Abstract
Much of the lower urinary tract, including the bladder, is lined by a stratified urothelium forming a highly differentiated, superficial umbrella cell layer. The apical plasma membrane as well as abundant cytoplasmic fusiform vesicles of the umbrella cells is covered by two-dimensional crystals that are formed by four membrane proteins named uroplakins (UPs) Ia, Ib, II, and III. UPs are synthesized on membrane-bound polysomes, and after several co- and posttranslational modifications they assemble into planar crystals in a post-Golgi vesicular compartment. Distension of the bladder may cause fusiform vesicles to fuse with the apical plasma membrane. We have investigated the early stages of uroplakin assembly by expressing the four uroplakins in 293T cells. Transfection experiments showed that, when expressed individually, only UPIb can exit from the endoplasmic reticulum (ER) and move to the plasma membrane, whereas UPII and UPIII reach the plasma membrane only when they form heterodimeric complexes with UPIa and UPIb, respectively. Heterodimer formation in the ER was confirmed by pulse-chase experiment followed by coimmunoprecipitation. Our results indicate that the initial building blocks for the assembly of crystalline uroplakin plaques are heterodimeric uroplakin complexes that form in the ER.
INTRODUCTION
Although much has been learned about the structure and function of plasma membrane proteins, their assembly and the mechanisms of their targeted intracellular transport are less well understood. Most plasma membrane proteins, including important cell surface receptors, ion channels, and components of cell junctions function as multisubunit complexes (Klausner et al., 1990; Berridge, 1993; Macdonald and Olsen, 1994; Goodenough et al., 1996; Berridge, 1997; Davies et al., 1997; Trimmer, 1998). In all of these cases, folding and oligomerization of the subunits start in the endoplasmic reticulum and are monitored by an intricate quality control system, thus preventing the expression of incomplete or abnormal multisubunit complexes at the cell surface (Hurtley and Helenius, 1989; Doms et al., 1993; Hammond and Helenius, 1995; Nagaya and Papazian, 1997; George et al., 1999).
The epithelium of the urinary bladder, also known as urothelium, provides a unique system to study the processes that ultimately result in the assembly of four membrane proteins (uroplakins) into a planar crystalline array, that has the appearance of an asymmetric unit membrane (AUM) (Porter and Bonneville, 1963; Porter et al., 1967; Hicks, 1975; Kachar et al., 1999). The AUM structure is also observed in the numerous cytoplasmic fusiform vesicles of the superficial urothelial cells, called umbrella cells (Alroy and Weinstein, 1980; Lin et al., 1994). It has been suggested that during bladder distension some of these AUM-containing vesicles fuse with the luminal membrane, thus contributing to an increase in apical surface area (Porter and Bonneville, 1963; Porter et al., 1967; Severs and Hicks, 1979; Truschel et al., 2002). A major advantage of this membrane as a model system for studying membrane assembly is that AUM crystals can be purified in large quantities, thus facilitating biochemical and cross-linking experiments that complemented the biosynthetic and assembly studies presented herein (Wu et al., 1995). Furthermore, structural analysis of the AUM was greatly facilitated by the fact that it naturally forms two-dimensional crystals (Vergara et al., 1969; Warren and Hicks, 1970; Brisson and Wade, 1983; Taylor and Robertson, 1984; Walz et al., 1995; Kachar et al., 1999; Min et al., 2002). A better understanding of the interactions among the uroplakin subunits will help us unravel principles involved in uroplakin assembly and eventually understand the detailed biological functions of the AUM.
Four major protein subunits of the AUM have been identified and they have been named uroplakin Ia, Ib, II, and III (Wu and Sun, 1993; Lin et al., 1994; Yu et al., 1994). They can be divided into two structurally related groups. UPIa and UPIb are 39% identical in their amino acid sequences (Yu et al., 1994). Both contain four transmembrane domains (TMDs) and belong to the family of “tetraspanins” that include several leukocyte differentiation markers such as CD9, CD37, CD53, and CD63 (Hemler, 2001). The other group is represented by the two type I transmembrane proteins UPII and UPIII (Wu and Sun, 1993; Lin et al., 1994). UPII is synthesized as a precursor protein that contains, aside from the 100-amino acid mature sequence (15 kDa), a cleavable signal peptide of ∼26 amino acids and a prosequence of ∼59 residues with three potential N-glycosylation sites (Lin et al., 1994). Mature UPIII also has a single transmembrane domain and has three N-linked complex sugars attached to the extracellular domain (Wu and Sun, 1993). The nearest neighbor relationships among the uroplakin subunits have been studied by chemical cross-linking. These studies demonstrated that UPIa and UPIb could be cross-linked to UPII and UPIII, respectively (Wu et al., 1995). Furthermore, separation of uroplakin subunits by ion-exchange chromatography demonstrated the existence of UPIa/UPII and UPIb/UPIII complexes (Liang et al., 2001). Recent UPIII knockout experiments provided additional evidence for the formation of these two uroplakin pairs (Hu et al., 2000).
