Abstract
IκBα and IκBβ are regulators of the nuclear factor-κB (NF-κB) transcription factor family. Both IκBs bind to the same NF-κB dimers and are widely expressed in different cells and tissues. To better understand how these two IκB isoforms differ biologically, we have characterized the expression of IκBβ in testis, a tissue in which IκBα is only minimally expressed. We have found that IκBβ expression is localized within the haploid spermatid stages of spermatogenesis and follows the expression of nuclear NF-κB. IκBβ expression in haploid spermatids is likely regulated by Sox family proteins, members of which are also expressed within spermatids. We have shown that both SRY and Sox-5 can bind to multiple Sox binding sites found within the IκBβ promoter and can enhance transcription of a reporter gene in transient transfection assays. We also demonstrate that IκBβ mRNA is strongly expressed in developing male gonads. These results therefore suggest that IκBβ may be a novel target for transcription factors of the HMG-box SRY/Sox family and imply a potential role for NF-κB/IκBβ in spermatogenesis.
INTRODUCTION
IκBβ belongs to a family of proteins that function as regulators of nuclear factor-κB (NF-κB), a transcription factor with a central role in the vertebrate immune system. A number of IκB family members have now been defined (for review, see Beg and Baldwin, 1993; Whiteside and Israel, 1997; Ghosh et al., 1998). These include IκBα, IκBβ, and IκBε; the precursor proteins of the p50 and p52 Rel protein family members, known as p105 and p100; and the C-terminal portion of the p105 precursor, IκBγ, which is synthesized from an internal promoter, and the proto-oncogene Bcl-3. All of these proteins share a common structure composed of six or seven ankyrin repeats, which form the ankyrin repeat domain. Collectively, these repeats have been shown to impart a cylindrical structure to the IκB protein (Huxford et al., 1998; Jacobs and Harrison, 1998). This cylinder sits within the groove formed by interaction of the two Rel proteins and forms multiple contacts with the NF-κB dimer. Outside of the ankyrin repeat domain, individual IκBs are more variable, although additional common features are shared between some of the family members. The three most prevalent IκB proteins in mammalian cells include IκBα, IκBβ, and IκBε.
The existence of multiple Rel and IκB family members is thought to allow for the regulation of subsets of genes in response to the many different signals that can activate NF-κB. For example, IκBα is rapidly degraded and resynthesized in response to signals, whereas IκBβ is more gradually degraded and resynthesized in response to a subset of signals that degrade IκBα. Newly synthesized IκBα enters the nucleus, binds and removes NF-κB from DNA, and thus actively terminates transcription (Zabel et al., 1993; Arenzana-Seisdedos et al., 1995; Read et al., 1996; Tran et al., 1997). In contrast, newly synthesized IκBβ is hypophosphorylated, enters the nucleus, and binds NF-κB but does not remove it from DNA or terminate its transcriptional activity (Suyang et al., 1996; Tran et al., 1997). This difference between the two IκBs has led to the suggestion that IκBα provides the cell with a means of rapidly and transiently activating NF-κB, whereas IκBβ provides the cell with a way to persistently activate NF-κB in the presence of newly synthesized IκBα. The results of gene-targeting experiments of IκBα has supported such a role, because the NF-κB response in embryonic fibroblasts from IκBα−/− mice are not terminated (Beg et al., 1995; Klement et al., 1996).
Although supportive evidence for the function of IκBβ in the persistent activation of NF-κB has come from several laboratories (Beg et al., 1995; Good and Sun, 1996; McKinsey et al., 1996; Weil et al., 1997; Bitko and Barik, 1998), conclusive evidence for this role for IκBβ is currently still lacking, and the results of IκBβ gene-targeting experiments remain unpublished. Interestingly, results from knockin experiments in which the IκBα gene was replaced by the IκBβ coding sequence, placing IκBβ under the control of the IκBα promoter, suggested that IκBβ could compensate for IκBα (Cheng et al., 1998). Unlike IκBα−/− mice, which show a significant phenotype, including severe runting, extensive granulopoiesis, severe dermatitis, and death by day 7 or 8 (postnatal) (Beg et al., 1995; Klement et al., 1996), IκBβ knockin mice show normal survival and no obvious phenotype. Embryonic fibroblasts from these mice failed to show prolonged activation of NF-κB in the absence of IκBα in response to signals, thus suggesting that IκBα and IκBβ proteins were biochemically equivalent and that any differences in function resulted from differences in their transcriptional regulation or expression. However, these conclusions do not account for the possibility that significant overexpression of IκBβ in the knockin mice may have titrated out important regulatory elements, e.g., the newly described κB-Ras proteins, required for IκBβ to perform its proposed function in the persistent activation of NF-κB (Fenwick et al., 2000). Therefore, we felt that it would be important to study the regulation of expression of IκBβ to better understand the underlying reasons for its difference from IκBα.
In this article, we report that IκBβ expression is higher in the testis than in any other tissue examined and occurs in the virtual absence of IκBα expression (Thompson et al., 1995), suggesting that IκBβ may play a unique role in testis. We have localized the expression of IκBβ in testes to the haploid stages of spermatogenesis and have cloned and characterized the IκBβ promoter, and compared its regulation with the promoter for IκBα (de Martin et al., 1993; Le Bail et al., 1993; Chiao et al., 1994). We have defined regulatory regions important for the constitutive expression of IκBβ, including two SP1 sites and a possible negative regulatory region within the upstream sequences. More interestingly, we have found numerous binding sites for testes-specific HMG-box transcription factors, SRY, and/or other Sox family proteins (Prior and Walter, 1996; Pevny and Lovell-Badge, 1997; Wegner, 1999). Our results suggest that Sox proteins likely play an important role in the expression of IκBβ in haploid sperm, and possibly also in the developing male gonad.
MATERIALS AND METHODS
Cloning of the IκBβ Promoter
The IκBβ promoter was cloned by polymerase chain reaction (PCR) after determination of the upstream sequence by primer walking on DNA from an IκBβ genomic clone isolated previously from a mouse genomic liver library (Budde and Ghosh, 2000). Primer walking was initiated using a 3′ primer to sequence found within the 5′ end of the mouse IκBβ cDNA, 3PI 75 βB, GCTCTGGGCCAAGCTCTGCGC. Additional 3′ primers were generated as needed until sequence was obtained for 879 nucleotides upstream of the ATG. This region was then cloned using primers NOTB5, GCGAATGGAGCGGCCGCGAGAGTTGA GTGTGGGAGAGG, and BP3, GATAGA TCTGGCCCCAGCCACCTCGGGTG. This product was TA cloned into the pCR2.1 vector (Invitrogen, Carlsbad, CA). An additional 500 bases of upstream sequence was later obtained in the same manner.
Reporter Constructs
The IκBβ promoter luciferase reporter constructs were made by use of convenient restriction sites or PCR as follows. For BP, the 879-nucleotide IκBβ promoter fragment (see above) was cloned into XhoI/HindIII sites in the pGL3-βasic reporter plasmid (Promega, Madison, WI). For DEL451, the BP construct was digested with SmaI and PstI, blunted with T4 DNA polymerase, and religated. For DEL356, the BP construct was digested with SmaI and ApaI, blunted with T4 DNA polymerase, and religated. For DEL32, the BP construct was digested with SmaI and EcoRI, blunted with Klenow, and religated. The DEL547, DEL318, DEL185, and DEL61 constructs were made via PCR by using the BP construct as a template and then cloned into XhoI/HindIII sites in pGL3-βasic by using the following primers: BP275 (CCAACCCTCGAGCGGACCACTTAGCAACACCC) and BP3HIND3 for DEL547; BP500 (GGAAGGCTCGAGGCAGCGGAAACAAGAAGAGG) and BP3HIND3 for DEL318; BP640 (GGAAGGCTCGAGGGCGGCCATATTGATA AAGG) and BP3HIND3 for DEL185; and BP760 (GGAAGGCTCGAGGATT GGGTATATGAGGGGGC) and BPHIND3 for DEL61.
SP1 sites were mutated by PCR by using the DEL318 construct as a template. Base changes were made as follows: M1SP1, GGGCGG to GAGAGT and M2SP1, GGGCGG to GTAATG. The M1SP1 construct was made using the primers BP500 and M1SP1 (TATCGGGAATTCCCCAACACGCCCCCTCATATACCCAATCAAAATGTTTTAAATAGCTACACCACTCTCCTGTACTGC). This PCR fragment was then used to replace the XhoI/EcoRI fragment in the wild-type DEL318 construct. The M2SP1 construct was made using the primers M2SP1 (TGTTGGGGAATT CCCGATAGAGAGCAAGCACTGGAGCTCATCG) and BP3HIND3. This PCR fragment was then used to replace the EcoRI/HindIII fragment in the wild-type DEL318 construct. Finally, the M1+M2SP1 construct was made by replacing the XhoI/EcoRI fragment from the M2SP1 construct with the same fragment from the M1SP1 fragment.
The BP/SILC construct was made by cloning the 879 base pairs IκBβ promoter fragment from pGL3-βasic into XhoI/HindIII sites in pBIISK+ (Stratagene, La Jolla, CA). The PstI/NcoI fragment was then removed and replaced with a control fragment made by PCR by using the 3′BpBSK plasmid containing 3′ sequence of the IκBβ gene (Budde and Ghosh, 2000) as a template and 5-CR1 (GTCATCGTCTGCAGAGCAG CAGATGGAGAGCGGTG) and 3-CR1 (GCTTCTCACCATGGGTCTTTCCCTA CCATCAAGCG) primers. A PstI/EcoRI fragment was then removed from the chimeric BP construct and cloned in place of the wild-type PstI/EcoRI fragment in the wild-type BP construct.
The κB site in the BP construct was changed from GGGGAATTCCC to ATATAATTCCC by PCR by using the BP construct as a template and pGL3 (CTAGGTACCGAGCTCTTACGCGTGCTAGC) and MUTKB (GCTCTGCGA TGAGCTCCAGTGCTTCCGCCCTATCGGGAATTATATAACACGCCCCCTCATATACC) primers. This product was TA cloned into the pCR2.1 vector (Invitrogen). A SacI fragment containing the mutated κB site was then removed and recloned into the BP construct in place of the wild-type SacI fragment.
