Abstract
Mutations in the mouse p (pink-eyed dilution) and human P genes lead to melanosomal defects and ocular developmental abnormalities. Despite the critical role played by the p gene product in controlling tyrosinase processing and melanosome biogenesis, its precise biological function is still not defined. We have expressed p heterologously in the yeast Saccharomyces cerevisiae to study its function in greater detail. Immunofluorescence studies revealed that p reaches the yeast vacuolar membrane via the prevacuolar compartment. Yeast cells expressing p exhibited increased sensitivity to a number of toxic compounds, including arsenicals. Similarly, cultured murine melanocytes expressing a functional p gene were also found to be more sensitive to arsenical compounds compared with p-null cell lines. Intracellular glutathione, known to play a role in detoxification of arsenicals, was diminished by 50% in p-expressing yeast. By using the glutathione-conjugating dye monochlorobimane, in combination with acivicin, an inhibitor of vacuolar gamma-glutamyl cysteine transpeptidase, involved in the breakdown of glutathione, we found that p facilitates the vacuolar accumulation of glutathione. Our data demonstrate that the pink-eyed dilution protein increases cellular sensitivity to arsenicals and other metalloids and can modulate intracellular glutathione metabolism.
INTRODUCTION
The product of the mammalian p gene is an integral membrane protein with a predicted 12-transmembrane domain structure, resembling a channel or transporter (Gardner et al., 1992; Rinchik et al., 1993; Rosemblat et al., 1994). Recessive mutations at the mouse p locus result in diminished coat color pigmentation and reduced ocular pigment deposition, which give rise to the pink-eyed dilution phenotype. Mutations in P, the human ortholog of p, cause oculocutaneous albinism type 2 (OCA2), the most common form of oculocutaneous albinism worldwide (Gardner et al., 1992; Rinchik et al., 1993; Durham-Pierre et al., 1994; Lee et al., 1994, 1995). Melanocytes from p-deficient mice or persons with OCA2 contain small, minimally pigmented melanosomes, indicating a function for p in melanosome biogenesis (Russell et al., 1995; Orlow and Brilliant, 1999). Recent data show that p plays a crucial role in the processing and trafficking of tyrosinase, the rate-limiting enzyme involved in pigmentation (Chen et al., 2002; Toyofuko et al., 2002).
Although mutations in p causes a reduction of eumelanin (black-brown pigment) and alter the morphology of black pigment granules (eumelanosomes), they have little effect on pheomelanin (yellow-red pigment) (Ito et al., 1984; Rinchik et al., 1993). The mechanisms governing the balance between pheomelanin and eumelanin are believed to be dependent upon l-cysteine, glutathione (GSH), and tyrosinase activity (Benedetto et al., 1981, 1982; Jara et al., 1988; del Marmol et al., 1993; Ito, 1993).
Despite the central role of p in pigmentation, its biological function remains unknown. Searching for conserved domains in the protein sequence of p by using BLAST algorithms revealed that p exhibits similarities to two protein families: Na+/sulfate symporters (accession number PF00939), members of which are transporters for sulfate, citrate, succinate, and dicarboxylate, and ArsB (accession number PF02040), members of which are arsenate translocating channels. ArsB is one of the two polypeptide components of the arsenite export pump (San Francisco et al., 1989). The second subunit, ArsA, functions as an oxyanion-stimulated ATPase (Rosen et al., 1988). Members of the ArsB family also include Na+/H+ symporters and numerous proteins with unknown function.
It has been suggested that p functions as a tyrosine transporter (Rosemblat et al., 1994; Gahl et al., 1995), a proton pump (Puri et al., 2000; Brilliant and Gardner, 2001) or that it is involved in thiol transport (Lamoreux et al., 1995). The p protein has also been proposed to play a significant role in stabilizing a melanosomal complex of tyrosinase and related proteins (Lamoreux et al., 1995; Manga et al., 2001).
Herein, we show that expression of p in the yeast Saccharomyces cerevisiae leads to a higher sensitivity to arsenical compounds, and certain other metalloids. Melanocytes cultured from mice expressing a functional p are also more sensitive to arsenical compounds than those lacking functional p. Glutathione is involved in the cellular detoxification of arsenicals and yeast expressing functional p have diminished glutathione content compared with yeast that do not. When expressed in yeast, mouse p protein localized to the vacuolar membrane and demonstrated the ability to transport or facilitate the transport of glutathione into the yeast vacuole. That transport triggers the degradation of glutathione initiated by gamma-glutamyl transpeptidase (γGT). Our results implicate p in melanocyte sensitivity to certain cytotoxic agents, and in the control of glutathione metabolism.
MATERIALS AND METHODS
Yeast p Expression Vectors
The open reading frame of mouse p was amplified by polymerase chain reaction (PCR) by using oligonucleotides that corresponded to the 5′ (5′-ATCGAGGATCCATGCGCCTAGAGAACAAAG-3′, BamHI site underlined) and 3′ (5′-CTCTAGATATCTTAATGGTGATGGTGATGATGATTCCATCCCACCACAAT-3′, EcoRV site underlined, HIS6 tag shown in italic). The PCR product was cut with BamHI and EcoRV and subcloned into the centromeric expression vector p413GPD (American Type Culture Collection, Manassas, VA), to generate p413GPD-p. The BamHI/ClaI fragment encompassing the p cDNA and His6 tag was subcloned into p416GPD expression plasmid (American Type Culture Collection) to yield p416GPD-p. Plasmids encoding the p gene with a 1-kb deletion resulting in p413GPD-pΔ and p416GPD-pΔ were created as follows: restriction of the p413GPD-p and p416GPD-p with HindIII, which include the region between nucleotides 625 and 1717 of p cDNA. All enzymes used in DNA manipulations were from Roche Applied Science (Indianapolis, IN).
Yeast Strains, Plasmids, and Media
The yeast strains used in these experiments are presented in Table 1. LS4 was obtained after ADE2 gene disruption. BJ3501 cells were transformed with pΔADE2 (American Type Culture Collection) and linearized with BamHI. Ura+ transformants were plated onto media containing 5-fluoroorotic acid (Boeke et al., 1984), and ade2 disruption was confirmed with PCR analysis. LS5 disruption in YCF1 gene was obtained after transformation with pJAW53, a gift from Scott Moye-Rowley (Szczypka et al., 1994) linearized with KpnI and MluII. The disruption was screened by sensitivity to 100 μM Cd as described previously (Szczypka et al., 1994). Ura+ transformants were plated onto 5-fluoroorotic acid-containing medium and Δ ycf1 colonies were confirmed with PCR analysis. Yeast strains were grown at 30°C on YEPD or selective SC medium (Bio 101, Vista, CA) with auxotrophic supplement for plasmid maintenance as described by Sherman et al. (1986).
Table 1.
Yeast strains
| Yeast strain | Genotype | Source |
|---|---|---|
| SF838-1D | MATa leu2 ura3 his4 ade6 pep4gal2 | Stevens, et al., 1986 |
| RPY12 | MATa vps45Δ leu2 ura3 his4 ade6 pep4gal2 | Piper, et al., 1994 |
| RPY1 | MATavps27Δ∷LEU2, ura3, his4, ade6, pep4gal2 | Piper, et al., 1995 |
| KHY31 | vph1∷LEU2, leu2, ura3, his4, ade6, pep4, gal2 | Stevens, T., personal stock |
| SH3866 | MAT ura3 leu2 trp1 his3 pho86∷LEU2 pho87∷URA3 | Harashima, S. personal stock |
| K601 | ade2, his3, leu2, trp1, ura,3can1 | Nass and Rao, 1998 |
| R100 | ade2, his3, leu2, trp1, ura,3can1, Δnhx1 | Nass and Rao, 1998 |
| BJ3501 | MATαpep4∷HIS3 prb1-Δ1.6R his3 ura3 gal2 can1 | Moehle, C., 1987 |
| LS4 | MATα ade2Δ∷hisG pep4∷HIS3 prb1-Δ1.6R his3 ura3 gal2 can1 Δycf1 | This study |
| LS5 | MATα ade2Δ∷hisG pep4∷HIS3 prb1-Δ1.6R his3 ura3 gal2 can1 | This study |
Yeast expression plasmids were as follows: pJAW49 (Wemmie et al., 1994), which contained YCF1 gene, was a gift from Scott Moye-Rowley (University of Iowa, Iowa City, IA); pMB192 (Bun-ya et al., 1996), which contained PHO87, was provided by Satoshi Harashima (Osaka University, Japan); pRin72 (Nass and Rao, 1998), which contained NHX1, was a gift from Rajini Rao (Johns Hopkins University, Baltimore, MD); and pYDJ95 (Dormer et al., 2000), which contained GSH1, was a gift from Derek Jamieson (University of Dundee, Dundee, United Kingdom).
Cell Lines and Media
Melan-a (P/P) is an immortalized melanocyte line derived from C57B/6J mice, wild-type at the p locus. Melan-p1 is an immortalized melanocyte line from mice lacking p gene transcripts due to overlapping deletions (pcp/p25h) (Sviderskaya et al., 1997). Melan-c is an immortalized melanocyte line homozygous for a point mutation (C85S) at the Tyr locus (Bennett et al., 1987). The melan-p1+p cell line is a clone of melan-p1 cells stably transfected with an expression plasmid encoding an epitope-tagged p protein (p-his-v5) as described previously (Manga et al., 2001). All cell lines were maintained in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) as described previously (Manga et al., 2001).