We demonstrate herein that the formation of UPIa/UPII or UPIb/UPIII pairs is a prerequisite for their exit from the endoplasmic reticulum (ER) compartment and that UPIb has the unique property of being able to exit from the ER compartment by itself. UPIb is also the only uroplakin known to exist in the absence of other known uroplakins in some nonurothelial tissues such as corneal epithelium (Adachi et al., 2000). These results indicate that the uroplakin pairs are indeed structurally meaningful, because they seem to play a role in the early stages of AUM assembly. Further investigations of these assembly processes should help us understand how the crystalline uroplakin arrays are formed during terminal stages of urothelial differentiation. The intracellular assembly of UP subunits may also serve as a paradigm for the oligomerization of other multimeric plasma membrane proteins.
MATERIALS AND METHODS
cDNA Constructs
Bovine urothelial cDNAs were used as the template for polymerase chain reaction (PCR). For the amplification of UP cDNAs, additional EcoRI and XhoI restriction sites were added to the sense and antisense primers, respectively. The following primers were used: UPIa: sense, 5′-CCC GAA TTC ACC ATG GCT TCT GCA GCA GCA GCA-3′; antisense, 5′-CCC CTC GAG TCA CAA CGT GGT GTA GAA ATA-3′; UPIb: sense, 5′-CCC GAA TTC ACC ATG GCC AAA GAC GAC TCC ACT-3′; antisense, 5′-CCC CTC GAG TTA ATA GTC AAT TCT GCT CCA-3′; UPII: sense, 5′-CCC GAA TTC ACC ATG GCA TCT CCG TGG CCT GTG TGG-3′; antisense, 5′-CCC CTC GAG TCA CTT TCG GGC GCC CAG TGC TAG-3′; and UPIII: sense, 5′-CCC GAA TTC ACC ATG CCT CCG CTC TGG GTA GTG-3′; antisense, 5′-CCC CTC GAG TCA GTC CTG GAG CTT GCT GGC GTA-3′. PCR was carried out in a mixture containing the cDNA template, 0.2 mM dNTP, 50 pM primers, and PWO polymerase (Roche Applied Science, Indianapolis, IN), in 10 mM Tris-HCl, pH 8.85, 25 mM KCl, 5 mM (NH4)2SO4, and 2 mM MgSO4. Reaction condition were as follows: initial cycle at 94°C for 5 min followed by 30 cycles at 94°C for 1 min, 56°C for 1.5 min, and 72°C for 1 min, and a final cycle at 72°C for 10 min. The PCR products were cloned into the pcDNA3 vector (Invitrogen, Carlsbad, CA) by using the EcoRI and XhoI sites, yielding plasmids UPIa-pcDNA3, UPIb-pcDNA3, UPII-pcDNA3, and UPIII-pcDNA3. The UPII-HA-pcDNA3 was constructed similarly except that an oligonucleotide encoding the hemagglutinin (HA) tag (underlined below) was inserted after the 3′ end of the UPII sequence, by using the UPII sense primer listed above and the following antisense primer: 5′-CCC CTC GAG CTA AGC GTA GTC TGG GAC GTC GTA TGG GTA CAA CGT GGT GTA GAA ATA CAT-3′. To generate UP constructs with HA tag at its N terminus, we used UPII-pcDNA3 as the template. A PCR was carried out using the UPII sense primer and the following antisense primer, which contains the HA sequence (underlined): 5′-AGC GTA GTC TGG GAC GTC GTA TGG GTA GCT CAC CAG CTC CCT GCG-3′. Another PCR was performed with the sense primer containing the HA sequence (underlined): 5′-TAC CCA TAC GAC GTC CCA GAC TAC GCT GTG GTG GAC AGC GGG TCT-3′ and UPII antisense primer. Extension of these two PCR products, which overlap in the HA sequence, was obtained by three cycles each at 94°C for 1 min, 37°C for 10 min, and 72°C for 10 min. The fragment obtained corresponded in size to the sum of the two initial products. Reamplification of this fragment with UPII sense and antisense primers resulted in a secondary PCR product, HA-UPII, encoding UPII tagged with HA at the +5 position (the N-terminal amino acid of mature UPII protein was designated as +1). The secondary PCR product was cloned into pcDNA3 by using the EcoRI and XhoI sites. This construct was named HA-UPII-pcDNA3. The DNA sequence encoding the myc tag (underlined) was