Mutations of Sox binding sites 4 M and 6 M were made by PCR by using the BP construct as a template to make 5′ [5XHOBP, GGTCCTCGAGAGTGTTGGTGGCTGAGAGAGGG + (3MUT4, AAGGGTCCGgcTGcCAAGTTCT or 3MUT6, GAGAGGCACgcTGcA CGAAAGC) and 3′ (5MUT4, ATTAAGAACTTGgCAgcCGGAC or 5MUT6, AACCGCTTTCGTgCAgcGTGCC), + BP3HIND3, GGAAGGAAGCTTGGCCCCAGCCACCTCGGGTG] fragments followed by overlapping PCR by using these two fragments as template for PCR with 5XHOBP + BP3HIND3 primers. The latter product was then cloned into pGL3-βasic. Construct 4 M was then used as a template for PCR for construct 4 M+6 M by using the same strategy with 3MUT6 and 5MUT6 primers. All constructs were sequenced before use in experiments.
Activators/Inhibitors of NF-κB
Activators and inhibitors of NF-κB were added at the following concentrations: 10 μg/ml lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO); 2 μg/ml leucoagglutin (PMA-L) (Sigma-Aldrich); 25 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich); and 100 μM pyrrolidinedithiocarbamate (PDTC) (Sigma-Aldrich), with 60 min pretreatment.
Antibodies
The polyclonal anti-mouse IκBβ antibody was raised in rabbits in our laboratory against bacterially expressed, affinity-purified, full-length mouse IκBβ (Thompson et al., 1995). The polyclonal rabbit anti-human IκBα antibody, MAD-3, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal mouse anti-actin antibody AC-40 was raised against a synthetic peptide and was purchased from Sigma-Aldrich. Anti-rabbit IgG-fluorescein isothiocyanate (FITC) was purchased from Santa Cruz Biotechnology.
Northern Hybridization
Multiple endocrine tissue Northern blot was purchased from CLONTECH (Palo Alto, CA) and was hybridized and washed using manufacturer's protocols. The actin control included with the blot was used as the loading control.
Normalized values for the induction of IκBα and IκBβ mRNA were obtained by densitometric analysis of phosphorimaging signals obtained for the IκBs vs. actin signals obtained on the same Northern blots. Normalized data were obtained using Molecular Analyst software (Bio-Rad, Hercules, CA).
Cloning of Sox-5 and SRY
Mouse Sox-5 was cloned by reverse transcription-PCR (Superscript Preamplification kit; Invitrogen) by using mouse testis RNA (CLONTECH) as template with the primers 5SOX (GCTTCCACAAGCTTGCAGTTCTTATGAAGCCTC) and 3SOX (GGAGAGCTTCT AGAAGAACAAACAGCCATAAAG). The PCR product was TA cloned into pCR2 (Invitrogen) and then into HindIII/XbaI sites in pcDNA3 (Invitrogen). Human SRY was cloned by reverse transcription-PCR by using human testis RNA (CLONTECH) as a template with the primers 5TDF (GCTTCCACAAGCTTACTCTCCTTGTTTTTGACAATGC) and 3TDF-XHO (GGAGAGCTCTCGAGCGATTGTCCTACAGCTTTGTCC) and cloned into HindIII/XhoI sites in pcDNA3. All constructs were sequenced.
Preparation of His-tagged Proteins
The coding regions of human SRY (HIS5TDF, CTGTTCAGGAAT TCTTAAGCGTATTCAACAGC + 3TDF-XHO; see above) and mouse Sox-5 (HISSOX5, CTGTTCAGGAATTCGCAGCTGCTGCTGCAGCAACACC + 3HISSox, CTGTTCAGAAGCTTTCAG TTGGCTTGTCCCGCAATGTGG) were made by PCR, cloned into the pET-30a(+) vector (Novagen, Madison, WI), and sequenced. SRY was cloned into the EcoRI/XhoI sites. Sox-5 was cloned into the EcoRI/HindIII sites. Plasmids were transformed into BL21 bacterial cells and grown in Luria Broth medium to OD600 nM = 0.5. Isopropyl β-d-thiogalactoside was added to 0.4 mM to induce production of the His-tagged proteins, and cultures were grown for 2 additional hours at 37°C. Cells were collected, chilled on ice, spun down for 10 min at 3000 × g at 4°C, resuspended in binding buffer (His-Bind Buffer kit; Novagen) plus protease inhibitors (as described above), and frozen at −70°C. Cells were sonicated using a Virsonic sonicator (Virtis Instruments, Gardinier, NY) and the supernatant clarified at 12,000 rpm × 30 min at 4°C. His-tagged proteins in the supernatants were purified over a nickel-agarose column by using the Novagen His-Bind Buffer kit according to the manufacturer's instructions. Samples were dialyzed against sodium phosphate dialysis buffer (25 mM sodium phosphate, pH 7.4, 50 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 10% glycerol). Protein concentration was determined using the Micro BCA Protein Assay Reagent kit (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions, and samples were electrophoresed on a 10% SDS-PAGE to check purity.
Electrophoretic Mobility Shift Assay
Oligonucleotides used for SRY/Sox binding sites were as follows: Control, GATCTATCCCAAACAATTTCAC and AGCTGTGAAATTGTTTGGGATA; SRY1, CTGGGGATTAGTACAATCTCCT and TCCCAGGAGATTGTACTAATCC; SRY2, GCAGAAGGGCTCACAATGGTGG and TCCTCCACCATTGTGAGCCCTT; SRY3, CAGTTGCCCTTAACAACAGACA and AGGTTGTCTGTTGTTAAGGGCA; SRY4, ATTAAGAACTTGACAATCGGAC and AAGGG-TCCGATTGTCAAGTTCT; SRY5, ACGGCAGCGGAAACAAGAAGAG and CGGCCTCTTCTTGTTTCCGCTG; SRY6, AACCGCTTT-CGTACAATGTGCC and GAGAGGCACATTGTACGAAAGC; SRY7, AGACGCCCTTTATCAATATGGC and GGCGGCCATATTGATAAAGGGC; MutSRY4, ATTAAGAACTTGgCAgcCGGAC and AAGGGTCCGgcTGcCAA GTTCT; MutSRY6, AACCGCTTTCGTgCAgcGTGCC and GAGAGGCACgcTGcACG AAAGC; and KB, GATCAGAGGGGACTTTCCGAGG and GATCCCTCGGAAAGT-CCCCTCT.
Complementary oligonucleotides (oligos) were annealed by heating to 90°C for 10 min followed by slow cooling to room temperature. Annealed oligos were then radiolabeled using Klenow enzyme (Roche Applied Science, Indianapolis, IN), [32P]α-dATP and α-dCTP (Amersham Biosciences, Piscataway, NJ), and cold dGTP and dTTP (Roche Applied Science), and the probe was purified on a nondenaturing polyacrylamide gel and resuspended in STE (0.1 M NaCl, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, pH 8.0). His-tagged protein (100 ng) was incubated with 20,000 cpm of labeled probe and 1 μg of competitor DNA [poly(dI-dC); Amersham Biosciences)] in binding buffer (10 mM HEPES, pH 7.9, 60 mM KCl, 1 mM dithiothreitol, 1 mM EDTA, 0.25 mg/ml bovine serum albumin, 12% glycerol) in a total volume of 20 μl for 20 min. Samples were then electrophoresed on a 4% nondenaturing polyacrylamide gel with 0.5× Tris borate-EDTA (0.045 M Tris borate, 0.001 M EDTA) as running buffer. The gel was then vacuum-dried for 60 min at 80°C and exposed to Biomax film overnight (Eastman Kodak, Rochester, NY).
For competition experiments, cold oligonucleotides were annealed as described above. Then 100-1000 times cold annealed oligonucleotide was preincubated with his-tagged protein + dIdC in binding buffer for 20 min at room temperature. Radiolabeled oligonucleotide (20,000 cpm) was then added and incubated for an additional 10 min. Samples were then run on a nondenaturing polyacrylamide gel as described above.
The p50 protein used as a control in the SRY/Sox-5 gel shift experiments was prepared by in vitro translation using the TNT T7 Quick Rabbit Reticulocyte Lysate kit (Promega).
Gel shifts that looked at NF-κB binding to the κB probe only were done as described above but with the following changes. Samples were prepared in Lipage binding buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM EDTA, pH 7.5, 1% glycerol). Samples also included 6 mM GTP, 2 μg of dIdC, and 20 μg of bovine serum albumin. Samples were run on a 4% nondenaturing polyacrylamide gel containing 1× Lipage buffer (6.7 mM Tris-HCl, pH 7.5, 3.3 mM NaOAc, pH 7.0, 1 mM EDTA, pH 8.0) with 1× Lipage running buffer.
In Situ Hybridization
Testes from male adult mice (4 wk) were isolated and fixed with 4% paraformaldehyde in diethyl pyrocarbonate-phosphate-buffered saline (PBS) (paraformaldehyde/PBS) overnight at 4°C. The fixed tissues were washed with PBS three times and then embedded in paraffin and sectioned. The slides were stored at 4°C and were dewaxed at 65°C for 24 h and then cooled down before hybridization. Tissues on the slides were further fixed in paraformaldehyde/PBS, acetylated to block positively charged free amino groups, and permeabilized with 1% Triton X-100. pcDNA3-IκBβ plasmid was linearized and purified by phenol/chloroform extraction, precipitated by ethanol, and dissolved in filtered TE buffer. Using the linearized plasmid as template, sense and antisense probes were made according to DIG RNA labeling kit (SP6/T7) ( Roche Applied Science) and purified using QuickSpin columns (Roche Applied Science). An antisense protamine probe was synthesized as a positive control. Yeast tRNA (100 μg) was added as carrier and to saturate any RNase present.
In situ hybridization of mouse embryos was done according to protocol provided with the DIG RNA labeling kit. Briefly, the slides were hybridized with probes in a humidified box at 65°C overnight and then washed according to DIG RNA labeling kit protocol. After color development by using BM Purple AP substrate (Roche Applied Science), slides were rinsed, ethanol-dehydrated, mounted with coverslips, and visualized under microscope.