Immunological Methods
Yeast protein samples were denatured and subjected to electrophoretic separation by SDS-PAGE and electroblotted onto polyvinylidene fluoride membranes. Membranes were sequentially incubated with primary antibodies and peroxidase-conjugated anti-mouse IgG (Amersham Biosciences, Piscataway, NJ) and visualized using the enhanced chemiluminescence method. Mouse antiserum specific for His6(C-term) conjugated to fluorescein isothiocyanate was from Invitrogen (Carlsbad, CA). Antisera to Vph1p, Alpp, Vma2p, Vps10p, and Dpm1p were from Molecular Probes (Eugene, OR). Antisera against BiPp was a gift from Jeffrey Brodsky (University of Pittsburgh, Pittsburgh, PA). Rabbit antiserum against Pma1p was a gift from John Aris and mouse antiserum against Pep12p was a gift from Tom Stevens (University of Oregon, Eugene, OR).
Subcellular Fractionation
Yeast were grown in selective medium to A600 of 1 and then incubated in 1 ml of 50 mM Tris-HCl, pH 8, 1% 2-mercaptoethanol for 10 min at 30°C. Cells were converted to spheroplasts by treatment with 150 μg/ml Zymolase 100T (Seikagaku, Falmouth, MA) 1.2 M sorbitol, 50 mM KPO4, pH 7.5, at 30°C for 40 min. Spheroplasts were washed in 1.2 M sorbitol and lysed in 1 ml of cold 0.2 M sorbitol, 50 mM Tris-HCl, pH 7.5, 1 mM EDTA. Unbroken cells were removed by centrifugation for 5 min at 500 × g, yielding the whole cell extract. The extract was subjected to centrifugation at 13,000 × g for 10 min to yield a P13 fraction. The resulting supernatant was subjected to centrifugation at 100,000 × g for 30 min to yield the P100 fraction. The P13 and P100 fractions were resuspended in 5% SDS, 40 mM Tris-HCl, pH 6.8, 0.1% EDTA, 0.4% bromphenol blue, 10% 2-mercaptoethanol before SDS-PAGE.
Sucrose Gradient Fractionation
Yeast cells were grown in selective medium to an A600 of 1. Cells were collected by centrifugation (3000 × g for 10 min), suspended in medium containing 1 M sorbitol, 5 mM NaN3, 5 mM NaF supplemented with 0.3 mg/ml Zymolase 100T and incubated for 40 min at 30°C. The spheroplasts were collected at 500 × g for 10 min and lysed in hypoosmotic buffer, 50 mM Tris-HCl pH 7, 200 mM sorbitol, 1 mM EDTA contained freshly prepared protease inhibitors (Roche Applied Science) by using 15 strokes in a Dounce homogenizer. The cell lysate was centrifuged at 500 × g for 10 min and the supernatant was layered on top of an 12–60% discontinuous sucrose gradient and spun for 15 h at 100,000 × g in a SW60 rotor. Sucrose gradient solutions were made in 0.8 M sorbitol, 30 mM Tris-HCl, 1 mM EDTA that contained complete mixture of protease inhibitors. Fractions were collected from the top and centrifuged at 100,000 × g for 1 h to collect the membrane fraction.
Immunofluorescence Studies
Yeast were grown in selective medium to A600 of 1 and then diluted 1:1 in YPD and allow to grow for an additional 2 h. Cells were treated with cycloheximide to a final concentration of 100 μg/ml for 10 min before fixation and fixed in 3% formaldehyde for 10 min followed by incubation in 2% paraformaldehyde/50 mM KPO4, pH 7, for 18 h. Cells were spheroplasted for 40 min with 150 μg/ml Zymolase 100T and permeabilized with 1% SDS for 3 min. Cell were washed with 1.2 M sorbitol and allowed to adhere to poly-l-lysine–coated slides (Polysciences, Warrington, PA). Incubation of cells with primary antibody was performed overnight at 4°C followed by 1-h incubation of biotinylated secondary antibody in combination with Texas Red–labeled streptavidin (Molecular Probes) and 2-h incubation with aniHis6(C-term) antibody conjugated with fluorescein isothiocyanate. Images were captured with 100× objectives on a confocal inverted (LSM 510; Carl Zeiss, Thornwood, NY) laser scanning microscope, equipped with Nomarski optics.
Yeast Drug/Toxin Sensitivity Assay
A quantitative drug/toxin sensitivity assay was performed using an adaptation of the method of Egner et al. (1998). Various concentrations of compounds prepared in 55°C selective medium-agar were plated in duplicate into 24-well plates. Actively growing cultures of cells were diluted to A600 values of 0.1, 0.01, 0.001, and then 10-μl aliquots of each dilution were spotted in duplicate onto drug containing medium. Plates were incubated at 30°C for 3 d. A quantitative measure of each drug or toxin's effect was determined based upon the concentration of compound that inhibited colony formation by 50% (IC50 value).
Toxicity Screening in Cultured Melanocytes
Cells were trypsinized and resuspended in RPMI 1640 (Invitrogen) medium to a final concentration of 1.5 × 105. Then 100 μl of the suspension was added per well in a 96-well dish (adjusted such that cells would be ∼80% confluent after overnight culture). After 24 h, the medium was removed and replaced with fresh medium containing an appropriate concentration of the test compound. After a 72-h incubation, a cell viability assay was performed using the Promega Cell Titer 96 proliferation assay (Promega, Madison, WI). The kit allows for colorimetric quantitation of viable cells by using bioreduction of a tetrazolium compound. The growth or death rate was expressed as OD490 of cells in treated well/OD490 of cells in untreated well. The IC50 was estimated by linear regression analysis.
Measurement of Intracellular GSH
Glutathione was assessed in yeast strains and melanocytes by the 5,5′ dithiobis-(2-nitrobenzoic acid)-glutathione reductase-coupled assay (Anderson, 1985) (Oxford Biomedical Research, Westbury, NY). Cells were harvested at the desired time intervals, washed twice with water, resuspended in 0.4 ml of 5% sulfosalicylic acid, mixed with an equal volume of glass beads, and broken by vigorous beating at 4°C for a total of 6 min. The extracts were spun down in a microfuge to remove the glass beads, cell debris, and protein precipitate. The supernatant was assayed to determine the amount of glutathione. In some experiments, 0.5 mM acivicin (Sigma-Aldrich) was added to the cell culture and cells were cultured for an additional 24 h. Glutathione was then estimated as described above.
High-Performance Liquid Chromatography (HPLC) Analysis of Bimane-labeled Thiols
Cells were labeled with 100 μM monochlorobimane (Molecular Probes), washed with water, and ground with ice-cold 200 mM methanesulfonic acid in a chilled mortar. Cell extracts were centrifuged (12,000 × g, 10 min) and supernatants were analyzed by HPLC (Hichrom 5C18, 300/4.6 mm; Hichrom, Reading, United Kingdom) by using 0.25% (vol/vol acetic acid) (pH 3.9) as solvent A and methanol as solvent B. The elution protocol used a linear gradient from 92% A to 85% A in 10 min and a subsequent hold for further 20 min. The flow rate was kept constant at 1 ml min−1. Bimane derivates were detected fluorometrically with excitation at 395 nm and emission at 477 nm.
Fluorimetry
Excitation and emission spectra were measured using a fluorescence spectrometer (650–10S: PerkinElmer Life Sciences, Boston, MA). After labeling with 100 μM monochlorobimane (Molecular Probes) for 6 h, the cells were washed with phosphate-buffered saline, 100 μl of the suspension was added to 1 ml of phosphate-buffered saline, and the fluorescence intensity was measured with λ excitation = 395 nm and λ emission = 477 nm. The fluorescence intensities were adjusted by subtracting the background after measuring the fluorescence at zero time point after adding the dye. Calibration standards were made by serial dilution from a 10 mM glutathione-S-bimane stock in the presence of glutathione S-transferase (rabbit liver glutathione S-transferase; Sigma-Aldrich).
Assessment of Vacuolar Fluorescence
Yeast were grown in selective maintaining medium for 24 h at 30°C to an OD600 of 1.4, and 200-μl aliquots of the suspension were transferred to 5-ml volumes of fresh medium containing 100 μM monochlorobimane (Molecular Probes). After incubation for 6 h (as described above), the cells were pelleted by centrifugation, washed twice with SC-his medium lacking monochlorobimane, and viewed without fixation with a fluorescence microscope equipped with a BP-490 UV excitation filter and Nomarski optics attachment. The quantitation of fluorescence intensity was analyzed with Simple PCI image processing software. The average fluorescence intensity for the vacuole was measured from manually delimited regions of interest in >15 cells. The average background signal measured from a nearby region was subtracted from the average fluorescence value.
Acid Phosphatase Assay
Yeast were cultured in selective synthetic high-phosphate medium (supplemented with K2HPO4-KH2PO4 at a concentration of 3000 mg/l) to an OD600 of 0.8. The cells were harvested and suspended in a solution containing 4% ethanol and 1% toluene and incubated at room temperature for 10 min (Toh-e and Oshima, 1974). After washing with water, the cell suspension was used for the assay. Acid phosphatase was measured directly by a spectrophotometric assay, by using 2.25 mg of p-nitrophenylphosphate in 0.5 ml of 0.1 M sodium acetate, pH 4.3. The reaction was run at 37°C for 10 min and stopped by the sequential addition of 0.12 ml of 25% (wt/vol) trichloroacetic acid and 0.6 ml of saturated sodium carbonate. Cells were removed by centrifugation, and the absorbance of the supernatant was measured at 420 nm.