integrated into the UPIII cDNA immediately after the signal peptide using the same strategy and the resulting construct was named myc-UPIII-pcDNA3. The primers used were as follows: sense, 5′-GAA CAA AAA CTT ATT TCT GAA GAA GAT CTG GTG AAC CTC CAG CCC CAA CTG-3′; and antisense, 5′-CAG ATC TTC TTC AGA AAT AAG TTT TTG TTC ACC GGA GCC AAG TCG-3′. A construct containing a cDNA encoding galactosyltransferase fused to yellow fluorescent protein (GalT-YFP) was a gift from Dr. Jennifer Lippincott-Schwartz (Ward et al., 2001). RI-GFP, a construct with ribophorin I cDNA inserted into pEGFPN3, was a gift from Dr. Anderi Nikonov from our laboratory.
Antibodies
The two His-tagged fusion proteins, one containing the large loop of UPIa (aa 113–232) and the other containing the large loop of UPIb (aa 108–232), were expressed in the E. coli strain DH5α by using the pPROEX HTb prokaryotic expression system (Invitrogen, Carlsbad, CA). The expressed proteins were affinity-purified using the TALON metal affinity resin (CLONTECH, Palo Alto, CA) and were used to raise two rabbit antisera. A rabbit antiserum that was raised using total bovine AUM proteins as antigen immunoprecipitated only UPIII (Wu et al., 1990). A rabbit antibody against the synthetic UPII peptide GASTESSREIPMSTFPRRK (Lin et al., 1994) and a mouse monoclonal antibody against UPIII (Riedel et al., 2001) were used for Western blot analysis. Monoclonal antibodies against the HA tag (16B12) and against myc (9E10) were purchased from Berkeley Antibody Company (Richmond, CA) and Santa Cruz Biotechnology (Santa Cruz, CA) respectively.
Transient Transfections
Eighteen hours before transfection, 293T cells, which are simian virus 40-transformed human embryonic kidney epithelial cells, were seeded in six-well plates (6 × 105 cells/well) in DMEM supplemented with 10% fetal bovine serum. The cells were transfected using a mixture of DNA and FuGENE6 (1:3, wt/vol) in serum-free DMEM that had been kept at room temperature for 30 min. The cells were analyzed 24 h posttransfection.
SDS-PAGE and Western Blotting
Cells were rinsed 24 h posttransfection with ice-cold phosphate-buffered saline (PBS) and lysed on ice in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% sodium deoxylcholate, 1% NP-40, 0.1% SDS, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). The cell lysates were centrifuged at 13,000 × g for 10 min at 4°C and the supernatants were stored at −20°C or used for experiments directly. Protein samples resolved by SDS-PAGE (Laemmli, 1970) were electrotransferred onto nitrocellulose membranes, which were blocked with 5% fat-free milk in PBS, pH 7.5, followed by incubation with the first antibody against the individual uroplakin subunits at room temperature for 2 h. Rabbit antisera against UPIa, UPIb, and UPII were used at 1:2000 dilution, whereas the mouse antibody against UPIII was used at 1:200. After washing, the membranes were incubated with a secondary antibody (horseradish peroxidase-conjugated donkey anti-rabbit or donkey anti-mouse IgG at 1:10,000; Jackson Immunoresearch Laboratories, West Grove, PA) for 1 h and visualized using the Supersignal chemiluminescent substrate (Pierce Chemical, Rockford, IL).
Endoglycosidase Treatment
Bovine AUM were prepared as described previously (Wu et al., 1990, 1994) and dissolved in 10 mM HEPES, pH 7.5, containing 0.1% SDS. Bovine AUM or cell lysates were treated with endoglycosidase H (Endo-H; Roche Applied Science) in 50 mM sodium citrate, pH 5.5, 1% octylglucoside, 0.05% NaN3, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride at 37°C for 16 h. Bovine AUM or cell lysates were treated with N-glycanase (GLYKO, Novato, CA) in 20 mM sodium phosphate, pH 7.5, at 37°C for 16 h. All samples were then analyzed by Western blotting.
