependent protein kinase (cAK) in human B cells: co-localization of type I cAK (RIα2 C2) with the antigen receptor during anti-immunoglobulin-induced B cell activation. Eur J Immunol. 1996;26:1290–1296. doi: 10.1002/eji.1830260617. [DOI] [PubMed] [Google Scholar]
- Lin RY, Moss SB, Rubin CS. Characterization of S-AKAP84, a novel developmentally regulated A kinase anchor protein of male germ cells. J Biol Chem. 1995;270:27804. doi: 10.1074/jbc.270.46.27804. [DOI] [PubMed] [Google Scholar]
- Mei X, Singh IS, Erlichman J, Orr GA. Cloning and characterization of a testis-specific, developmentally regulated A-kinase-anchoring protein (TAKAP-80) present on the fibrous sheath of rat sperm. Eur J Biochem. 1997;246:425–432. doi: 10.1111/j.1432-1033.1997.t01-1-00425.x. [DOI] [PubMed] [Google Scholar]
- Miki K, Eddy EM. Single amino acids determine specificity of binding of protein kinase A regulatory subunits by protein kinase A anchoring proteins. J Biol Chem. 1999;274:29057–29062. doi: 10.1074/jbc.274.41.29057. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Barrera N, Uribe P, Mora C, Villalon M. Functional cross talk after activation of P2 and P1 receptors in oviductal ciliated cells. Am J Physiol Cell Physiol. 2000;279:C658–C669. doi: 10.1152/ajpcell.2000.279.3.C658. [DOI] [PubMed] [Google Scholar]
- Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol Cell Physiol. 2001;280:C1485–C1497. doi: 10.1152/ajpcell.2001.280.6.C1485. [DOI] [PubMed] [Google Scholar]
- Reed W, Carson JL, Moats-Staats BM, Lucier T, Hu P, Brighton L, Gambling TM, Huang CH, Leigh MW, Collier AM. Characterization of an axonemal dynein heavy chain expressed early in airway epithelial ciliogenesis. Am J Respir Cell Mol Biol. 2000;23:734–741. doi: 10.1165/ajrcmb.23.6.4045. [DOI] [PubMed] [Google Scholar]
- Renthal R, Schneider BG, Miller MM, Luduena RF. Beta IV is the major beta-tubulin isotype in bovine cilia. Cell Motil Cytoskeleton. 1993;25:19–29. doi: 10.1002/cm.970250104. [DOI] [PubMed] [Google Scholar]
- Rubin CS, Erlichman J, Rosen OM. Cyclic adenosine 3′,5′-monophosphate-dependent protein kinase of human erythrocyte membranes. J Biol Chem. 1972;247:6135–6139. [PubMed] [Google Scholar]
- Salathe M, Pratt MM, Wanner A. Cyclic AMP-dependent phosphorylation of a 26 kD axonemal protein in ovine cilia isolated from small tissue pieces. Am J Respir Cell Mol Biol. 1993;9:306–314. doi: 10.1165/ajrcmb/9.3.306. [DOI] [PubMed] [Google Scholar]
- Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, Milgram SL, Goldenring JR. AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J Biol Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- Scott JD, Glaccum MB, Fischer EH, Krebs EG. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1986;83:1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- Skalhegg BS, Tasken K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- Stommel EW, Stephens RE. Cyclic AMP and calcium in the differential control of Mytilus gill cilia. J Comp Physiol A. 1985;157:451–459. doi: 10.1007/BF00615145. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shibata H, Shimakawa M, Miyamoto M, Mukai H, Ono Y. Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J Biol Chem. 1999;274:17267–17274. doi: 10.1074/jbc.274.24.17267. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Kondo M, Takizawa T. Effect of cAMP on ciliary function in rabbit tracheal epithelial cells. J Appl Physiol. 1989;66:1035–1039. doi: 10.1152/jappl.1989.66.3.1035. [DOI] [PubMed] [Google Scholar]
- Trotter KW, Fraser ID, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J Cell Biol. 1999;147:1481–1492. doi: 10.1083/jcb.147.7.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Liberty GA, Mohan J, Winfrey VP, Olson GE, Carr DW. Isolation and molecular characterization of AKAP110, a novel, sperm-specific protein kinase A-anchoring protein. Mol Endocrinol. 1999;13:705–717. doi: 10.1210/mend.13.5.0278. [DOI] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–L835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, O'Neal WK, Randell SH, Blackburn K, Moyer MB, Boucher RC, Ostrowski LE. Identification of dynein heavy chain 7 as an inner arm component of human cilia that is synthesized but not assembled in a case of primary ciliary dyskinesia. J Biol Chem. 2002;277:17906–17915. doi: 10.1074/jbc.M200348200. [DOI] [PubMed] [Google Scholar]