Detection of Vacuole Acidification by Quinacrine Fluorescence
Yeast were grown in selective medium to an OD600 of 0.8. The cells were cooled on ice for 5 min, and 1 ml of the cells was sedimented by centrifugation and resuspended in 100 μl of medium containing 100 mM HEPES, pH 7.6, and 200 μM freshly prepared quinacrine (Sigma-Aldrich). The suspension was incubated for 5 min at 30°C and cooled on ice for 15 min. After centrifugation, the cells were resuspended in 1 ml of 100 mM HEPES, pH 7.6, 2% glucose, and washed twice and resuspended in 0.1 ml of the same buffer. Then 4 μl of the cell suspension was mixed on the microscope slide with 4 μl of 0.5% low-melting agarose and a glass coverslip was applied. The accumulation of quinacrine in the vacuole was followed by fluorescence microscopy with excitation at 423 nm and emission through a filter of 503-nm maximal transmission.
Quantitation of Red Pigment
Red pigment formation in yeast was assessed by examination of the spectra of extracts made in 5% sulfosalicylic acid. An equal amount of cells grown in complete minimal medium containing limiting adenine were harvested at OD600 of 3.0, washed with water, and broken in a bead-beater with glass beads as described previously (Chaudhuri et al., 1997). To determine the relative concentrations of the pigment, absorbances at the peak value (530 nm) were taken.
RESULTS
Expression and Localization of p in Yeast Cells
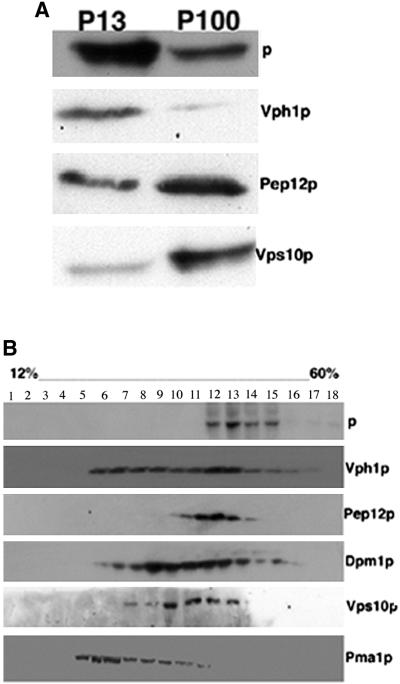
His6-tagged mouse p was cloned into a low copy yeast expression vector, and its expression was examined by immunoblot analysis. A single band of 110 kDa, corresponding to the molecular mass reported for the mammalian protein, was observed (our unpublished data). To assess the localization of p, we performed subcellular fractionation studies (Figure 1A). The majority of p appeared in the P13 fraction, which contains markers for the vacuole, endoplasmic reticulum (ER), and plasma membrane (Marcusson et al., 1994). We found lower levels of p in the P100 fraction, which is highly enriched for membranes of the Golgi and endosomes (Vida et al., 1993). To further define the distribution of p we also performed sucrose gradient fractionation studies. As shown in Figure 1B, upon sucrose gradient fractionation, p cosediments with markers for Golgi (Vps10p), the prevacuolar compartment (PVC) (Pep12p), and the vacuole (Vph1p), but not with those for the plasma membrane (Pma1p) and ER (Dpm1p). When these studies are taken together, the results are most consistent with a vacuolar localization for p. Colocalization of p with vacuolar markers was confirmed by immunofluorescence. Wild-type strain yeast was transformed with a plasmid encoding epitope-tagged p and double labeled with antibodies against the His6 epitope, and markers for different cellular compartments. We observed colocalization of p with Vph1p (Figure 2A) and two other different vacuolar proteins, Alpp and Vma2p, whereas no colocalization of p was observed with BiPp, a resident ER protein in yeast (our unpublished data). However, we do not exclude the possibility that a portion of p protein also localizes to Golgi-derived and PVC endosome vesicles.
Figure 1.
Subcellular localization of p in yeast. (A) Wild-type (SF838-1D) yeast transformed with p were lysed and subjected to differential centrifugation to yield low-speed pellet (P13) and high-speed pellet (P100) as described in MATERIALS AND METHODS. (B) Wild-type (SF838-1D) yeast transformed with p were lysed, homogenized, and layered on the top of a discontinuous 12–60% (wt/vol) sucrose gradient. Equal portions of gradient fractions were subjected to SDS-PAGE and immunoblotted using anti-His3 antibodies or antibodies specific for markers of the plasma membrane (Pma1p), Golgi (Vps10p), endoplasmic reticulum (Dpm1p), vacuole (Vph1p), and prevacuolar compartment (Pep12p).
Figure 2.
Immunolocalization of His6-tagged p protein in wild-type and ΔVps27 yeast. Wild-type (SF838-1D) and ΔVps27 strain (RPY1) yeast were fixed, labeled with antibodies, and viewed under a confocal microscope equipped with Nomarski optics as described in MATERIALS AND METHODS. (A) Colocalization of Vph1p (red) and p-His3p (green) in the wild-type cells. (B) Colocalization of Pep12p (red) and p-His3p (green) in a Δ Vps27 strain.
To gain insight into the trafficking of p in yeast we examined the immunofluorescence staining of p in strains disrupted in genes involved in the secretory and endocytic pathways. Strain RPY1 is disrupted in VPS27, a member of a class of genes (class E) in which mutations cause proteins that follow the secretory biosynthetic pathway to be trapped in an enlarged PVC (Piper et al., 1995). Strain RPY12 is disrupted in VPS45, in which mutations lead to accumulation of Golgi-derived transport vesicles (Piper et al., 1994). We observed a punctate, dispersed pattern for p in RPY 12 resembling the distribution of the Golgi apparatus staining (our unpublished data). The staining pattern for p in strain RPY1 resembled that of the prevacuolar compartment. A prevacuolar localization for p in RPY1 was confirmed by additional immunofluorescence studies, which revealed colocalization of p with Pep12p, a resident protein of the PVC (Figure 2B). These data suggest that p traverses the pathway from the Golgi to the vacuole that passes through the PVC.
Expression of p Confers Sensitivity to Arsenical Compounds in Yeast
Based on the sequence homology of p to bacterial arsenite transporters as revealed by BLAST search algorithms, we sought to determine whether the expression of p would confer altered resistance or sensitivity to arsenite. By use of a colony formation assay, we found that the expression of p in a wild-type yeast strain conferred increased sensitivity not only to arsenite but also to other arsenical compounds, including arsenate and arsenic trioxide. No difference in sensitivity was seen with steroid hormones (progesterone), antibiotics (cyhloheximide and erythromycin), cations (lithium, copper, and iron), anions (fluoride), hydrophilic charged nucleobases (fluorouracil and 5-fluorouridine), selenite, or a cysteine analog (vinylglycine). In contrast, cells transformed with empty plasmid did not exhibit any differential sensitivity to the arsenical compounds compared with control cells. Sensitivity to selenate and resistance to another cysteine analog, allylglycine, was also observed in p-expressing cells. As a further control, we used a p construct carrying a 1-kb in-frame deletion (pΔ), encoding a protein lacking four of the predicted 12 transmembrane domains of the full-length protein. Cells expressing p were more sensitive to the same compounds compared with pΔ-expressing cells. No significant difference in sensitivity of the cells transformed with the empty plasmid compared with those expressing pΔ was observed with any of the different compounds tested. The data demonstrate that the sensitivity of p-expressing cells to the arsenical compounds is a specific function of p expression and is not due to the expression of heterologous membrane protein.
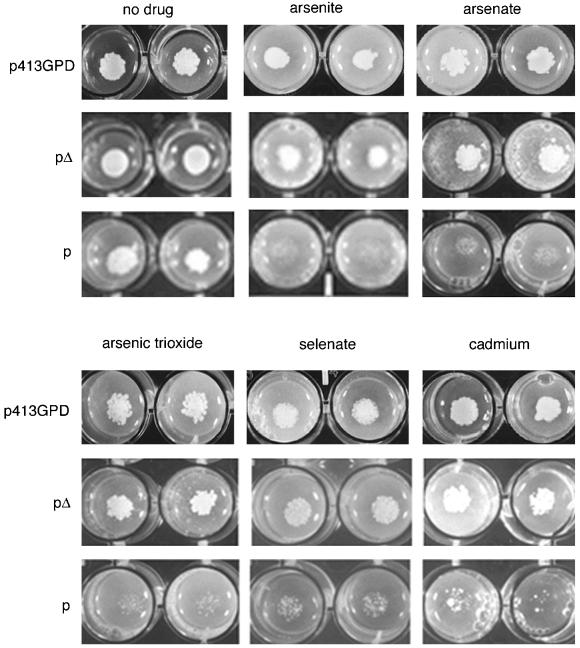
To construct an even more sensitive assay for p protein we used a strain disrupted in PHO87 and PHO86 genes. The yeast PHO87 gene encodes a low-affinity phosphate transporter (Bun-ya et al., 1996) and PHO86 encodes an ER-resident protein that facilitates the exit of the plasma membrane transporter Pho84p from the ER (Lau et al., 2000). The combined Δpho87Δpho87 mutation leads to increased resistance of yeast to arsenate (Bun-ya et al., 1996). We capitalized upon this effect, using a double mutant Δpho87Δpho86 strain (SH3866) as a sensitive drug sensitivity assay. Strain SH3866 yeast cells expressing p were more sensitive to arsenical compounds than cells expressing the mutant pΔ plasmid or the empty plasmid as shown in Figure 3. The concentration of a number of arsenical compounds required to inhibit colony formation by 50% (IC50) determined for pΔ and p-expressing Δpho87Δpho86 yeast are presented in Table 2.