Immunostaining
Cells were plated on coverslips the day before transfection. Twenty-four hours posttransfection, cells were fixed with 3% paraformadehyde in PBS, pH 7.5, and blocked with 5% nonfat milk in PBS. Some samples were permeabilized by adding 0.05% saponin to the blocking solution. Fixed cells were incubated with a first antibody at 37°C for 90 min and then with a secondary antibody (Texas Red-conjugated donkey anti-mouse IgG, and Texas Red or fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG; Jackson Immunoresearch Laboratories). Immunostained cells were examined using an LSM510 confocal microscope (Carl Zeiss, Thornwood, NY).
Metabolic Labeling of Cells
Twenty-four hours after transfection, cells were first incubated with methionine-free DMEM (Mediatech, Herndon, VA) containing 5% dialyzed fetal bovine serum for 30 min at 37°C and then pulse labeled with 150 μCi of [35S]methionine (ICN Pharmaceuticals, Costa Mesa, CA) for 10 min at 37°C. At the end of the pulse period, cells were washed once with serum-free medium and chased at 37°C for the indicated time with complete DMEM containing 5 mM methionine. The labeled cells were then washed twice with ice-cold PBS, pH 7.4, and subjected to immunoprecipitation.
Immunoprecipitation
Transfected cells (2 × 106) were lysed in 0.5 ml of 1% NP-40, 0.5% sodium deoxycholate, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl. The total cell lysates were centrifuged at 13,000 × g for 10 min, at 4°C and the supernatants were incubated overnight with the first antibody (HA antibody at 1:150 and anti-AUM antibody at 1:200) at 4°C. The complexes were precipitated by adding 25 μl of protein G-agarose beads (Roche Applied Science) to the lysates and incubated for 60 min at 4°C. The beads were sedimented (5000 × g for 3 min at 4°C) and washed three times with ice-cold lysis buffer. The samples resuspended in SDS loading buffer with 2% β-mercaptoethanol were denatured by boiling for 5 min, followed by SDS-PAGE and autoradiography.
RESULTS
To study the assembly of the four UP subunits, we expressed them individually or in pairwise combinations in 293T cells. We chose 293T cells for these transfection experiments because they can be easily transfected, and they do not express any endogenous UP subunits that can complicate the analysis. The assumption was that these nonpolarized cells express the basic mechanisms concerned with the early assembly steps that affect oligomeric membrane proteins. The subcellular location of the UP subunits was assessed by immunofluorescence microscopy and by analyzing their state of glycosylation. Conversion of N-linked oligosaccharides from an Endo-H–sensitive to an Endo-H–resistant form indicates that they have been processed by enzymes contained in the medial compartment of the Golgi apparatus. Therefore, Endo-H resistance was used as a criterion that uroplakins have left the cis-Golgi compartment.
Only UPIb Can Reach the Plasma Membrane When Expressed Alone
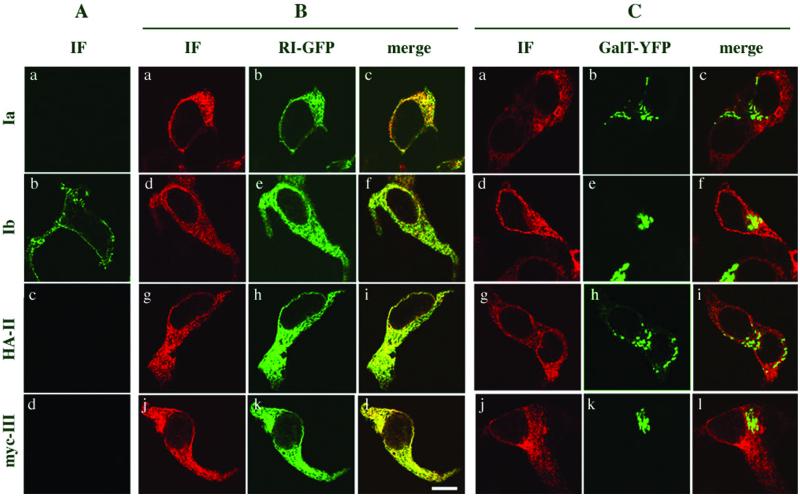
Initially, cDNAs encoding each of the four uroplakin subunits were used singly in transfection experiments to determine their intracellular location. UPIa and UPIb ectopically expressed in 293T cells comigrated during SDS-PAGE with the corresponding uroplakins found in purified bovine AUM (Figure 1, A and B, lanes 1 and 4). Most N-linked oligosaccharides attached to plasma membrane proteins are modified in post-ER compartments such that they cannot be removed by Endo-H; for this class of proteins, Endo-H resistance indicates that they have been transported out of the ER and reached the medial-Golgi cisternae (Dong et al., 1998). Native UPIa and UPIb isolated from AUM plaques are exceptional in that their N-linked sugar moieties remain