Immunofluoresence
Ejaculated bull sperm (Pel-Freeze) were incubated in poly-l-lysine–coated (Sigma-Aldrich) chamber slides (Nalge Nunc, Naperville, IL) for 60 min at room temperature. Chambers were then washed several times with PBS. Sperm were permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature and rinsed with PBS. Sperm were incubated with blocking solution (1% fetal calf serum in Hanks' without phenol red, 5% nonfat dry milk in PBS) for 60 min. FITC-conjugated anti-IκBαC21 or anti-IκBβC20 (Santa Cruz Biotechnology) without or with preincubation with specific blocking peptide (Santa Cruz Biotechnology) (1:5, antibody-to-blocking peptide ratio) was added at 1:100 dilution with PBS containing 5% goat serum. After 1-h incubation, slides were washed with PBS containing 5% goat serum several times. Gel mount (Biomedia Corp., Foster City, CA) mounting medium was added on the sample areas and coverslips were placed onto the slides. Slides were viewed and photographed with a fluorescence microscope.
Transient Transfection Assays
Cells were seeded into 12-well tissue culture plates (Falcon Plastics, Oxnard, CA) 24 h before transaction. HeLa (American Type Tissue Collection, Manassas, VA) or 293 (American Type Tissue Collection) cells were plated to reach ∼70% confluence by the time of transfection. Jurkat (American Type Tissue Collection) cells were plated at 1–1.5 million cells per well in 1 ml of serum-supplemented medium. Then 250 ng of each construct was incubated with 1.5 μl of FuGENE 6 (Roche Applied Science) in 50 μl of unsupplemented medium for 20 min in a 96-well tissue culture plate (Falcon Plastics) before its addition to cells in the 12-well plates. The total amount of DNA tranfected per well was not >1 μg. If more than one construct was transfected into a well, the appropriate vector alone construct was added to bring up the total DNA concentration to the same amount for all wells. After addition of the transfection reagent and DNAs, cells were replaced into the incubator for 36 h. After this time, cells were either harvested, or activators of NF-κB were added for the specified lengths of time, and then harvested.
Whole cell extracts were prepared by collecting and washing the cells in PBS followed by lysis in TNT buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100) supplemented with protease inhibitors. Protease inhibitors were added at the following concentrations: 1 μg/ml aprotinin (Sigma-Aldrich), 1 μg/ml leupeptin (Roche Applied Science), 100 μg/ml phenylmethylsulfonyl fluoride (Sigma), and 1 μg/ml pepstatin (Roche Applied Science). Then 5 μl of supernatant from lysed cells was added to 50 μl of luciferase substrate (Luciferase Assay System; Promega) and assayed for light units in a LUMAT luminometer (Perkin Elmer, Gaithersburg, MD). Protein concentrations for each sample were determined using the Micro BCA Protein Assay Reagent kit (Pierce Chemical). Luciferase units obtained for each sample were normalized for micrograms of protein in each sample and plotted for each construct. Figures shown are representative examples of assays performed in triplicate and repeated three or more times.
NF-κB-Luciferase Transgenic Mice
Transgenic mice carrying a luciferase reporter gene under the control of a minimal fos promoter and two κB sites from the Igκ immunoglobulin enhancer were created by the Immunobiology Transgenic Mouse Facility at Yale University according to standard procedures. The pBIIX-luciferase reporter gene construct described previously was used to make the mice (Kopp and Ghosh, 1994).
RESULTS
IκBβ and IκBα Are Differentially Expressed in Testes
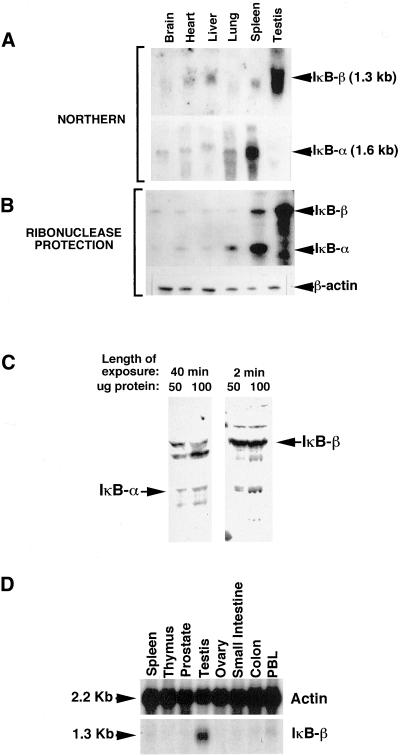
To identify potential unique roles for IκBβ that were different from those of IκBα resulting from differential expression patterns of the two IκBs, levels of mRNA expression were determined for multiple tissues by Northern hybridization and ribonuclease protection analysis. The results demonstrated that IκBα and IκBβ were both expressed at low levels in brain, heart, liver, and lung and that IκBα was more highly expressed in spleen than IκBβ. Strikingly, however, IκBβ was more highly expressed in testis than in any other tissue examined, and this high level of expression occurred in the virtual absence of IκBα expression (Figure 1, A and B). This suggested that IκBβ might have a role in testis that was different from that of IκBα. To verify that this difference held up at the level of protein expression, a testis protein extract was analyzed by immunoblotting with antibodies against IκBα or IκBβ. As expected, the results showed that IκBβ protein was abundantly expressed and detectable after a very brief exposure of the blot to film (Figure 1C). In contrast, a small amount of IκBα protein was detectable but only after prolonged exposure. To determine whether high-level IκBβ expression is a testis-specific phenomenon or common to reproductive tissues, Northern hybridization of a human multiple tissue blot, including testis and ovary RNA samples, was done using a radiolabeled IκBβ cDNA probe. The results showed that IκBβ mRNA was virtually undetectable in ovary in comparison with expression in testis (Figure 1D).
Figure 1.
IκBβ but not IκBα is highly expressed in mouse testes. Multiple tissue Northern blot (A) and ribonuclease protection (B) analyses show that IκBβ mRNA is highly expressed within the testis more than in any other tissue tested. In contrast, IκBα mRNA is barely detectable within the testis. (C) Western blot analysis of total testis extracts shows that this difference in IκBβ and IκBα expression holds up at the protein level. The left panel was probed with an IκBα antibody and the right panel was probed with an IκBβ antibody. (D) Northern blot analysis of a multiple tissue blot shows that high level IκBβ expression does not occur in ovary, suggesting that this expression is not a general phenomenon of reproductive tissues.
A High Level of NF-κB Activity Is Found within Mouse Testes
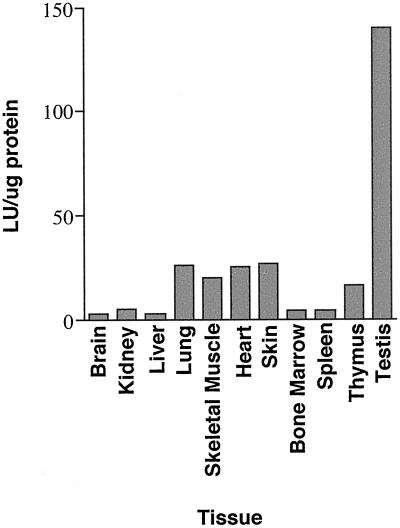
It is likely that the high level of IκBβ expression that occurs within the testis serves to regulate NF-κB within this tissue. To demonstrate that NF-κB activity is found within the testis, we took advantage of transgenic mice available in our laboratory that express a luciferase reporter gene under the control of a minimal fos promoter and two κB sites. We assayed a variety of tissues for endogenous NF-κB activity by determining levels of reporter gene activity. Strikingly, luciferase reporter activity was higher in testis extracts than in extracts from any other tissue assayed (Figure 2). These results support observations of Delfino and Walker (1998) who showed by gel shift and immunohistochemistry assays that nuclear NF-κB is present in Sertoli cells and particular stages of developing germ cells. Given that Delfino and Walker (1998) identified nuclear NF-κB within late meiotic and early haploid spermatid stages of developing germ cells we wanted to determine whether IκBβ was expressed in later stages of germ cell development, e.g., in haploid spermatids, thereby explaining the loss of NF-κB activity in those stages.
Figure 2.
Tissue distribution of luciferase activity in mice containing an NF-κB–dependent reporter transgene. Total cell extracts were made from the tissues indicated and assayed for luciferase reporter gene activity. The data shown are representative of results obtained in repeated experiments.
IκBβ Expression Occurs in Haploid Spermatids
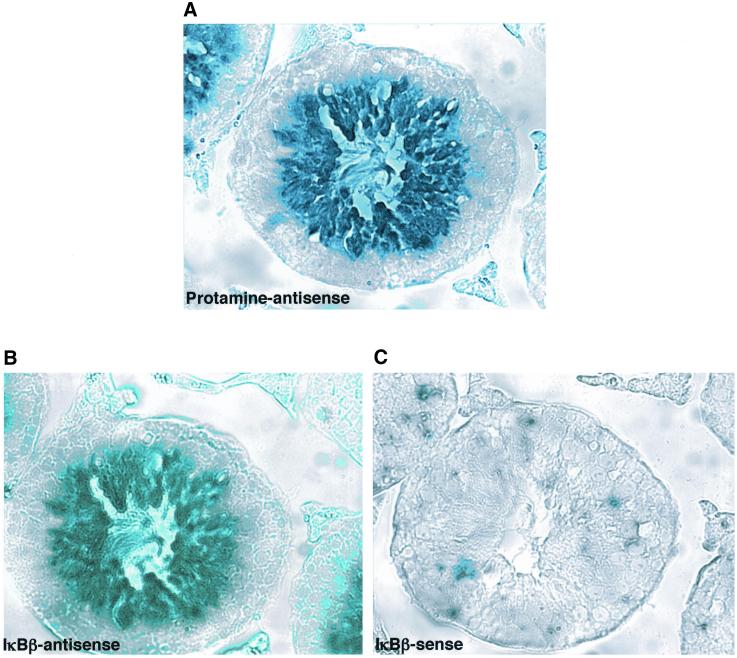
To localize the cell type in which IκBβ expression occurs within the testis, in situ hybridization was done on mouse testis sections by using a IκBβ antisense probe. Protamine antisense and IκBβ sense probes were included as positive and negative controls, respectively. Protamine is a sperm-specific histone that replaces the somatic and transitional histones within the developing sperm cells during the process of spermiogenesis, the third and final stage of spermatogenesis during which substantial cellular remodeling occurs to produce mature haploid sperm cells (Hecht, 1998). Protamine is a highly basic protein and allows the DNA to become extremely condensed for packaging within the tiny sperm head. Its expression is limited to the haploid stages of spermatogenesis. As expected, staining with the protamine antisense probe was limited to the haploid spermatid stages of spermatogenesis located within the center of the seminiferous tubules (Figure 3A). Interestingly, staining with the IκBβ antisense probe was also restricted to the same stages of sperm development (Figure 3, A and B). The IκBβ sense probe produced no staining (Figure 3C). Therefore, these results suggest that IκBβ expression in haploid spermatids may serve to terminate the NF-κB that is activated in preceding stages of germ cell development.