Figure 3.
Quantitation of drug/toxin sensitivity of p-expressing yeast by plating assay. A quantitative estimation of the drug/toxin resistance or sensitivity was determined by measuring the colony formation of 103 diluted cultures of strain Δpho87Δpho86 (SH3866) containing p, pΔ, or the empty vector (p413GPD) as a control. Cells were aliquoted onto selective media that contained either drug carrier (Me2SO) or increasing concentrations of compound in Me2SO. Shown are examples with 3 mM sodium arsenite, 0.2 mM sodium arsenate, 0.2 mM arsenic trioxide, 60 mM cadmium, and 30 mM selenate.
Table 2.
Quantitation of p-mediated hypersensitivity to metalloids
| Drug | IC50 in pΔ-expressing cells (mM) | IC50 in p-expressing cells (mM) |
|---|---|---|
| Arsenite | 5 ± 0.5 | 3 ± 0.2 |
| Arsenate | 1.2 ± 0.1 | 0.2 ± 0.02 |
| Arsenic trioxide | 1 ± 0.08 | 0.2 ± 0.01 |
Δpho86Δpho87 (SH3866) yeast transformed with p or pΔ plasmid as a control were subjected to the quantitative plating assay using graded concentrations of each compound. The IC50 for colony formation is indicated. The experiment was repeated 5 times.
Melanocytes Expressing p Are More Sensitive to Arsenical Compounds
We confirmed these results in cultured melanocytes. We found that p-expressing melan-a (wild-type), melan-c (tyrosinase negative-albino), and melan-p1+p (p-null cells stably transformed with epitope-tagged p) melanocytes were all more sensitive to arsenical compounds compared with (p-null) melan-p melanocytes. Thus, our results show that both yeast cells and melanocytes expressing p exhibit a specific sensitivity to arsenical compounds compared with cells that lack p expression.
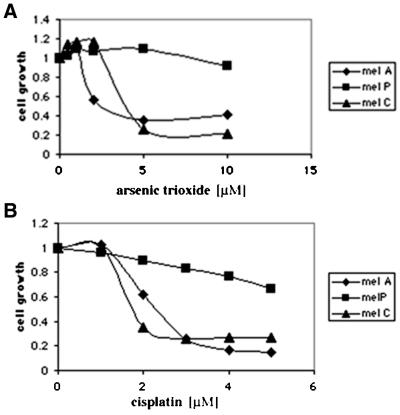
Because glutathione plays a key role in the detoxification of arsenical compounds in yeast (Ghosh et al., 1999; Tamas and Wysocki, 2001) we hypothesized that p-expressing cells might be more sensitive to other cytotoxic agents that regulate GSH for their detoxification. We found that cadmium and antimony, also detoxified by GSH in yeast, are also more toxic to p-expressing cells. We also tested the sensitivity of cultured melanocytes to cisplatin, antimony, and doxorubicin, three cytotoxic agents known to be detoxified in mammalian cells as glutathione-conjugates (Rinchik et al., 1993; Awasthi et al., 1994; Commandeur et al., 1995). As shown in Figure 4, A and B, p-expressing melan-a cells are more sensitive to these drugs compared with p-null melan-p melanocytes. The concentration of a number of arsenical compounds required to inhibit colony formation by 50% (IC50) determined for p-null melanocytes (melan-p) and wild-type cells expressing p (melan-a) are shown in Table 3.
Figure 4.
Melanocytes expressing p are more sensitive to arsenic trioxide and cisplatin. Melanocytes were treated with arsenic trioxide (A) and cisplatin (B) as described in MATERIALS AND METHODS. Melan-c and melan-a cells, both of which express p, are more sensitive to cytotoxic agents than are melan-p cells, which lack p. The experiment was repeated five times with similar results. The data shown are from representative experiment.
Table 3.
Quantitation of p-mediated arsenical hypersensitivity in melanocytes
| Drug | IC50 melan-a (μM) | IC50 melan-p (μM) | IC50 melan-c (μM) | IC50 melan-pl+P (μM) |
|---|---|---|---|---|
| Arsenite | 36 ± 3 | 69 ± 6 | 20 ± 2 | 25 ± 2 |
| Arsenate | 42 ± 3.6 | 131 ± 10 | 48 ± 4 | 53 ± 4 |
| Arsenic trioxide | 7 ± 0.5 | 19 ± 1 | 3 ± 1.5 | 8 ± 0.5 |
Shown are IC50 values for melanocytes treated with increased concentrations of arsenical compounds as described in Materials and Methods. The experiment was performed 5 times.
Expression of p Does Not Complement Δpho87
Because the expression of p confers increased sensitivity to arsenical compounds in a Δpho87Δpho86 strain and because p shares some weak homology to Pho87p (E value of 5.1) we asked whether p could complement other phenotypic features of the lack of PHO87. Mutations in PHO87 typically do not have a phenotype unless they are combined with mutations in PHO86 (Bun-ya et al., 1996). In addition to increased resistance to arsenate, Δpho87Δpho86 strains express constitutively repressible acid phosphatase when grown in high-phosphate medium. We found that yeast disrupted in both PHO87 and PHO86 genes, and transformed with p or pΔ showed the same elevated levels of acid phosphatase (our unpublished data). In contrast, the replacement of the absent PHO87 by transformation with a PHO87 plasmid resulted in a low (repressed) level of acid phosphatase activity in high-phosphate medium. In other experiments when a wild-type yeast strain was transformed with p or pΔ, the acid-phosphatase activity was also low (our unpublished data). The data demonstrate that expression of p fails to complement phenotypic features other than arsenate resistance in a double mutant Δpho87Δpho86 strain.
Depletion of Intracellular Glutathione by p Is a Function of Vacuolar Transport and Degradation
The increased sensitivity of p-expressing cells to compounds detoxified by glutathione suggested the possibility of depletion or sequestration of glutathione in p-expressing cells. The results in Tables 4 and 5 show that yeast cells expressing p have 50% less GSH compared with cells expressing a pΔ plasmid. To address the question of how the expression of p leads to GSH depletion, we assessed the effect of drugs known to modulate GSH metabolism. Acivicin has been shown to γ-GT, the enzyme involved in the initial breakdown of GSH in the yeast vacuole (Chittur et al., 2001; Mehdi et al., 2001). Addition of 0.5 mM acivicin to p-expressing yeast cells completely reversed the depletion of GSH (Table 4). These data suggested that p may transport or facilitate the transport of GSH into the yeast vacuole where it is subsequently degraded and that acivicin can block this degradation. We observed that control cells treated with acivicin also exhibit higher levels of glutathione compared with control cells untreated with acivicin (Table 4). Because degradation of glutathione by γGT in the vacuole has been proposed to be activated by Ycf1p, a vacuolar glutathione transporter (Mehdi et al., 2001), we reasoned that a Δycf1 strain treated with acivicin would have the same glutathione level as the same strain in the absence of acivicin treatment. As expected, acivicin treatment of Δycf1 cells expressing pΔ did not result in an additional increase in the glutathione level. Expression of p in the Δycf1 yeast diminished the level of glutathione to 50% compared with the same strain expressing pΔ (Table 5). Treatment of p-expressing Δycf1 cells with acivicin, on the other hand, fully reversed the p-induced decrease in the glutathione content. The results confirm that p, localized to the yeast vacuolar membrane, facilitates glutathione transport into the vacuole where it is degraded via a Ycf1-independent mechanism.
Table 4.
Comparison of intracellular glutathione levels of wild-type yeast transformed with p and pΔ
| Transformed yeast cells | Glutathione nmol/107 cells | Relative glutathione content (%) |
|---|---|---|
| pΔ | 2.9 ± 0.25 | 70 |
| p | 1.3 ± 0.21 | 30 |
| pΔ+ 0.5 mM acivicin | 4.2 ± 0.9 | 100 |
| p+ 0.5 mM acivicin | 4.4 ± 1.2 | 105 |
Shown are the glutathione levels in wild type strain SF838 transformed with p and pΔ, treated and untreated, with 0.5 mM acivicin. Glutathione was determined as described in MATERIALS AND METHODS. 100% value was 4.2 nmol GSH/107 cells.
Table 5.
Comparison of intracellular glutathione levels of Δycf1 yeast transformed with p and pΔ
| Transformed yeast cells | Glutathione nmol/107 cells | Relative glutathione content (%) |
|---|---|---|
| pΔ | 4.9 ± 0.80 | 100 |
| p | 2.5 ± 0.31 | 50 |
| pΔ+ 0.5 mM acivicin | 4.9 ± 0.1 | 100 |
| p+ 0.5 mM acivicin | 4.9 ± 1.2 | 100 |
Shown are the glutathione levels in Δycf1 (LS5) cells transformed with p and pΔ, treated and untreated with 0.5 mM acivicin. Glutathione was determined as described in MATERIALS AND METHODS. 100% value was 4.9 nmol GSH/107 cells.
To confirm our observation that p specifically affected glutathione, we also coexpressed Gsh1p, the rate-limiting enzyme in glutathione biosynthesis, together with p in yeast. Coexpression of Gsh1p reversed completely the sensitivity of p-expressing cells to arsenical compounds (our unpublished data). We have shown previously that in mammalian cells p is localized to the ER, and thus GSH transported by p would not be subject to γGT-induced degradation. In fact, no significant difference was observed in glutathione levels between melan-a (42.2 ± 4 nmol/106 cells) and melan-p cells (49.9 ± 4 nmol/106).