Endo-H sensitive. This Endo-H sensitivity was also observed when the UPIa and UPIb subunits were individually expressed in 293T cells (Figure 1, A and B, lanes 3 and 6). Therefore, based on these data we could not determine whether the ectopically expressed UPIa and UPIb had exited from the ER. The subcellular location of these two uroplakins was therefore assessed by immunofluorescence microscopy. As shown in Figure 2A, intact cells transfected with UPIa cDNA were not immunostained (a), indicating that this uroplakin subunit was not transported to the cell surface. Immunolabeling of the transfected cells after saponin-permeabilization demonstrated that UPIa was indeed expressed, as indicated by the typical lace-like staining of the ER (Figure 2B, a and C, a). The ER localization of UPIa was confirmed by colocalzation with the ER marker RI-GFP (Figure 2B, b and c). No significant colocalization was observed with the Golgi marker GalT-YFP (Figure 2C, b and c), a chimera targeted to the Golgi apparatus due to the presence of the transmembrane domain of the Golgi resident enzyme galactosyltransferase. We therefore concluded that UPIa expressed alone was unable to exit from the ER. In striking contrast, expression of UPIb, which is structurally closely related to UPIa, resulted in surface staining of nonpermeabilized 293T cells (Figure 2A, b), indicating that UPIb was delivered to the plasma membrane. As may be expected from its site of synthesis, the typical ER staining pattern was observed in permeabilized cells (Figure 2B, d–f).
Figure 1.
Uroplakins remain Endo-H sensitive when expressed individually in 293T cells. 293T cells were transfected with cDNAs encoding one of the four uroplakin subunits, UPIa (A), UPIb (B), UPII (C), or UPIII (D). Equal aliquots of cell lysates obtained 24 h after transfection were kept as untreated controls (lane 4), incubated in the absence of Endo-H (−, lane 5) or treated with Endo-H (+, lane 6). For comparison, purified bovine AUM was subjected to the same treatment (lanes 1, 2, and 3, respectively). Western blot analysis was performed using the antibodies indicated at the bottom of each panel. Endo-H removed the high-mannose oligosaccharide of UPIa (A) or UPIb (B). (C) Pro-UPII remained Endo-H sensitive (lane 6), indicating that the prosequence was not removed. (D) ER form of UPIII remained Endo-H sensitive (lane 6) in contrast to the mature form of UPIII in AUM (lane 3).
Figure 2.
Only UPIb is expressed on the cell surface. Expression of the individual uroplakin subunits in 293T cells was carried out as described in MATERIAL AND METHODS. An HA-tagged form of UPII (HA-UPII) or a myc-tagged form of UPIII (myc-UPIII) was used because antibodies against UPII or UPIII were not available for immunostaining. (A) Transfected cells were immunostained under nonpermeabilized condition. fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG (a and b) or Texas Red-conjugated donkey anti-mouse IgG (c and d) was used. Only UPIb was stained at the plasma membrane. (B) To determine the intracellular location of individual uroplakins, 293T cells were cotransfected with the ER marker ribophorin I-GFP (a–l) and UPIa (a–c), or UPIb (d–f), or HA-UPII (g–i) or myc-UPIII (j–l). Cells were permeabilized by adding 0.05% saponin to the blocking solution and immunostained. All the secondary antibodies were conjugated with Texas Red. Uroplakins retained intracellularly colocalized with RI-GFP. (C) Cells were cotransfected with the Golgi marker galactosyl transferase tagged with YFP (GalT-YFP; a–l) with UPIa (a–c), or UPIb (d–f), or HA-UPII (g–i) or myc-UPIII (j–l). No significant colocalization between GalT-YFP and uroplakins was observed. All the immunostained cells were analyzed by confocal microscopy.