Figure 3.
Expression of IκBβ mRNA in mouse testes occurs in haploid spermatids. Tissue sections of adult mouse testes were hybridized to antisense protamine (A), antisense IκBβ (B), or sense IκBβ (C) probes. Similar to the staining seen for protamine mRNA, staining for IκBβ mRNA occurs within the haploid spermatids, located toward the lumen of the seminiferous tubules. As expected, no staining is seen with the IκBβ sense probe.
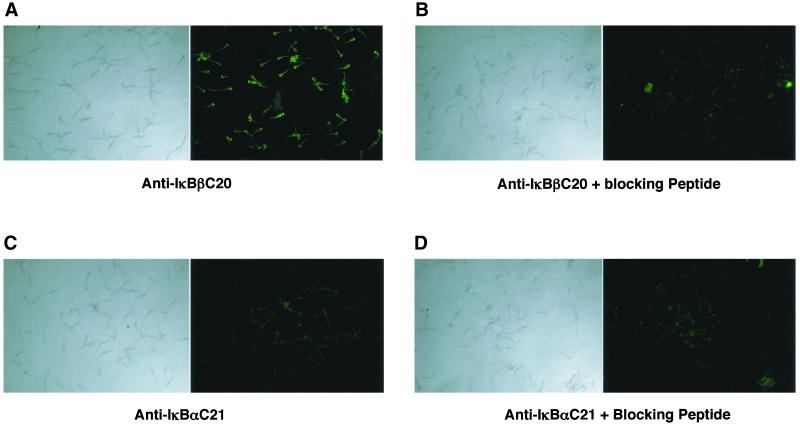
Because it has been previously reported that much of the mRNA isolated from haploid spermatids is not associated with polysomes, it is possible that this RNA is never translated (Ivell, 1992). We thus felt it was important to demonstrate that IκBβ protein could be specifically identified within haploid sperm, despite having demonstrated previously that substantial amounts of IκBβ protein were detectable in total testis extracts on a Western blot (Figure 1C). We thus did immunocytochemical analysis for IκBβ protein in ejaculated bull sperm by using a polyclonal antibody raised against mouse IκBβ protein. As expected, the results demonstrated that IκBβ protein was found in bull sperm (Figure 4A). The immunostaining could be abolished upon preincubation of the antibody with the peptide immunogen used to raise the antibody (Figure 4B). Also consistent with our prior results, an antibody against IκBα failed to immunostain the sperm (Figure 4, C and D). Immunocytochemical analysis of sperm isolated from mouse epididymi gave identical results (our unpublished data), demonstrating that the results were not species specific.
Figure 4.
Immunostaining for IκBβ in bull sperm. (A) Ejaculated bull sperm were stained with antibody against IκBβ protein and visualized with FITC-conjugated secondary antibody. (B) IκBβ antibody was preincubated with the blocking peptide before use. (C) Sperm were stained with antibody against IκBα protein and visualized with a FITC-conjugated secondary antibody. (D) IκBβ antibody was preincubated with the blocking peptide before use. Bright field microscopy of sperm shown in the left-hand panels.
Cloning and Characterization of the IκBβ Promoter
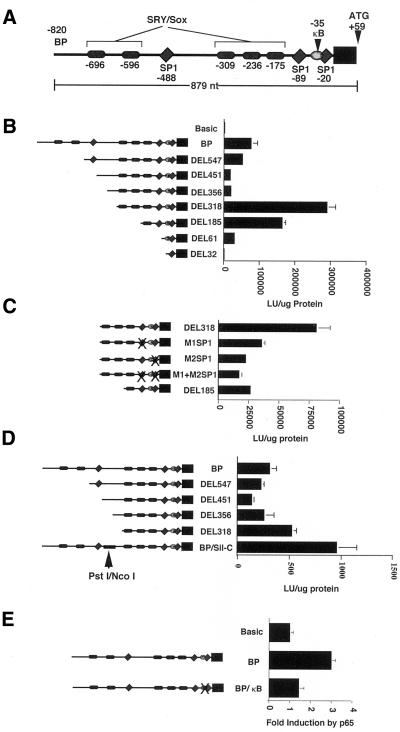
To identify and characterize the regulatory elements important for the expression of the IκBβ gene, particularly in testis, sequences upstream of the IκBβ coding region were obtained from a clone of the IκBβ gene, isolated from a SV29 mouse genomic liver library by using the IκBβ cDNA as probe (Budde and Ghosh, 2000). Sequences upstream from the initiating methionine were sequenced and analyzed for potential transcription factor binding sites by using the MatInspector 2.1 program (Quandt et al., 1995). This analysis revealed several binding sites for transcription factors that seemed to be relevant for the regulation of the IκBβ gene. These included three SP1 sites, a single κB site, and five SRY/Sox protein binding sites. These are schematically represented in Figure 5A.
Figure 5.
Characterization of the IκBβ promoter. (A) Schematic representation of putative binding sites identified in the IκBβ promoter. The initiating methionine (ATG) is located at +59. (B) Boundaries of the IκBβ promoter were established using luciferase reporter constructs in which the 879 nucleotides upstream of the ATG were progressively deleted at the 5′ end. Data shown are from transient transfection of HeLa cells. Maximal reporter activity was observed upon deletion of 503 of the most 5′ nucleotides in the DEL318 construct, suggesting important positive regulatory elements were located upstream and that a negative regulatory element existed upstream. (C) Contribution of the two downstream SP1 sites to the reporter activity observed for the DEL318 construct was established by mutating the SP1 sites individually and together. Both SP1 sites contribute significantly to reporter activity in Jurkat cells. (D) Demonstration that a negative regulatory element is located between nucleotides −449 and −376. Replacement of this sequence in the BP construct with another segment of DNA of equivalent size results in increased reporter activity in Jurkat cells. (E) NF-κB activation by p65 cotransfection in Jurkat cells leads to increased reporter activity of the BP construct through NF-κB binding to the κB site, as mutation of this site inhibits increased reporter activity.
The location of the transcription initiation site had previously been established to be 59 nucleotides upstream of the initiating methionine (Budde and Ghosh, 2000). To further characterize the boundaries of the IκBβ promoter, reporter constructs were generated in which various amounts of sequence upstream of the initiating methionine in the IκBβ coding sequence (Thompson et al., 1995) were placed upstream of a luciferase reporter gene. The largest construct, BP, contained all 879 nucleotides initially sequenced upstream of the initiating methionine. Progressive 5′ deletions of this sequence were then generated using a combination of convenient restriction sites and PCR to make the remaining constructs. Interestingly, transfection of these constructs into several cell types including HeLa, Jurkat, and 293 cells revealed a biphasic pattern of reporter gene activity (Figure 5B). Deletion of sequences upstream of nucleotide −318 resulted in a three- to fourfold increase in reporter activity in repeated experiments in comparison to that obtained with the full 879 nucleotides (compare DEL318 and BP constructs). This suggested that important positive regulatory elements were located downstream of −318 and that a silencer or negative regulatory element was located upstream of −318.
Two of the three SP1 binding sites identified within the sequences upstream of the IκBβ coding sequence were located downstream of −318 where the strongest reporter activity was detected. Because IκBβ expression is widespread among cells and tissues (Thompson et al., 1995) and because SP1 has been shown to direct the constitutive expression of many genes (Lania et al., 1997), we wanted to determine whether the SP1 sites located within this region were driving the robust reporter gene expression seen for the DEL318 construct. We mutated nucleotides within each SP1 site individually and together within the DEL318 construct. Wild-type and mutated constructs were then assayed for reporter gene activity in transient transfection assays (Figure 5C). Reporter activity was reduced to 40 and 30% of that seen for the wild-type DEL318 construct after mutation of the distal or proximal SP1 sites in the M1SP1 and M2SP1 constructs, respectively. Mutation of both SP1 sites together in the M1+M2SP1 construct reduced reporter activity to 25% of that seen for the DEL318 construct. This suggested that both SP1 sites contribute significantly to IκBβ promoter activity to drive its basal transcription. Interestingly, construct DEL185, which lacks nucleotides −318 to −184 but still contains both wild-type SP1 sites shows only 35–50% of the reporter activity seen with the DEL318 construct. This suggests that additional positive regulatory elements located between −318 to −184 also contribute to the maximal reporter gene activity seen with the DEL318 construct, likely in cooperation with the two downstream SP1 sites.
Removal of the 500 most 5′ nucleotides from the BP reporter construct produced an increase in reporter activity (Figure 5B), suggesting that a classic silencer or a negative regulatory element existed within these upstream sequences. To establish whether a position-independent classic silencer was present upstream of nucleotide −318, we removed DNA sequences located between −547 to −319 and placed them in front of heterologous promoters driving a reporter gene. Placement of these sequences in front of simian virus 40 and cytomegalovirus promoters did not reduce reporter activity, suggesting that a classic silencer was not present within the IκBβ promoter (our unpublished data). However, removal of nucleotides −449 to −376 by PstI/NcoI digestion and replacement of this sequence with a DNA fragment of equivalent size that did not positively or negatively influence activity of heterologous promoters (see MATERIALS AND METHODS; our unpublished data), resulted in an increase in reporter activity (Figure 5D, compare BP, DEL318, and BP/Sil-C constructs). This suggested that a negative regulatory element functioning within the context of the IκBβ promoter was located upstream within these sequences.