Decreased Red Pigment Formation in p-expressing Yeast
Genes involved in glutathione metabolism play an important role in red pigment formation in yeast. Mutations in gsh1+ in Schizosaccharomyces pombe have been shown to completely abolish red pigmentation (Chaudhuri et al., 1997). Disruption in the second gene in glutathione biosynthesis, gsh2+, leads to a partial defect in pigment formation. We reasoned that since the expression of p leads to diminished glutathione level in yeast, p might affect the formation of red pigment. Lysates from cells disrupted in ADE2 gene (in which red pigment formation occurs) and transformed with p and pΔ were measured spectrometrically for red pigment formation. We found that expression of p caused a 30% decrease in red pigmentation in the Δade2 strain. Interestingly, when the cells were additionally disrupted in the YCF1 gene, known to transport pigment precursors conjugated to glutathione into the vacuole (Chaudhuri et al., 1997), p-expressing cells show an additional 40% decrease in accumulation of red pigment compared with control cells (also shown as a plate assay; Figure 5). The results confirm the ability of p to modulate glutathione metabolism.
Figure 5.
Red pigment formation in a ΔYcf1 strain (LS5) transformed with pΔ and p. Cells transformed with pΔ and p were plated on minimal selective medium having limiting amounts of adenine (10 mg/l) and allowed to grow for 5–6 d as described in MATERIALS AND METHODS.
Diminished Accumulation of Bimane-labeled Glutathione in p-expressing Cells
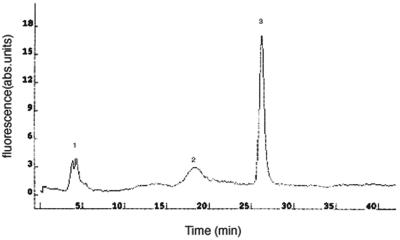
We took an additional complementary approach to measure the GSH levels in p-expressing cells, by using monochlorobimane (MCB), a membrane-permeant nonfluorescent compound, that is specifically conjugated to GSH by intracellular glutathione S-transferase to generate the intensely fluorescent, membrane-impermeant monochlorobimane S-conjugate (Shrieve et al., 1988; Zadzinski et al., 1996). To determine the specificity of the dye for glutathione, we separated the labeled thiols in the yeast extracts by HPLC. In vivo labeling with 100 μM MCB for 6 h resulted in a single major peak (GSH) and two minor peaks (cysteine and γ-glutamylcysteine) (Figure 6).
Figure 6.
HPLC profile of low-molecular-weight thiols. Cells were labeled with 100 μM MCB as described in MATERIALS AND METHODS and the bimane-labeled thiols were then separated by HPLC. Peak 1, cysteine S-bimane; 2, γ-glutamylcysteine S-bimane; and 3, glutathione S-bimane.
We measured the fluorescence intensities of yeast transformed with p and pΔ and labeled with MCB. To quantify the amount of GSH-S-bimane, the fluorescence was calibrated against a series of solutions with known concentrations of GSH-S-bimane. The results shown in Table 6 confirmed that p-expressing cells have diminished amounts of glutathione.
Table 6.
Fluorimetric determination of glutathione content
| Yeast strain transformed with | GSH nmol/107 cells | Relative GSH content (%) |
|---|---|---|
| pΔ | 3.25 ± 0.3 | 65 |
| p | 1.75 ± 0.2 | 35 |
| pΔ + acivicin | 5 ± 0.5 | 100 |
| p + acivicin | 5 ± 0.4 | 100 |
The wild type cells (SF838) were labeled with MCB as described in MATERIALS AND METHODS. The fluorescence was measured and converted to glutathione levels using a standard curve.
Increased Vacuolar Glutathione Transport in p-expressing Yeast
We further investigated the mechanism by which p expression could lead to depletion of cellular GSH in yeast cells. We took advantage of the observation that yeast cells disrupted in the vacuolar glutathione transporter Ycf1p do not accumulate MCB in their vacuole (Li et al., 1996). We examined the accumulation of MCB in a Δycf1 strain. Yeast transformed with either the pΔ plasmid (Figures 6B and 7) or p (Figure 7, C and D) fail to exhibit vacuolar accumulation of MCB. However, when cells were preincubated with 0.5 mM acivicin to inhibit vacuolar GSH breakdown, p-expressing Δycf1 cells exhibited an intense fluorescence, corresponding to the vacuole (Figure 7, G and H), compared with the absence of fluorescence in Δycf1 cells expressing the control pΔ plasmid (Figure 7, E and F). In contrast, Δycf1 cells transformed with YCF1 as a positive control exhibited robust accumulation of MCB in the vacuole (our unpublished data). The quantitation of the vacuolar fluorescence intensity signals of cells labeled with MCB glutathione is presented in Table 7. These data confirm the observations that p facilitates the transport of GSH into the yeast vacuole and that this transport triggers γGT-dependent degradation.
Figure 7.
Expression of p facilitates vacuolar accumulation of monochlorobimane. Δycf1 yeast (LS4) transformed with pΔ (A, B, E, and F) and p (C, D, G, and H) were grown in SC-ura for 24 h at 30°C. The cells were diluted to an OD600 of 0.01, and 0.5 mM acivicin was added to the strain transformed with pΔ (E and F) and p (G and H), and all the cells were allowed to grow for additional 24 h. Aliquots (200 μl) of the cell suspension were then transferred into 5 ml of fresh medium containing 100 μM monochlorobimane. After incubation for 6 h, the cells were washed and examined in fluorescence (A, C, E, and G) or Nomarski mode (B, D, F, and H).
Table 7.
Quantitative evaluation of vacuolar fluorescence values for GSH-S-bimane
| ΔYcfl transformed with | Mean fluorescence arbitrary intensity units |
|---|---|
| pΔ | 130 ± 10 |
| p | 144 ± 8 |
| pΔ + 0.5 mM acivicin | 134 ± 9 |
| p + 0.5 mM acivicin | 200 ± 18 |
Δycf1 (LS5) yeast were transformed with p or pΔ as a control. The cells were labeled with MCB and visualized as described in MATERIALS AND METHODS. The quantitation of the vacuolar fluorescence intensity was measured with Simple PCI image software (>15 cells for each experimental group).
Vacuolar pH Is Not Altered by p in Yeast Cells
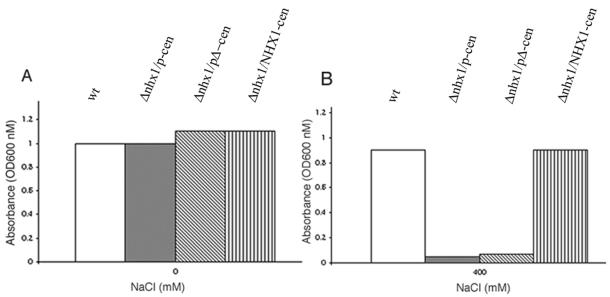
A role for p in regulating intramelanosomal pH has been suggested (Puri et al., 2000; Brilliant and Gardner, 2001; Halaban et al., 2002). The vacuolar localization of p protein in yeast allowed us to determine whether expression of p could alter the vacuolar pH. Cells transformed with p and pΔ as a control were examined for fluorescence after treatment with quinacrine, a fluorescent dye that accumulates in acidic compartments (Weisman et al., 1987). As shown in Figure 8, we observed equally intense fluorescence in p- and pΔ-expressing cells exposed to quinacrine. No vacuolar accumulation of quinacrine was observed under either condition in Δvph1 strain in keeping with previously reported inability of Δvph1 yeast to accumulate quinacrine in their vacuoles (Preston et al., 1989). We also sought to determine whether p could complement defects in yeast disrupted in NHX1, encoding a Na+/H+ transporter involved in the regulation of intracellular pH (Nass et al., 1997). Yeast cells disrupted at NHX1 are highly sensitive to increased levels of NaCl (Nass and Rao, 1998). We transformed Δnhx1 yeast with p or pΔ and examined for the ability to grow at 400 mM NaCl at pH 4. No growth at 400 mM NaCl was in either group. In contrast, transformation with a control plasmid encoding NHX1 resulted in tolerance to 400 mM NaCl (Figure 9). Thus, under conditions where p can be demonstrated to alter vacuolar GSH content, no effect of p on the pH of the vacuolar or prevacuolar compartments could be observed.
Figure 8.
Accumulation of quinacrine. Yeast cells were labeled with quinacrine and viewed by confocal microscopy as described in MATERIALS AND METHODS. Shown are wild-type strain (SF838-1D) transformed with pΔ (A and B) and p (C and D) and strain Δvph1 (E and F), which fails to accumulate quinacrine to its vacuole.
Figure 9.
Evaluation of the sodium tolerance of Δnhx1 cells transformed with p. Δnhx1 yeast (R100) transformed with pΔ and p or NHX1::HA were grown to saturation at 30°C in the absence (A) or presence of 400 mM NaCl (B) in pH 4-buffered medium as described previously (Nass and Rao, 1998).
DISCUSSION
The pink-eyed dilution locus (p) plays a central role in controlling mammalian pigmentation and encodes a 12-transmembrane domain protein lacking significant homology to any other vertebrate proteins. We took advantage of the ease of manipulation of S. cerevisiae as a tool for studying the function of p protein in greater detail.