Uroplakin II is a small nonglycosylated type I transmembrane protein with an apparent molecular mass of 15 kDa (Figure 1C, lane 1–3). UPII expressed in 293T cells was, however, represented by a 28-kDa band (lane 4) that could be converted to an 18-kDa form after Endo-H treatment due to the removal of three high-mannose oligosaccharides linked to its propeptide (Lin et al., 1994). The difference in molecular mass between mature UPII found in AUM and the Endo-H–trimmed UPII expressed in 293T cells (lane 6) corresponded to the approximate size of the propeptide, which was presumably cleaved by furin-like endoproteases in a late Golgi compartment (Bresnahan et al., 1990; Misumi et al., 1991; Lin et al., 1994). The finding that the propeptide was not cleaved and that the high-mannose oligosaccharide moieties remained Endo-H sensitive indicated that UPII expressed alone in 293T cells did not reach the medial-Golgi cisternae. As expected, in nonpermeabilized cells the plasma membrane was not stained (Figure 2A, c), whereas after permeabilization a staining pattern typical for the ER was obtained (Figure 2B, g–i).
Like UPII, UPIII has only a single transmembrane domain; however, mature UPIII has three potential N-glycosylation sites (Wu and Sun, 1993) and the attached oligosaccharides are Endo-H resistant (Figure 1D, lanes 1–3). UPIII expressed in 293T cells had an apparent molecular size of ∼37 kDa (lane 4), which was reduced to ∼29 kDa after Endo-H digestion due to the removal of N-linked, high-mannose oligosaccharides (lanes 4–6). Immunostaining showed that UPIII expressed in 293T cells was ER-associated (Figure 2B, j–l) and did not reach the cell surface (Figure 2A, d), indicating that UPIII expressed by itself was unable to exit from the ER.
Uroplakins Exit from ER in Heterodimeric Pairs
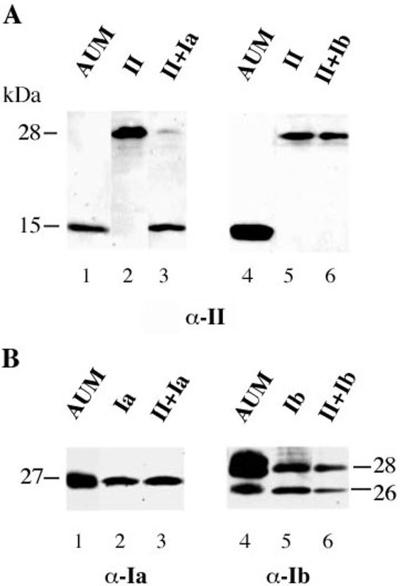
It is well known that oligomerization of membrane proteins, as well as proteins contained in the lumen of the endomembrane system, takes place in the ER and that in most instances, the unassembled proteins are unable to exit from this compartment (Green and Millar, 1995; Nagaya and Papazian, 1997; Reddy and Corley, 1998; Ellgaard et al., 1999; Green, 1999). To test whether the expression of more than one UP subunit may lead to the formation of properly folded uroplakin oligomers, we expressed pairs of UP subunits in 293T cells and assessed their posttranslational modifications and their subcellular location. Although UPII expressed alone has an apparent molecular mass of 28 kDa (Figure 3A, lane 2), its coexpression with UPIa resulted in a shift of the UPII band to ∼15 kDa (lane 3), which comigrated with the authentic mature UPII of AUM (lane 1). This drastic shift in the apparent molecular mass of UPII was, however, not observed when uroplakin II was coexpressed with UPIb (Figure 3A, compare lanes 3 and 6) or UPIII (our unpublished data). Because cleavage of prosequences occurs normally in a late Golgi compartment, it is reasonable to assume that UPII had reached the cell surface, as confirmed by immunostaining of the nonpermeabilized cells (see below).
Figure 3.
Coexpression of UPII with UPIa results in the maturation of UPII. UPII was coexpressed with UPIa (lane 1–3) or UPIb (lane 4–6) in 293T cells. The expression of the uroplakins was analyzed by Western blotting with the antibodies directed against UPII (A) and UPIa or UPIb (B). (A) When UPII was coexpressed with UPIa, mainly its mature form (15 kDa) was observed (lane 3), whereas only its precursor (28 kDa) was detected when UPII was coexpressed with UPIb (lane 6). (B) Expression of UPIa (lane 3) or UPIb (lane 6) in cotransfected cells was confirmed by Western blotting. Purified AUM (lane 1 and 4) and total cell lysates from single transfections (lane 2 and 5) were used as controls.