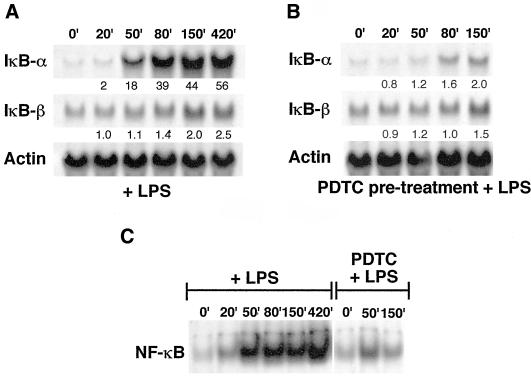
The existence of a κB site within the IκBβ promoter was curious, because the expression of IκBβ has previously been shown not to be dependent on NF-κB (Thompson et al., 1995). To test whether this site was functional in transient transfecton assays, reporter activity of the wild-type BP construct and a BP/κB construct, in which the κB site was mutated, were compared upon cotransfection of a p65 encoding plasmid (Figure 5E). The results showed that activation of NF-κB by p65 cotransfection-induced reporter activity of the wild-type BP construct by three- to fourfold in repeated experiments and that this induction was inhibited by mutation of the κB site. This suggested that NF-κB could bind to this site and induce transcription, although induction through this site is modest. Indeed, comparison of the in vivo induction of IκBα vs. IκBβ in 70Z/3 cells stimulated with LPS showed the difference in induction by NF-κB that occurs through the three κB sites found in the IκBα promoter (de Martin et al., 1993; Le Bail et al., 1993; Chiao et al., 1994) vs. the single κB site identified in the IκBβ promoter (Figure 6). IκBα was induced 56-fold after 7 h, whereas IκBβ was induced only 2.5-fold after the same amount of time. Additionally, some of this induction of IκBβ likely occurred due to other factors induced by LPS, because PDTC-pretreated cells (an inhibitor of NF-κB), did not show complete suppression of this induction. Thus, although the κB site in the IκBβ promoter seems to be functional, it does not serve the same role as the κB sites found in the IκBα promoter, which allow for an autoregulatory feedback inhibition of NF-κB.
Figure 6.
Comparison of IκBβ and IκBα mRNA induction by NF-κB in 70Z/3 cells stimulated with LPS. (A) Total RNA was isolated from cells stimulated with 10 μg/ml LPS for the indicated times and probed with radiolabeled fragments of IκBα, IκBβ, or actin cDNAs after Northern hybridization. Fold induction was determined by normalization to actin signals by densitometric analysis and is indicated below each time point. (B) Cells were pretreated for 1 h with 100 μM PDTC to inhibit the activation of NF-κB by LPS treatment of cells to establish that mRNA induction was due to NF-κB. (C) Gel shift analysis of total cell extracts incubated with a κB probe to establish that NF-κB was activated by LPS and inhibited by PDTC pretreatment in A and B.
SRY and Sox-5 Bind to Seven SRY/Sox Sites within the IκBβ Promoter
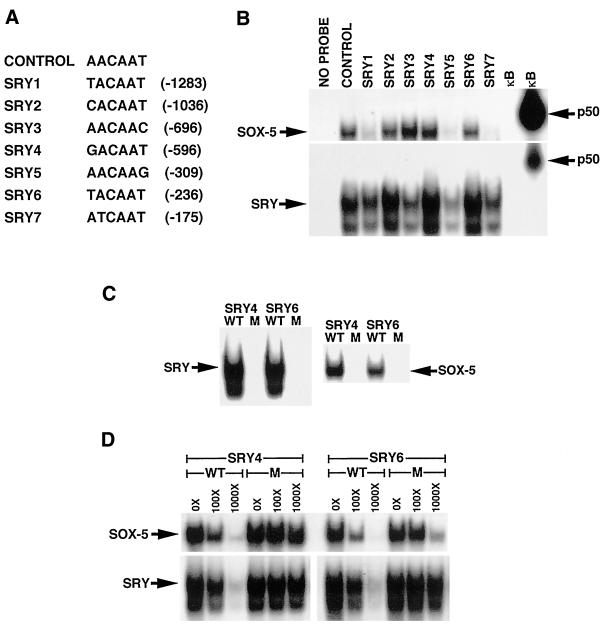
Our identification of multiple binding sites for SRY/Sox proteins upstream of the IκBβ coding sequence immediately suggested how high-level expression of IκBβ mRNA might be achieved in haploid sperm, because SRY and several Sox family members are known to be expressed in the testes (Prior and Walter, 1996; Pevny and Lovell-Badge, 1997; Wegner, 1999). Although five sites were initially identified upon analysis of the 879 nucleotides upstream of the initiating methionine in the IκBβ coding sequence as indicated above, two additional binding sites were identified when another 500 nucleotides were analyzed just upstream of the 879 nucleotides. The sequence and location of the individual sites are indicated in Figure 7A. To determine whether Sox family proteins regulate IκBβ expression in sperm, we initially made polyhistidine-tagged Sox proteins and incubated them with radiolabeled oligonucleotides containing each of the seven binding sites found within the IκBβ promoter, to determine whether such proteins would bind to DNA in a gel shift assay. Although several Sox family proteins are known to be expressed within the testis, we chose to look at SRY and Sox-5 based on the expression of these proteins within the appropriate cell type and stages of spermatogenesis (Denny et al., 1992; Zwingman et al., 1994; Capel, 1998). Our results indicated that both SRY and Sox-5 proteins bound to all seven sites found within the IκBβ promoter, although to varying degrees (Figure 7B). SRY bound to all sites better than Sox-5, when equal amounts of protein were tested. SRY bound best to sites 2, 4, and 6; moderately to sites 1, 3, and 7; and least to site 5. Sox-5 bound best to sites 2, 3, 4, and 6, and poorly to sites 1, 5, and 7. Both proteins also bound well to an oligonucleotide containing the ideal consensus binding site previously determined for several Sox family proteins (Connor et al., 1994; Harley et al., 1994; Kanai et al., 1996) and failed to bind to an oligonucleotide containing a κB binding site.
Figure 7.
SRY and Sox-5 bind to the SRY/Sox protein binding sites in the IκBβ promoter. (A) Sequence of the individual Sox protein binding sites within the IκBβ promoter. The sequence of the previously determined preferred Sox protein binding site used as the positive control is also listed. (B) Purified His-tagged SRY or Sox-5 (100 ng) was incubated with a radiolabeled oligonucleotide of each putative SRY/Sox family binding site and run on a nondenaturing polyacrylamide gel. Oligonucleotides containing the perfect Sox protein consensus and a κB consensus sequence were included as positive and negative controls, respectively. In vitro-translated p50 protein was incubated with the κB oligonucleotide as an additional control. (C) His-tagged SRY or Sox-5 (100 ng) bound to radiolabeled oligonucleotides containing wild-type but not mutant Sox protein binding sites SRY4 and SRY6. (D) Cold oligonucleotides containing wild-type but not mutant Sox protein binding sites SRY4 and SRY6 competed for binding to 100 ng of His-tagged SRY or Sox-5 bound to wild-type radiolabeled oligonucleotides SRY4 and SRY6.
Because each oligonucleotide contained sequences in addition to that found within the actual Sox binding site, we wanted to establish that binding of the his-tagged proteins was occurring specifically to those nucleotides within the binding site. Specificity of binding by SRY and Sox-5 was tested in two ways for sites SRY4 and SRY6, sites to which both proteins bound well. First, several bases within the SRY/Sox site of each oligonucleotide were mutated. Neither protein was able to bind to either mutated oligonucleotide in a gel shift assay (Figure 7C). Second, addition of unlabeled wild-type SRY4 or SRY6 oligonucleotide but not mutated SRY4 or SRY6 oligonucleotide competed for binding of his-tagged SRY or Sox-5 to radiolabeled wild-type SRY4 and SRY6 oligonucleotides in a gel shift assay (Figure 7D). Competition assays for the remaining binding sites also showed similar results (our unpublished data), demonstrating that both proteins were binding specifically to each SRY/Sox site within the oligonucleotides.
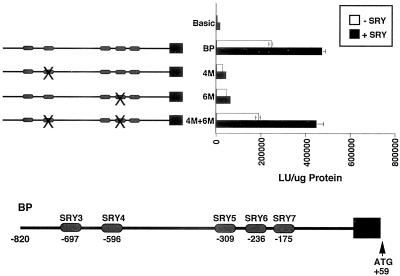
Having demonstrated that both SRY and Sox-5 bind to the individual SRY/Sox sites found within the IκBβ promoter, we next tested whether these two proteins could activate transcription of an IκBβ promoter-driven luciferase reporter gene in transient transfection assays in HeLa cells. The BP construct used previously for characterization of the IκBβ promoter and containing the five downstream SRY/Sox binding sites (SRY3 to SRY7) was used for the experiments. Mutation of sites SRY4 and SRY6 individually and in combination within the BP construct were included in the assay, because these sites were shown to efficiently bind both SRY and Sox-5 proteins. Results of these experiments showed a complex pattern of reporter activity. Cotransfection of SRY activated transcription of the BP construct two- to fourfold in repeated experiments (Figure 8). Mutation of site 4 or 6 (4 M or 6 M) resulted in inhibition of reporter activity, whereas mutation of both sites together gave a level of reporter activity similar to the wild-type BP construct. We believe that this pattern of reporter activity is consistent with the function of these proteins as architectural transcription factors (Giese et al., 1992), so that mutation of individual and combinations of binding sites lead to different DNA conformations upon protein binding. The different DNA conformations result from the dramatic bending of DNA, which these proteins are known to induce (van de Wetering and Clevers, 1992; Love et al., 1995; Werner et al., 1995). This in turn leads to increased or decreased transcriptional activity, depending on which conformations are assumed by the DNA. Cotransfection of Sox-5 in these experiments gave similar results, although the increase in reporter activity was not more than twofold in repeated experiments (our unpublished data). Taken together, results of the gel shift and transient transfection experiments suggest that the SRY/Sox binding sites within the IκBβ promoter are functional sites.
Figure 8.
SRY increases activity of an IκBβ promoter luciferase reporter construct. Cotransfection of an SRY-encoding plasmid and the BP reporter construct into HeLa cells results in a twofold increase in reporter activity. Mutation of Sox protein binding sites 4 (4 M) or 6 (6 M) in the BP construct inhibited the increase in reporter activity, whereas mutation of both sites 4 and 6 together (4 M+6 M) did not prevent an increase in reporter activity.