In view of the resemblance of the 12-transmembrane structure of p to that of a transporter or channel (Gardner et al., 1992; Rinchik et al., 1993; Rosemblat et al., 1994) and in light of the homology of p to the ArsB family of bacterial transporters, we assessed the response of p-expressing cells to arsenical compounds. We found unexpectedly that expression of p conferred increased sensitivity to arsenical compounds. Pentavalent arsenate in yeast is reduced to trivalent arsenite by glutathione (Mukhopadhyay et al., 2000). The arsenite is subsequently detoxified by two mechanisms: transport out of the cell by Acr3p, an arsenite permease (Wysocki et al., 1997) and by conjugation with GSH and transport into the vacuole by Ycf1p, a 12-transmembrane pump (Szczypka et al., 1994; Ghosh et al., 1999). The vacuolar localization of heterologously expressed p suggested to us that if p transported arsenite into the vacuole it should confer a resistant phenotype, yet we observed an exaggerated arsenite-sensitive phenotype. A possible explanation for the sensitive phenotype conferred by p expression was the sequestration or depletion of intracellular glutathione, needed for conjugation with arsenite as a prelude to its detoxification. That possibility was further supported by finding increased sensitivity of both p-expressing yeast cells and melanocytes to other compounds known to require GSH for detoxification. In addition, we found that p-expressing yeast cells exhibit greatly diminished levels of intracellular GSH compared with control cells confirmed by 5,5′ dithiobis-(2-nitrobenzoic acid)-glutathione coupled assay and fluorometry after labeling with monochlorobimane. The involvement of p in glutathione metabolism was further confirmed by the observation that Gsh1p, the rate-limiting enzyme in glutathione biosynthesis, completely reverses the arsenical sensitivity conferred by p expression in yeast.
We also found that p-expressing yeast cells and melanocytes are more sensitive to selenate, but not to selenite. We attribute this difference to the different mechanisms of action of these two metalloids in causing cell toxicity. Because selenate is a structural analog of sulfate, its toxic effect is believed to be due to its incorporation into analogs of sulfur-containing compounds such as cysteine, methionine, and glutathione (Reuveny, 1977). It is possible that p could differentially influence the consumption of glutathione in different cellular pools and that could result in increased cell sensitivity to selenate. On the other hand, selenite toxicity is associated with oxidative stress, which causes DNA damage in the form of single-strand breaks, chromosome breaks, and spindle disturbances in mammalian cells (Sinha et al., 1996) and in yeast (Pinson et al., 2000). Murine B16 melanoma cells are more sensitive to selenite but not to selenate (Siwek et al., 1994). It has been suggested that p is not transcribed in melanoma cells (Gardner et al., 1992), again consistent with the hypothesis that increased sensitivity of p-expressing melanocytes to selenate could be due to a function of p in glutathione transport. The reported increased sensitivity to selenite in melanoma cells compared with the lack of a difference in sensitivity in melanocytes could be due to the differential response to oxidative damage in both cells.
To gain a better understanding of how the expression of p could result in the depletion of intracellular GSH, we treated yeast cells with acivicin, a drug that blocks the enzyme γGT known to initiate the breakdown of GSH in the yeast vacuole (Chittur et al., 2001; Mehdi et al., 2001). After treatment of yeast with acivicin for 24 h, the GSH levels in p-expressing cells and in the control cells were the same. These data are consistent with a model in which p facilitates GSH sequestration from the cytoplasm to the vacuole, where the glutathione is subsequently degraded (Figure 10). In mammalian cells, p is localized to the ER, and we ascribe the vacuolar localization of p when expressed in protease-deficient yeast cells to the well-established default pathway for heterologous membrane proteins in yeast (Conibear and Stevens, 2002; Murray et al., 2002). In light of this difference in distribution, it is perhaps not at all surprising that we did not observe any significant difference in total glutathione levels in wild-type melan-a cells and p-null melanocytes. The degradation of glutathione in yeast is triggered by vacuolar accumulation; there is no evidence that such a system exists in the mammalian ER. In melanocytes, p could alter the sequestration of glutathione between different subcellular pools, resulting in altered detoxification of, and increased sensitivity to arsenicals, cisplatin, and doxorubicin.
Figure 10.
Model for effect of p on GSH metabolism in yeast. Glutathione normally enters the yeast vacuole through Ycf1p in the form of glutathione conjugates or as free glutathione. The expression of p triggers additional transport of glutathione into the vacuole. In the vacuole, the glutathione is degraded by γGT to l-Glu and the dipeptide l-Cys-Gly, resulting in an overall dimension in GSH levels in the yeast.
In agreement with the observation that p modulates glutathione metabolism, we observed a decrease in red pigment formation in Δade2 cells as well as in cells additionally disrupted in the YCF1 gene, involved in the transport of pigment-conjugated glutathione to the vacuole (Chaudhuri et al., 1997). These results suggest that glutathione plays other roles in red pigment formation in yeast in addition to that of transport of pigment precursors as GSH conjugates into the vacuole.
In confirmation of the assumption that p could be involved in glutathione transport, we performed fluorescence studies with the dye monochlorobimane (Shrieve et al., 1988). The results in Figure 7 revealed that p-expressing Δycf1 cells, which otherwise fail to accumulate this dye in their vacuole, exhibit intense vacuolar fluorescence, but only if they are pretreated with acivicin to prevent intravacuolar GSH degradation. Together, these data suggest that p alters intracellular GSH metabolism in yeast by transporting GSH into the vacuole where as a secondary phenomenon it is subsequently degraded in a γGT-dependent manner. We cannot exclude the possibility that p may transport GSH-conjugates, even although the expression of p in the Δycf1 strain failed to complement the arsenic- and cadmium-sensitive phenotype of that strain, as had previously been achieved with the mammalian drug transporter MRP1, another 12-transmembrane domain protein (Tommasini et al., 1996). A Δycf1 strain transformed with either p or pΔ exhibited the same growth in the presence of cadmium, arsenite, and arsenate compared with cells transformed with YCF1 (our unpublished data).
Thiol compounds have been suspected to play a significant role in melanin pigmentation, but the exact mechanisms have remained unknown (Benedetto et al., 1981, 1982; Jara et al., 1988; del Marmol et al., 1993; Ito, 1993). Pheomelanin is synthesized from cysteinyl-DOPA building blocks, and the p gene is not transcribed during the pheomelanic phase of the murine agouti hair cycle (Tamate et al., 1989; Rinchik et al., 1993). Low levels of reduced GSH have been found to be associated with eumelanin production (Benedetto et al., 1981, 1982). Tyrosinase activity is also important in the eumelanin/pheomelanin switch: high tyrosinase activity had been associated with eumelanogenesis and lower tyrosinase activity with pheomelanogenesis (Ito, 1993; Lamoreux et al., 1995). Glutathione has been reported to have two effects on tyrosinase activity: first, a direct one by inhibition of tyrosinase activity via the reduction of copper atoms at the active site of the enzyme; and second, indirectly via interference with melanin formation by redox mechanisms (Jara et al., 1988). The effect of p on the transport or intracellular sequestration of GSH directly or indirectly might alter intraorganellar levels of GSH in such a way as to increase the formation of eumelanin.
Roles for p as an anion transporter, in the control of melanosomal pH, or in regulating the pH of other intracellular compartments have been suggested (Puri et al., 2000; Ancans et al., 2001; Brilliant and Gardner, 2001; Halaban et al., 2002). We have shown previously that inhibitors of vacuolar-type ATPases such as bafilomycin A1 and concanamycin, the ionophore monensin, and ammonium chloride all restore pigmentation in p-null cells (Manga and Orlow, 2001). In the current studies, we did not observe an effect of p-expression upon the pH of the yeast vacuole. However, we note that involvement of p in intracellular GSH metabolism might alter intracellular pH indirectly by redox mechanisms or directly by regulating the activity of V-ATPases as has been shown previously (Oluwatosin and Kane, 1997). On the other hand, evidence exists to suggest that certain V-ATPases can themselves transport glutathione, because it has been shown that bafilomycin A1 decreases the transport of GSH in isolated yeast vacuoles (Mehdi et al., 2001).
Glutathione is also known to be required for the folding of cysteine-rich proteins (Cuozzo and Kaiser, 1999). Tyrosinase passage through the ER is much slower than for the related protein Tyrp1, suggesting that tyrosinase ER folding and processing are highly regulated (Branza-Nichita et al., 1999). Tyrosinase as well as the other members of the tyrosinase-related protein family have 15 cysteine residues in well-conserved positions along the polypeptide chain (Hearing and King, 1993; Spritz and Hearing, 1994; King et al., 1995) and may be especially vulnerable to oxidative damage. Data exist that folding and maturation of tyrosinase-related protein-1 are also regulated by formation of disulfide bonds (Negroiu et al., 2000). Because glutathione is a major redox buffer in the secretory pathway (Hwang et al., 1992; Frand et al., 2000) it may play an important role in the proper folding and processing of tyrosinase. Recently, we have shown that abnormal ER processing of tyrosinase, followed by aberrant trafficking and release of the tyrosinase into the medium, occurs in the absence of p (Chen et al., 2002). Together with our recent observations that in melanocytes the majority of p is found in the ER (Chen et al., 2002), the current data suggest a possible role for p in regulating the folding of tyrosinase via control of glutathione.
Finally, our results have implications for chemotherapeutic approaches to melanoma. Melanoma cells are recognized to be resistant to many chemotherapeutic agents, including those known to be detoxified by glutathione-dependent mechanisms. We have found that resistance to chemotherapeutic agents such as arsenic trioxide (Dai et al., 1999; Yang et al., 1999; Zhang et al., 2001) cisplatin (Eton et al., 2002; Gibbs et al., 2002), and doxorubicin (Cho et al., 2001) is modulated by p expression. Agents that mimic p function in the cell might well increase the sensitivity of melanoma to useful chemotherapeutic agents.
ACKNOWLEDGMENTS
We thank Sandra Lemmon for generously providing strains RPY1 and RPY12; Scott Moye-Rowley for plasmids pJAW49 and pJAW53; Satoshi Harashima for the strains SH3866 and SH3412 and the plasmid pMB192; Rajini Rao for the strains R100 and K601 and the plasmid pRin72; Tom Stevens for the antisera against Pep12p and strain KHY31, John Aris for the antisera against Pma1p; Derek Jamieson for the plasmid pYDJ95; and Jeffrey Brodsky for antisera against BiPp. We thank Elizabeth Conibear for helpful advice in the fluorescence studies. We acknowledge the experienced contribution to the experimental work of Sharon Pifko-Hirst and Bao Kang Zhou. We also thank Bradley States for help with Simple PCI software and Leonard Liebs' group for performing HPLC analysis. This work was supported in part by funding from the Anaderm Research Corporation and by Public Health Service grant AR41880.
Abbreviations used:
- ER endoplasmic reticulum
GSH, glutathione
- γGT
γ-glutamyl transpeptidase
- MCB
monochlorobimane
- OCA2
oculocutaneous albinism type 2
- PVC
prevacuolar compartment
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0282. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0282.
REFERENCES
- Ancans J, Hoogduijn MJ, Thody AJ. Melanosomal pH, pink locus protein and their roles in melanogenesis. J Invest Dermatol. 2001;117:158–159. doi: 10.1046/j.0022-202x.2001.01397.x. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Srivastava SK, Zimniak P, Bajpai KK, Saxena M, Sharma R, Ziller SA, 3rd, Frenkel EP, Singh SV. Adenosine triphosphate-dependent transport of doxorubicin, daunomycin, and vinblastine in human tissues by a mechanism distinct from the P-glycoprotein. J Clin Invest. 1994;93:958–965. doi: 10.1172/JCI117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto JP, Ortonne JP, Voulot C, Khatchadourian C, Prota G, Thivolet J. Role of thiol compounds in mammalian melanin pigmentation: Part I. Reduced and oxidized glutathione. J Invest Dermatol. 1981;77:402–405. doi: 10.1111/1523-1747.ep12494592. [DOI] [PubMed] [Google Scholar]
- Benedetto JP, Ortonne JP, Voulot C, Khatchadourian C, Prota G, Thivolet J. Role of thiol compounds in mammalian melanin pigmentation. II. Glutathione and related enzymatic activities. J Invest Dermatol. 1982;79:422–424. doi: 10.1111/1523-1747.ep12530631. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumor promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Bittinger F, Gonzalez-Garcia JL, Klein CL, Brochhausen C, Offner F, Kirkpatrick CJ. Production of superoxide by human malignant melanoma cells. Melanoma Res. 1998;8:381–387. doi: 10.1097/00008390-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Branza-Nichita N, Petrescu AJ, Dwek RA, Wormald MR, Platt FM, Petrescu SM. Tyrosinase folding and copper loading in vivo: a crucial role for calnexin and alpha-glucosidase II. Biochem Biophys Res Commun. 1999;261:720–725. doi: 10.1006/bbrc.1999.1030. [DOI] [PubMed] [Google Scholar]
- Brilliant M, Gardner J. Melanosomal pH, pink locus protein and their roles in melanogenesis. J Invest Dermatol. 2001;117:386–387. doi: 10.1046/j.0022-202x.2001.01462.x. [DOI] [PubMed] [Google Scholar]
- Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr Genet. 1996;29:344–351. [PubMed] [Google Scholar]
- Chaudhuri B, Ingavale S, Bachhawat AK. apd1+, a gene required for red pigment formation in ade6 mutants of Schizosaccharomyces pombe, encodes an enzyme required for glutathione biosynthesis: a role for glutathione and a glutathione-conjugate pump. Genetics. 1997;145:75–83. doi: 10.1093/genetics/145.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. The pink eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittur SV, Klem TJ, Shafer CM, Davisson VJ. Mechanism for acivicin inactivation of triad glutamine aminotransferases. Biochemistry. 2001;40:876–887. doi: 10.1021/bi0014047. [DOI] [PubMed] [Google Scholar]
- Cho JE, Kim HS, Ahn WS, Park YS. Enhanced cytotoxicity of doxorubicin encapsulated in liposomes with reconstituted Sendai F-proteins. J Microencapsul. 2001;18:421–431. doi: 10.1080/02652040010019550. [DOI] [PubMed] [Google Scholar]
- Commandeur JN, Stijntjes GJ, Vermeulen NP. Enzymes and transport systems involved in the formation and disposition of glutathione S-conjugates. Role in bioactivation and detoxication mechanisms of xenobiotics. Pharmacol Rev. 1995;47:271–330. [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Studying yeast vacuole. Methods Enzymol. 2002;351:408–432. doi: 10.1016/s0076-6879(02)51861-9. [DOI] [PubMed] [Google Scholar]
- Cuozzo JW, Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol. 1999;1:130–135. doi: 10.1038/11047. [DOI] [PubMed] [Google Scholar]
- Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- del Marmol V, Solano F, Sels A, Huez G, Libert A, Lejeune F, Ghanem G. Glutathione depletion increases tyrosinase activity in human melanoma cells. J Invest Dermatol. 1993;101:871–874. doi: 10.1111/1523-1747.ep12371709. [DOI] [PubMed] [Google Scholar]
- Dormer UH, Westwater J, McLaren NF, Kent NA, Mellor J, Jamieson DJ. Cadmium-inducible expression of the yeast GSH1 gene requires a functional sulfur-amino acid regulatory network. J Biol Chem. 2000;275:32611–3266. doi: 10.1074/jbc.M004167200. [DOI] [PubMed] [Google Scholar]
- Durham-Pierre D, Gardner JM, Nakatsu Y, King RA, Francke U, Ching A, Aquaron R, del Marmol V, Brilliant MH. African origin of an intragenic deletion of the human P gene in tyrosinase positive oculocutaneous albinism. Nat Genet. 1994;7:176–179. doi: 10.1038/ng0694-176. [DOI] [PubMed] [Google Scholar]
- Egner R, Rosenthal FE, Kralli A, Sanglard D, Kuchler K. Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol Biol Cell. 1998;9:523–543. doi: 10.1091/mbc.9.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eton O, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol. 2002;20:2045–2052. doi: 10.1200/JCO.2002.07.044. [DOI] [PubMed] [Google Scholar]
- Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000;10:203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- Gahl WA, Potterf B, Durham-Pierre D, Brilliant MH, Hearing VJ. Melanosomal tyrosine transport in normal and pink-eyed dilution murine melanocytes. Pigment Cell Res. 1995;8:229–233. doi: 10.1111/j.1600-0749.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Nakatsu Y, Gondo Y, Lee S, Lyon MF, King RA, Brilliant MH. The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science. 1992;257:1121–1124. doi: 10.1126/science.257.5073.1121. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Shen J, Rosen BP. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P, Anderson C, Pearlman N, LaClaire S, Becker M, Gatlin K, O'Driscoll M, Stephens J, Gonzalez R. A phase II study of neoadjuvant biochemotherapy for stage III melanoma. Cancer. 2002;94:470–476. doi: 10.1002/cncr.10186. [DOI] [PubMed] [Google Scholar]
- Halaban R, Patton RS, Cheng E, Svedine S, Trombetta ES, Wahl ML, Ariyan S, Hebert DN. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem. 2002;25:1–25. doi: 10.1074/jbc.M111497200. [DOI] [PubMed] [Google Scholar]
- Hearing VJ, King RA. In: In: Pigmentation and Pigmentary Abnormalities. Levine N, editor. Boca Raton, FL: CRC Press; 1993. pp. 3–32. [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Ito S. High-performance liquid chromatography (HPLC) analysis of eu- and pheomelanin in melanogenesis control. J Invest Dermatol. 1993;100:166S–171S. [PubMed] [Google Scholar]
- Ito S, Fujita K, Takahashi H, Jimbow K. Characterization of melanogenesis in mouse and guinea pig hair by chemical analysis of melanins and of free and bound dopa and 5-S-cysteinyldopa. J Invest Dermatol. 1984;83:12–14. doi: 10.1111/1523-1747.ep12261634. [DOI] [PubMed] [Google Scholar]
- Jara JR, Aroca P, Solano F, Martinez JH, Lozano JA. The role of sulfhydryl compounds in mammalian melanogenesis: the effect of cysteine and glutathione upon tyrosinase and the intermediates of the pathway. Biochim Biophys Acta. 1988;967:296–303. doi: 10.1016/0304-4165(88)90023-2. [DOI] [PubMed] [Google Scholar]
- King RA, Oettting WS, Hearing VJ. In: In: Metabolic and Molecular Bases of Inherited Disease. Scriver SR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw-Hill Book Company; 1995. pp. 4353–4392. [Google Scholar]
- Lamoreux ML, Zhou BK, Rosemblat S, Orlow SJ. The pink eyed-dilution protein and the eumelanin/pheomelanin switch: in support of a unifying hypothesis. Pigment Cell Res. 1995;8:263–270. doi: 10.1111/j.1600-0749.1995.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Lau WT, Howson RW, Malkus P, Schekman R, O'Shea EK. Pho86p, an endoplasmic reticulum (ER) resident protein in Saccharomyces cerevisiae, is required for ER exit of the high-affinity phosphate transporter Pho84p. Proc Natl Acad Sci USA. 2000;97:1107–1012. doi: 10.1073/pnas.97.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994;330:529–534. doi: 10.1056/NEJM199402243300803. [DOI] [PubMed] [Google Scholar]