High Level IκBβ Expression Occurs in Embryonic Male Gonad
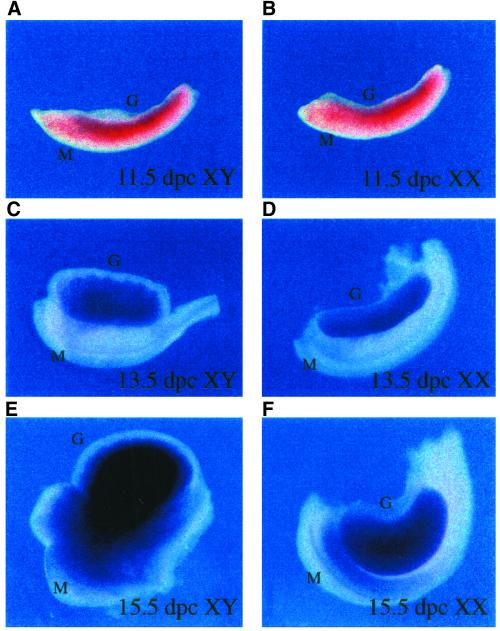
SRY encodes the testis-determining factor, a protein encoded by a gene on the Y chromosome that is essential for directing differentiation of the bipotential gonad into the testes. Although its role in testis development is well established and is believed to function as a transcription factor that regulates downstream genes important for testis formation, no target genes for it have yet been clearly identified, despite its discovery >10 years ago (Capel, 1998). We thus felt it was important to establish whether IκBβ might be a target gene of SRY in the developing male gonad. SRY is expressed during embryonic days 10.5–12.0 within the gonadal ridge of the developing male mouse (Koopman et al., 1990; Hacker et al., 1995; Jeske et al., 1995). Thus, any direct downstream target gene controlled by SRY should be expressed around this time in the gonadal ridge of male but not female embryos. In situ hybridization analysis by using a IκBβ cDNA antisense probe was performed on gonadal tissue that had been dissected from male or female embryos on embryonic days 11.5, 13.5, and 15.5. Low levels of IκBβ mRNA were detected in both male and female gonadal ridges at day 11.5 (Figure 9, A and B), suggesting that IκBβ expression at this stage of gonadal development was not an SRY-regulated process. Interestingly however, a dramatic male-specific up-regulation of IκBβ expression was observed at day 15.5 within the developing testis cords (Figure 9, E and F). This dramatic expression of IκBβ likely occurs within germ cells or Sertoli cells, as these are the two cell types found within the developing testis cords. The transcription factor responsible for the increased level of IκBβ expression remains to be identified but could be other members of the Sox family of proteins. These results suggest that IκBβ also plays some stage-specific role within the developing male testis during embryogenesis.
Figure 9.
Expression of IκBβ in embryonic mouse gonads. The developing gonadal ridge was dissected from male (A, C, and E) and female (B, D, and F) mouse embryos at the indicated number of days postcoitum (p.c.), sectioned, and in situ hybridization performed using an IκBβ antisense probe. Although IκBβ mRNA is expressed at 11.5 d p.c. in the male gonad (A), it is also expressed in the female gonad at the same time (B), suggesting that IκBβ is not an SRY target gene. IκBβ expression continues in the gonads of both sexes at low levels at day 13.5 p.c. (C and D). Strikingly, a dramatic male-specific increase in expression occurs at day 15.5 p.c. (E), with an apparent localization to the developing testis cords.
DISCUSSION
In this study, we have have examined the tissue-specific expression and regulation of IκBβ to understand how the function of IκBβ may differ from that of IκBα in the regulation of NF-κB. We have found that two SP1 sites and a promoter-specific negative regulatory element direct the constitutive expression of IκBβ that occurs in many cells and tissues. We have also identified a single NF-κB site within the IκBβ promoter that binds NF-κB and can modestly activate transcription of a reporter gene in transient transfection assays. However, this κB site was unable to strongly up-regulate transcription in comparison to the κB sites within the IκBα promoter. We believe this difference in the transcriptional regulation of IκBα vs. IκBβ reflects differences in the function of the two IκBs. We have also found that IκBβ is highly expressed within the testis, more than in any other tissue and that this high level of expression occurs in the virtual absence of IκBα expression. This differential expression for the two IκBs suggests the existence of a unique role for IκBβ within testis tissue. IκBβ mRNA and protein expression is restricted to the haploid spermatid stages of spermatogenesis and follows a wave of nuclear NF-κB expression within earlier stages of spermatogenesis identified previously (Delfino and Walker, 1998), suggesting that IκBβ serves to inactivate NF-κB at subsequent stages of spermatogenesis. We have also demonstrated that a high level of NF-κB activity is found within mouse testes through the analysis of tissues from mice expressing a luciferase reporter transgene under the control of two κB sites. This suggests that NF-κB is highly active within the testes and likely plays a role in this tissue. We have shown that multiple binding sites for Sox family proteins are found within the IκBβ promoter and that SRY and Sox-5 proteins can bind to these sites in gel shift assays and can activate a IκBβ promoter reporter gene in transient transfection assays. Our data suggest that these sites are functional and that stage-specific expression of IκBβ in developing sperm are directed by particular Sox proteins expressed during these stages. To date, few bona fide target genes have been identified for the rapidly growing family of Sox proteins and no target genes have been identified for the group of Sox proteins now identified within developing sperm cells. Thus, our results may extend the short list of Sox-regulated genes and provide the first evidence for a potential target gene for sperm-specific Sox proteins. Finally, we have found that a high level of IκBβ expression also occurs within the testis cords of the developing male embryonic gonad. Although the timing of this expression does not coincide with that of SRY, other Sox proteins expressed later may direct the expression of IκBβ in male gonads. Thus, these results suggest that IκBβ plays a role in both adult and embryonic testes.
Role for IκBβ and NF-κB in Testis
The virtual absence of IκBα mRNA and protein in the face of dramatically increased expression of IκBβ mRNA and protein in testis extracts suggested the existence of a unique role for IκBβ within this tissue. Because the only known role of the IκB proteins is in the regulation of NF-κB, we assume that IκBβ expression in the testes serves to regulate NF-κB. Gel shift and immunohistochemical data from Delfino and Walker (1998) support our findings, because they observed constitutively nuclear NF-κB in Sertoli cells, a peak of nuclear NF-κB in pachytene spermatocytes during stages VII to XI, and lesser amounts of nuclear NF-κB in stage I to VII spermatids. Our finding that IκBβ is strongly expressed during the latter stages of spermatogenesis and is restricted to haploid spermatids suggests that IκBβ probably serves to terminate the active NF-κB present in earlier stages.
The role of NF-κB within developing germ cells is unknown. However, given that a significant amount of apoptosis occurs among germ cells within the testis (Bartke, 1995; Billig et al., 1995; Blanco-Rodriguez and Martinez-Garcia, 1996; Hsueh et al., 1996) and that a role for NF-κB in preventing apoptosis has been supported by data from numerous studies (Sonenshein, 1997), we suggest that NF-κB may be activated in those cells which have successfully completed meiosis and will progress further in development. Because the induction of apoptosis in germ cells may be an active process controlled by the supporting Sertoli cells, occurring through the differential expression of Fas ligand on Sertoli cells and Fas on germ cells (Lee et al., 1997, 1999), the expression of nuclear NF-κB within those cells may serve as a survival signal. NF-κB would presumably regulate the expression of genes required for cell survival and might also induce the expression of genes that promote further differentiation of the developing germ cells. The absence of effects on fertility seen in mice in which various Rel or IκB proteins have been gene-targeted may still be consistent with a role for NF-κB in sperm survival. The predominant NF-κB complexes within the developing germ cells in the testis have been identified as p50 and p65 heterodimers (Delfino and Walker, 1998). The fact that p65 is the transcriptionally active member of the p50/p65 heterodimer and that p65−/− mice die during embryogenesis may explain the inability to observe effects on fertility in these experiments. As work on IκBβ gene-targeted mice has not been published, and IκBβ is the predominant IκB expressed within the testis, defects in fertility may be revealed upon careful analysis of these mice.
The expression of IκBβ in haploid spermatids may serve to terminate the transcription of genes regulated by NF-κB beyond a certain stage of germ cell development to avoid the accumulation of unnecessary transcripts in late spermatids. It is known that all transcription ceases by the last week of spermiogenesis, the 2-wk process during which haploid spermatids undergo dramatic cellular remodeling to become mature, fully differentiated sperm (Kierszenbaum and Tres, 1978; Hecht, 1998). This occurs because the DNA becomes progressively condensed as the spermatids differentiate, making the DNA inaccessible to transcription factors. However, much translation still occurs during this last week of spermiogenesis when the DNA is transcriptionally inactive, because cellular remodeling is still incomplete. This is accomplished through the accumulation of transcripts synthesized during the preceding week, which are stored in an inactive form via a variety of mechanisms and then translated at a later time (Braun et al., 1995; Schafer et al., 1995; Sassone-Corsi, 1997; Hecht, 1998). Thus, IκBβ may serve to inactivate NF-κB at earlier stages of spermiogenesis, to avoid the synthesis of transcripts that are no longer needed and would otherwise compete with needed transcripts for the translational machinery.
Transcriptional Regulation of IκBβ in Haploid Spermatids by Sox Proteins
The identification of multiple binding sites for testes-specific transcription factors within the IκBβ promoter immediately suggested how the differential expression of IκBβ and IκBα expression within the testes was achieved. The Sox proteins are a recently defined family of DNA binding proteins, with several members being expressed within the testis (for review, see Prior and Walter, 1996; Pevny and Lovell-Badge, 1997; Wegner, 1999). The Sox family was initially defined through their homology with the HMG domain of SRY, a gene encoding the testis-determining factor (Gubbay et al., 1990; Sinclair et al., 1990). Sox family members have been identified in every species examined and >20 members of this family are now known. Functions for most of them remain undefined. However, detailed study of several family members suggests that these proteins play important roles in various developmental processes. All of these proteins contain an 80-amino acid motif known as the HMG box, placing them within the larger family of HMG proteins (Laudet et al., 1993). This domain is required for DNA binding, which occurs within the minor groove and results in significant bending of the DNA. These proteins are thus thought to function as architectural transcription factors. Some HMG proteins also contain a transactivation domain, including many of the Sox proteins, and so may function as classical transcription factors. Sox proteins all bind to highly similar sequences on DNA. Their specificity is thus thought to occur through their highly restricted patterns of expression and through their interaction with other transcription factors.
The expression of several Sox family members has been localized to various stages of developing sperm, including Sox-17 (Kanai et al., 1996), Sox-6 (Connor et al., 1995), Sox-5 (Denny et al., 1992), and SRY (Sinclair et al., 1990; Foster et al., 1992; Capel et al., 1993; Zwingman et al., 1994). Our rationale for choosing to examine whether SRY and Sox-5 regulated IκBβ expression was based on their expression within haploid spermatids. We chose not to look at Sox-17 and Sox-6 because a truncated form of Sox-17 is found in haploid spermatids that has been shown not to bind DNA, and Sox-6, although it is expressed within haploid spermatids, has been shown not to bind DNA unless a leucine zipper motif is deleted from its structure. Since this work was completed, however, another Sox protein has been identified in haploid spermatids, Sox-30 (Osaki et al., 1999), which might also play a role in regulating IκBβ expression. The function of all of these proteins within the testis remains unknown.
To date, few target genes for the Sox proteins have been defined. Among the well-defined target genes are the human and mouse collagen type II genes regulated by Sox-9 (Lefebvre et al., 1996, 1997), the lens-specific chicken δ1-crystallin and mouse γ-crystallin genes regulated by Sox1/2/3 (Kamachi et al., 1995, 1998), and the fibroblast growth factor 4 gene regulated by Sox2 (Yuan et al., 1995). Detailed study of these genes has revealed that the transcriptional induction of these genes by Sox proteins requires the cooperation of Sox proteins with other transcription factors. This interaction stabilizes the binding of Sox proteins to DNA and also allows for specificity in target gene selection (Kamachi et al., 1999). Several examples of cooperative interactions between specific Pou family proteins and Sox family proteins have now been reported. These include the interaction of Sox2 with Oct3/4 (Yuan et al., 1995), Sox10 with Tst-1/Oct6/SCIP (Kuhlbrodt et al., 1998a), and Sox11 with Brn-1 or Brn-2 (Kuhlbrodt et al., 1998b). Whether cooperative interaction between various Pou and Sox family members will be a common theme for target genes regulated by Sox proteins remains to be determined. Our finding that SRY and Sox-5 proteins could bind to all of the Sox binding sites within the IκBβ promoter and could induce activity of an IκBβ promoter reporter gene suggests that these sites are functional in vivo. Although we expected to obtain a more robust activation of the IκBβ reporter gene by Sox proteins, the modest induction of reporter activity by SRY and Sox-5 may reflect the absence of a cooperating binding partner in HeLa cells. Interestingly, a Pou family binding site is located immediately downstream of Sox binding site SRY4 in the IκBβ promoter (our unpublished data), suggesting that such cooperative interactions could occur. A number of Pou family members are expressed in germ cells (Scholer et al., 1989), including a sperm-specific Pou protein known as Sprm-1 (Andersen et al., 1993; Pearse et al., 1997). The absence of sperm cell lines did not allow us to do our experiments in the proper cellular context, which has been shown to be important for appropriate levels of transcription of other Sox-regulated target genes. It remains possible that other Sox proteins within haploid sperm and other transcription factors also contribute to the high level of IκBβ expression in sperm. Further experiments will be required to establish conclusively which proteins collectively contribute to the expression of IκBβ in haploid sperm.
The likely role of IκBβ in testis as an inhibitor of NF-κB that is active in earlier stages of developing sperm raises the question of why IκBβ performs this role rather than IκBα. Because IκBα functions to rapidly terminate activated NF-κB through an autoregulatory loop driven by three κB sites within its promoter, it may be that it is an inappropriate inhibitor within developing sperm that may require active NF-κB for some period of time during particular stages of germ cell development. An inhibitor whose expression is regulated by transcription factors that are also developmentally expressed at particular stages may make more sense for controlling NF-κB activity involved in a precisely orchestrated developmental process. The developmentally regulated expression of Sox proteins within sperm provides a means by which stage-specific inactivation of NF-κB by IκBβ in developing sperm cells could be achieved.
Expression of IκBβ in Embryonic Testis
Although the timing and lack of sex-specific expression of IκBβ within the gonadal ridge of mouse embryos at day 11.5 suggested that IκBβ was not a direct target gene of SRY during the initiation of testis formation, the male-specific up-regulation of IκBβ expression at day 15.5 was unexpected. IκBβ expression is localized within the testis cords and thus is occurring within Sertoli or germ cells. It is unknown whether NF-κB is active within these cell types during embryogenesis. However, we presume that IκBβ expression at this time occurs to regulate NF-κB. What role NF-κB and IκBβ might play at this time and whether IκBβ expression is also regulated by Sox proteins, is unclear but would be worth exploring. It is known that primordial germ cells migrate into the developing gonads around embryonic days 10.5–11.5 and undergo proliferation to establish the germ cell population that will serve to produce sperm or oocytes after pubertal development (Buehr, 1997). By approximately embryonic day 13.5, colonization of the gonads by germ cells is complete and proliferation gradually ceases as ovarian germ cells enter into meiotic prophase and male germ cells undergo mitotic arrest. A substantial amount of apoptosis also occurs during this period of colonization and proliferation (Matsui, 1998; Wang et al., 1998). If a role for NF-κB in preventing apoptosis of developing sperm cells in the adult testis is established and IκBβ expression in the embryonic testis cords is found to occur within the germ cells, it may be that an earlier expression of NF-κB in the germ cells may serve a similar antiapoptotic role.
Transcriptional Regulation of the IκBβ Promoter
Previous observations by our laboratory suggested that IκBβ was constitutively expressed within a number of cell types and tissues and that unlike IκBα, its expression was not induced by NF-κB (Thompson et al., 1995). Autoregulation of IκBα expression by NF-κB via three κB sites in the IκBα promoter (de Martin et al., 1993; Le Bail et al., 1993; Chiao et al., 1994) fits with the functional role of IκBα in the rapid termination of activated NF-κB. The cloning and characterization of the IκBβ promoter revealed that the constitutive expression of IκBβ is driven by two SP1 sites that both contribute substantially to IκBβ promoter reporter gene activity in transient transfection assays in several cell types. These SP1 sites likely cooperate with other positive regulatory elements located farther upstream (between −318 and −185), given that deletion of this region in reporter constructs also significantly decreases activity despite the presence of both downstream SP1 sites. Although we did not characterize the additional upstream elements, inspection of the sequence revealed potential binding sites for AP1 and Oct 1, transcription factors which are also widely expressed among cells. Our experiments also suggested that the constitutive expression of the IκBβ gene is influenced by a negative regulatory element, as progressive deletion of 5′ sequence up to −318 increased reporter activity in transient transaction assays in several cell types. The nature of this element is unclear. Inspection of the sequence upstream of −318 did not reveal binding sites for proteins previously identified as negative regulatory proteins. However, we did observe that the sequence between nucleotides −547 to −319 was highly GC-rich (76%). It may be that this confers some structure to the DNA that is inhibitory to maximal transcriptional activation from this promoter in the context of the positive regulatory elements found downstream. The existence of this combination of elements within the IκBβ promoter may serve to limit the overall level of transcription in most cell types. This type of control would make sense if the role of IκBβ is to allow for a persistent activation of NF-κB through the resynthesis of a hypophosphorylated form of IκBβ after signal-induced degradation that then binds nuclear NF-κB to prevent its binding to newly synthesized IκBα (Suyang et al., 1996; Tran et al., 1997). A high level of IκBβ transcription might be undesirable because it would be more difficult to terminate the prolonged activation of NF-κB driven by hypophosphorylated IκBβ.
The existence of a single κB site within the IκBβ promoter that binds to NF-κB and modestly activates transcription of an IκBβ promoter reporter gene was unexpected, given that IκBβ expression has not been observed to be regulated by NF-κB (Thompson et al., 1995). If IκBβ plays a role in the persistent activation of NF-κB, the modest induction of IκBβ expression we observed in our experiments may serve to increase IκBβ levels just enough to compete with the strongly up-regulated IκBα, but not so much so that IκBβ is overexpressed, which would then make it difficult to terminate a prolonged NF-κB response. There is one report in the literature that shows that IκBβ is strongly up-regulated in mouse peritoneal macrophages in which NF-κB had been induced by LPS stimulation (Velasco et al., 1997). This suggests that the κB site in the IκBβ promoter may serve to up-regulate IκBβ in response to NF-κB activation in some cell types with some signals. This may occur because certain signals in some cell types may induce another transcription factor that can then cooperate with NF-κB bound at the κB site to maximally up-regulate IκBβ expression. Indeed, up-regulation of the interleukin-8 gene by NF-κB that contains a single κB site has been shown to require the cooperation of NF-κB and another transcription factor (Stein and Baldwin, 1993). Why IκBβ expression is differentially induced by NF-κB in different cell types remains unclear. Further examination of this phenomenon may further elucidate differences in function between IκBβ and IκBα.
ACKNOWLEDGMENTS
We thank Drs. Blanche Capel for thoughtful comments and suggestions, Changchun Xiao for help and advice, and Michael May for carefully reading the manuscript. The work was supported by Howard Hughes Medical Institute and National Institutes of Health (R37-AI33443), and the Anna Fuller Fund.
Footnotes
DOI: 10.1091/mbc.01–07–0373.