- Lee ST, Nicholls RD, Jong MT, Fukai K, Spritz RA. Organization and sequence of the human P gene and identification of a new family of transport proteins. Genomics. 1995;26:354–363. doi: 10.1016/0888-7543(95)80220-g. [DOI] [PubMed] [Google Scholar]
- Li ZS, Szczypka M, Lu YP, Thiele DJ, Rea PA. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem. 1996;271:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- Manga P, Boissy RE, Pifko-Hirst S, Zhou BK, Orlow SJ. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2, Exp. Eye Res. 2001;72:695–710. doi: 10.1006/exer.2001.1006. [DOI] [PubMed] [Google Scholar]
- Manga P, Orlow SJ. Inverse correlation between pink-eyed dilution protein expression and induction of melanogenesis by bafilomycin A1. Pigment Cell Res. 2001;14:362–367. doi: 10.1034/j.1600-0749.2001.140508.x. [DOI] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Mehdi K, Thierie J, Penninckx MJ. γ-Glutamyl transpeptidase in the yeast Saccharomyces cerevisiae, and its role in the vacuolar transport, and metabolism of glutathione. Biochem J. 2001;359:631–637. doi: 10.1042/0264-6021:3590631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle CM, Tizard R, Lemmon SK, Smart J, Jones EW. Protease B of the lysosome-like vacuole of the yeast Saccharomyces cerevisiae is homologuous to the subtilisin family of serine proteases. Moll Cell Biol. 1987;7:4390–4399. doi: 10.1128/mcb.7.12.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Shi J, Rosen BP. Purification and characterization of ACR2p, the Saccharomyces cerevisiae arsenate reductase. J Biol Chem. 2000;275:21149–21157. doi: 10.1074/jbc.M910401199. [DOI] [PubMed] [Google Scholar]
- Murray BP, Zgoda VG, Correia MA. Native CYP2C11. Heterologous expression in Saccharomyces cerevisiae reveals a role for vacuolar proteases rather than the proteasome system in the degradation of this endoplasmic reticulum protein. Mol Pharmacol. 2002;61:1146–1153. doi: 10.1124/mol.61.5.1146. [DOI] [PubMed] [Google Scholar]
- Nass R, Cunningham KW, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- Nass R, Rao R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J Biol Chem. 1998;273:21054–1060. doi: 10.1074/jbc.273.33.21054. [DOI] [PubMed] [Google Scholar]
- Negroiu G, Dwek RA, Petrescu SM. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J Biol Chem. 2000;275:32200–32207. doi: 10.1074/jbc.M005186200. [DOI] [PubMed] [Google Scholar]
- Oluwatosin YE, Kane PM. Mutations in the CYS4 gene provide evidence for regulation of the yeast vacuolar H+-ATPase by oxidation and reduction in vivo. J Biol Chem. 1997;272:28149–28157. doi: 10.1074/jbc.272.44.28149. [DOI] [PubMed] [Google Scholar]
- Orlow SJ, Brilliant MH. The pink-eyed dilution locus controls the biogenesis of melanosomes and levels of melanosomal proteins in the eye. Exp Eye Res. 1999;68:147–154. doi: 10.1006/exer.1998.0599. [DOI] [PubMed] [Google Scholar]
- Pinson B, Sagot I, Daignan-Fornier B. Identification of genes affecting selenite toxicity and resistance in Saccharomyces cerevisiae. Mol Microbiol. 2000;36:679–687. doi: 10.1046/j.1365-2958.2000.01890.x. [DOI] [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Preston RA, Murphy RF, Jones EW. Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc Natl Acad Sci USA. 1989;86:7027–7031. doi: 10.1073/pnas.86.18.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N, Gardner JM, Brilliant MH. Aberrant pH of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol. 2000;115:607–613. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Reuveny Z. Derepression of ATP sulfurylase by the sulfate analogs molybdate and selenate in cultured tobacco cells. Proc Natl Acad Sci USA. 1977;74:619–622. doi: 10.1073/pnas.74.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik EM, Bultman SJ, Horsthemke B, Lee ST, Strunk KM, Spritz RA, Avidano KM, Jong MT, Nicholls RD. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993;361:72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Rosemblat S, Durham-Pierre D, Gardner JM, Nakatsu Y, Brilliant MH, Orlow SJ. Identification of a melanosomal membrane protein encoded by the pink-eyed dilution (type II oculocutaneous albinism) gene. Proc Natl Acad Sci USA. 1994;91:12071–12075. doi: 10.1073/pnas.91.25.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BP, Weigel U, Karkaria C, Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite (antimonate)-stimulated ATPase. J Biol Chem. 1988;263:3067–3070. [PubMed] [Google Scholar]
- Russell LB, Montgomery CS, Cacheiro NL, Johnson DK. Complementation analyses for 45 mutations encompassing the pink-eyed dilution (p) locus of the mouse. Genetics. 1995;141:1547–1562. doi: 10.1093/genetics/141.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco MJ, Tisa LS, Rosen BP. Identification of the membrane component of the anion pump encoded by the arsenical resistance operon of R-factor R773. Microbiology. 1989;3:15–21. doi: 10.1111/j.1365-2958.1989.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks GB. Laboratory Course Manual for Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- Shrieve DC, Bump EA, Rice GC. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988;263:14107–14114. [PubMed] [Google Scholar]
- Sinha R, Said TK, Medina D. Organic and inorganic selenium compounds inhibit mouse mammary cell growth in vitro by different cellular pathways. Cancer Lett. 1996;107:277–284. doi: 10.1016/0304-3835(96)04373-x. [DOI] [PubMed] [Google Scholar]
- Siwek B, Bahbouth E, Serra MA, Sabbioni E, de Pauw-Gillet MC, Bassleer R. Effect of selenium compounds on murine B16 melanoma cells and pigmented cloned pB16 cells. Arch Toxicol. 1994;68:246–254. doi: 10.1007/s002040050064. [DOI] [PubMed] [Google Scholar]
- Spritz RA, Hearing VJ. Genetic disorders of pigmentation. In: Hirschhorn K, Harris H, editors. Advances in Human Genetics. New York: Plenum Press; 1994. pp. 1–45. [DOI] [PubMed] [Google Scholar]
- Stevens TH, Rothman JH, Payne GS, Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986;102:1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskaya EV, Bennett DC, Ho L, Bailin T, Lee ST, Spritz RA. Complementation of hypopigmentation in p-mutant (pink-eyed dilution) mouse melanocytes by normal human P cDNA, and defective complementation by OCA2 mutant sequences. J Invest Dermatol. 1997;108:30–34. doi: 10.1111/1523-1747.ep12285621. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem. 1994;269:22853–22857. [PubMed] [Google Scholar]
- Tamas MJ, Wysocki R. Mechanisms involved in metalloid transport and tolerance acquisition. Curr Genet. 2001;40:2–12. doi: 10.1007/s002940100234. [DOI] [PubMed] [Google Scholar]
- Tamate HB, Hirobe T, Wakamatsu K, Ito S, Shibahara S, Ishikawa K. Levels of tyrosinase and its mRNA in coat-color mutants of C57BL/10J congenic mice: effects of genic substitution at the agouti, brown, albino, dilute, and pink-eyed dilution loci. J Exp Zool. 1989;250:304–311. doi: 10.1002/jez.1402500310. [DOI] [PubMed] [Google Scholar]
- Toh-e A, Oshima Y. Characterization of a dominant, constitutive mutation, PHOO, for the repressible acid phosphatase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1974;120:608–617. doi: 10.1128/jb.120.2.608-617.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R, Evers R, Vogt E, Mornet C, Zaman GJ, Schinkel AH, Borst P, Martinoia E. The human multidrug resistance-associated protein functionally complements the yeast cadmium resistance factor 1. Proc Natl Acad Sci USA. 1996;93:6743–6748. doi: 10.1073/pnas.93.13.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuko K, Valencia JC, Kushimoto T, Costin G, Virado VM, Viera WD, Ferras VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA2) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res. 2002;15:1–10. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Vida TA, Huyer G, Emr SD. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J Cell Biol. 1993;6:1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman LS, Bacallao R, Wickner W. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J Cell Biol. 1987;105:1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Szczypka MS, Thiele DJ, Moye-Rowley WS. Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J Biol Chem. 1994;269:32592–32597. [PubMed] [Google Scholar]
- Wysocki R, Bobrowicz P, Ulaszewski S. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J Biol Chem. 1997;272:30061–30066. doi: 10.1074/jbc.272.48.30061. [DOI] [PubMed] [Google Scholar]
- Yang CH, Kuo ML, Chen JC, Chen YC. Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer. 1999;81:796–799. doi: 10.1038/sj.bjc.6690766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadzinski R, Maszewski J, Bartosz G. Transport of Glutathione S-Conjugates in the Yeast Saccharomyces cerevisiae. Cell Biol Int. 1996;20:325–330. doi: 10.1006/cbir.1996.0035. [DOI] [PubMed] [Google Scholar]
- Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20:7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]