REFERENCES
- Andersen B, Pearse RV 2nd, Schlegel PN, Cichon Z, Schonemann MD, Bardin CW, Rosenfeld MG. Sperm 1: a POU-domain gene transiently expressed immediately before meiosis I in the male germ cell. Proc Natl Acad Sci USA. 1993;90:11084–11088. doi: 10.1073/pnas.90.23.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F, Thompson J, Rodriguez MS, Bachelerie F, Thomas D, Hay RT. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology. 1995;136:3–4. doi: 10.1210/endo.136.1.7828545. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. [DOI] [PubMed] [Google Scholar]
- Bitko V, Barik S. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IkappaBbeta, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J Virol. 1998;72:5610–5618. doi: 10.1128/jvi.72.7.5610-5618.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Rodriguez J, Martinez-Garcia C. Spontaneous germ cell death in the testis of the adult rat takes the form of apoptosis: re-evaluation of cell types that exhibit the ability to die during spermatogenesis. Cell Prolif. 1996;29:13–31. doi: 10.1111/j.1365-2184.1996.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Braun RE, Lee K, Schumacher JM, Fajardo MA. Molecular genetic analysis of mammalian spermatid differentiation. Recent Prog Horm Res. 1995;50:275–286. doi: 10.1016/b978-0-12-571150-0.50016-0. [DOI] [PubMed] [Google Scholar]
- Budde LM, Ghosh S. Cloning and characterization of the gene encoding mouse IkappaBbeta. Gene. 2000;247:279–286. doi: 10.1016/s0378-1119(00)00051-2. [DOI] [PubMed] [Google Scholar]
- Buehr M. The primordial germ cells of mammals: some current perspectives. Exp Cell Res. 1997;232:194–207. doi: 10.1006/excr.1997.3508. [DOI] [PubMed] [Google Scholar]
- Capel B. Sex in the 90s: SRY and the switch to the male pathway. Annu Rev Physiol. 1998;60:497–523. doi: 10.1146/annurev.physiol.60.1.497. [DOI] [PubMed] [Google Scholar]
- Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- Cheng JD, Ryseck RP, Attar RM, Dambach D, Bravo R. Functional redundancy of the nuclear factor kappa B inhibitors I kappa B alpha and I kappa B beta. J Exp Med. 1998;188:1055–1062. doi: 10.1084/jem.188.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao PJ, Miyamoto S, Verma IM. Autoregulation of I kappa B alpha activity. Proc Natl Acad Sci USA. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor F, Cary PD, Read CM, Preston NS, Driscoll PC, Denny P, Crane-Robinson C, Ashworth A. DNA binding and bending properties of the post-meiotically expressed Sry-related protein Sox-5. Nucleic Acids Res. 1994;22:3339–3346. doi: 10.1093/nar/22.16.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor F, Wright E, Denny P, Koopman P, Ashworth A. The Sry-related HMG box-containing gene Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res. 1995;23:3365–3372. doi: 10.1093/nar/23.17.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martin R, Vanhove B, Cheng Q, Hofer E, Csizmadia V, Winkler H, Bach FH. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J. 1993;12:2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino F, Walker WH. Stage-specific nuclear expression of NF-kappaB in mammalian testis. Mol Endocrinol. 1998;12:1696–1707. doi: 10.1210/mend.12.11.0194. [DOI] [PubMed] [Google Scholar]
- Denny P, Swift S, Connor F, Ashworth A. An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J. 1992;11:3705–3712. doi: 10.1002/j.1460-2075.1992.tb05455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick C, Na SY, Voll RE, Zhong H, Im SY, Lee JW, Ghosh S. A subclass of Ras proteins that regulate the degradation of IkappaB. Science. 2000;287:869–873. doi: 10.1126/science.287.5454.869. [DOI] [PubMed] [Google Scholar]
- Foster JW, Brennan FE, Hampikian GK, Goodfellow PN, Sinclair AH, Lovell-Badge R, Selwood L, Renfree MB, Cooper DW, Graves JA. Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials. Nature. 1992;359:531–533. doi: 10.1038/359531a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Giese K, Cox J, Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992;69:185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Good L, Sun SC. Persistent activation of NF-kappa B/Rel by human T-cell leukemia virus type 1 tax involves degradation of I kappa B beta. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Harley VR, Lovell-Badge R, Goodfellow PN. Definition of a consensus DNA binding site for SRY. Nucleic Acids Res. 1994;22:1500–1501. doi: 10.1093/nar/22.8.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht NB. Molecular mechanisms of male germ cell differentiation. Bioessays. 1998;20:555–561. doi: 10.1002/(SICI)1521-1878(199807)20:7<555::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Eisenhauer K, Chun SY, Hsu SY, Billig H. Gonadal cell apoptosis. Recent Prog Horm Res. 1996;51:433–455. [PubMed] [Google Scholar]
- Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- Ivell R. ‘All that glisters is not gold’ – common testis gene transcripts are not always what they seem. Int J Androl. 1992;15:85–92. doi: 10.1111/j.1365-2605.1992.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- Jeske YW, Bowles J, Greenfield A, Koopman P. Expression of a linear Sry transcript in the mouse genital ridge. Nat Genet. 1995;10:480–2. doi: 10.1038/ng0895-480. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Cheah KS, Kondoh H. Mechanism of regulatory target selection by the SOX high-mobility-group domain proteins as revealed by comparison of SOX1/2/3 and SOX9. Mol Cell Biol. 1999;19:107–120. doi: 10.1128/mcb.19.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Sockanathan S, Liu Q, Breitman M, Lovell-Badge R, Kondoh H. Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 1995;14:3510–3519. doi: 10.1002/j.1460-2075.1995.tb07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development. 1998;125:2521–2532. doi: 10.1242/dev.125.13.2521. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Kanai-Azuma M, Noce T, Saido TC, Shiroishi T, Hayashi Y, Yazaki K. Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol. 1996;133:667–681. doi: 10.1083/jcb.133.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL, Tres LL. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed Proc. 1978;37:2512–2516. [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA, Stewart CL. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin [see comments] Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Enderich J, Hermans-Borgmeyer I, Wegner M. Cooperative function of POU proteins and SOX proteins in glial cells. J Biol Chem. 1998a;273:16050–16057. doi: 10.1074/jbc.273.26.16050. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998b;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lania L, Majello B, De Luca P. Transcriptional regulation by the Sp family proteins. Int J Biochem Cell Biol. 1997;29:1313–1323. doi: 10.1016/s1357-2725(97)00094-0. [DOI] [PubMed] [Google Scholar]
- Laudet V, Stehelin D, Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993;21:2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bail O, Schmidt-Ullrich R, Israel A. Promoter analysis of the gene encoding the I kappa B-alpha/MAD3 inhibitor of NF-kappa B: positive regulation by members of the rel/NF-kappa B family. EMBO J. 1993;12:5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140:852–858. doi: 10.1210/endo.140.2.6479. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Zhou G, Mukhopadhyay K, Smith CN, Zhang Z, Eberspaecher H, Zhou X, Sinha S, Maity SN, de Crombrugghe B. An 18-base-pair sequence in the mouse proalpha1(II) collagen gene is sufficient for expression in cartilage and binds nuclear proteins that are selectively expressed in chondrocytes. Mol Cell Biol. 1996;16:4512–4523. doi: 10.1128/mcb.16.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love JJ, Li X, Case DA, Giese K, Grosschedl R, Wright PE. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature. 1995;376:791–795. doi: 10.1038/376791a0. [DOI] [PubMed] [Google Scholar]
- Matsui Y. Developmental fates of the mouse germ cell line. Int J Dev Biol. 1998;42:1037–1042. [PubMed] [Google Scholar]
- McKinsey TA, Brockman JA, Scherer DC, Al-Murrani SW, Green PL, Ballard DW. Inactivation of IkappaBbeta by the tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-kappaB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki E, Nishina Y, Inazawa J, Copeland NG, Gilbert DJ, Jenkins NA, Ohsugi M, Tezuka T, Yoshida M, Semba K. Identification of a novel Sry-related gene and its germ cell-specific expression. Nucleic Acids Res. 1999;27:2503–2510. doi: 10.1093/nar/27.12.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RV, 2nd, Drolet DW, Kalla KA, Hooshmand F, Bermingham JR, Jr, Rosenfeld MG. Reduced fertility in mice deficient for the POU protein sperm-1. Proc Natl Acad Sci USA. 1997;94:7555–7560. doi: 10.1073/pnas.94.14.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7:338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- Prior HM, Walter MA. SOX genes: architects of development. Mol Med. 1996;2:405–412. [PMC free article] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read MA, Neish AS, Gerritsen ME, Collins T. Postinduction transcriptional repression of E-selectin and vascular cell adhesion molecule-1. J Immunol. 1996;157:3472–3479. [PubMed] [Google Scholar]
- Sassone-Corsi P. Transcriptional checkpoints determining the fate of male germ cells. Cell. 1997;88:163–166. doi: 10.1016/s0092-8674(00)81834-6. [DOI] [PubMed] [Google Scholar]
- Schafer M, Nayernia K, Engel W, Schafer U. Translational control in spermatogenesis. Dev Biol. 1995;172:344–352. doi: 10.1006/dbio.1995.8049. [DOI] [PubMed] [Google Scholar]
- Scholer HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989;8:2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sonenshein GE. Rel/NF-kappa B transcription factors and the control of apoptosis. Semin Cancer Biol. 1997;8:113–119. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- Stein B, Baldwin AS., Jr Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized I kappa B beta in persistent activation of NF-kappa B. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- Tran K, Merika M, Thanos D. Distinct functional properties of IkappaB alpha and IkappaB beta. Mol Cell Biol. 1997;17:5386–5399. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Clevers H. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. EMBO J. 1992;11:3039–3044. doi: 10.1002/j.1460-2075.1992.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco M, Diaz-Guerra MJ, Martin-Sanz P, Alvarez A, Bosca L. Rapid Up-regulation of IkappaBbeta and abrogation of NF-kappaB activity in peritoneal macrophages stimulated with lipopolysaccharide. J Biol Chem. 1997;272:23025–23030. doi: 10.1074/jbc.272.37.23025. [DOI] [PubMed] [Google Scholar]
- Wang RA, Nakane PK, Koji T. Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol Reprod. 1998;58:1250–1256. doi: 10.1095/biolreprod58.5.1250. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R, Laurent-Winter C, Israel A. Regulation of IkappaBbeta degradation. Similarities to and differences from IkappaBalpha. J Biol Chem. 1997;272:9942–9949. doi: 10.1074/jbc.272.15.9942. [DOI] [PubMed] [Google Scholar]
- Werner MH, Huth JR, Gronenborn AM, Clore GM. Molecular basis of human 46X,Y sex reversal revealed from the three-dimensional solution structure of the human SRY-DNA complex. Cell. 1995;81:705–714. doi: 10.1016/0092-8674(95)90532-4. [DOI] [PubMed] [Google Scholar]
- Whiteside ST, Israel A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- Zabel U, Henkel T, Silva MS, Baeuerle PA. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993;12:201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwingman T, Fujimoto H, Lai LW, Boyer T, Ao A, Stalvey JR, Blecher SR, Erickson RP. Transcription of circular and noncircular forms of Sry in mouse testes. Mol Reprod Dev. 1994;37:370–381. doi: 10.1002/mrd.1080370403. [DOI] [PubMed] [Google Scholar]