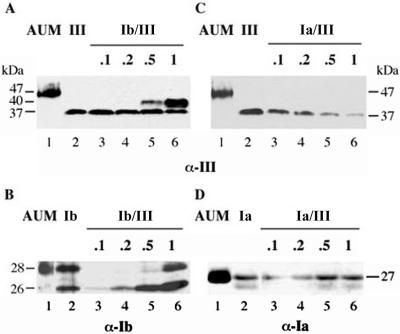
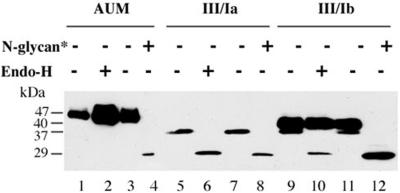
If the formation of specific uroplakin heterodimers is a prerequisite for their exit from ER, it might be expected that the expression of two subunits in optimal ratios would improve their transport out of the ER. We, therefore, cotransfected 293T cells with a fixed amount of UPIII cDNA (1 μg) together with various amounts of UPIb cDNA (0.1–1 μg) (Figure 4A). We found that at a low input of UPIb cDNA (0.1 and 0.2 μg; lanes 3 and 4), UPIII was expressed as a single band of 37 kDa that remained Endo-H sensitive, indicating that it did not exit from the ER. However, at a high input of UPIb cDNA (0.5 and 1 μg; lanes 5 and 6), most of the UPIII was converted to the 40-kDa Endo-H–resistant form (Figure 5), suggesting that it had exited from the ER. Furthermore, a high UPIb input greatly increased the amount of the 40-kDa form of UPIII (lane 6), suggesting stabilization of the uroplakin subunits as a result of heterodimer formation and exit from the ER. Because we have not observed a significant accumulation of UPIII in the Golgi apparatus (our unpublished data), the ratio of the two forms reflected most likely the steady-state distribution of UPIII at the plasma membrane and ER. At this point, we do not understand the mechanism by which UPIII expression affected the glycosylation of UPIb (Figure 4B). The specificity of the UPIII–UPIb interaction was shown by a control experiment demonstrating that coexpression of UPIa did not result in the conversion of UPIII into the Endo-H–resistant 40-kDa form (Figure 4C). As can be seen in Figure 4A, native UPIII in AUM has a significantly lower electrophoretic mobility than UPIII coexpressed with UPIb (compare lanes 1 and 6). To determine whether this is due to differences in the modification of the complex oligosaccharides we performed Endo-H or N-glycanase digestion on purified AUM or lysates obtained from cotransfected cells. As previously shown, UPIII in purified AUM does not carry O-linked sugar residues, but only N-linked complex oligosaccharides (Wu and Sun, 1993). Accordingly, digestion of AUM with N-glycanase removed all N-linked oligosaccharides from UPIII, resulting in a polypeptide of 29 kDa (Figure 5, lane 4). This was also the case when UPIII coexpressed with UPIa or UPIb was digested (lane 8 and 12). A band with the same electrophoretic mobility was also obtained when UPIII expressed alone (Figure 1D, lane 6) or together with UPIa (Figure 5, lane 6) was digested with Endo-H. It seems, therefore, that the difference in the electrophoretic mobility between UPIII in AUM and the Endo-H–resistant form of UPIII obtained when coexpressed with UPIb was due to differences in posttranslational modification of the N-linked oligosaccharides of UPIII.
Figure 4.
Cotransfection of UPIII cDNA with varying amounts of UPIb or UPIa cDNA. (A) 293T cells were cotransfected with UPIII and UPIb cDNA. Although the amount of UPIII cDNA was kept constant (1.0 μg, lane 2–6), the amount of UPIb cDNA varied from 0.1 to 1.0 μg (lane 3–6, corresponding to 0.1, 0.2, 0.5, and 1.0 μg of UPIb cDNA, respectively). Western blot analysis using an antibody directed against UPIII revealed conversion of the 37-kDa high-mannose form of UPIII into the mature form (40 kDa) when at least 0.5 μg of UPIb cDNA was used in the double transfection (lanes 5 and 6). (B) That the coexpressed UPIb was indeed synthesized (lane 3–6) was confirmed by Western blot analysis by using an antibody directed against UPIb. In cells cotransfected with UPIb and UPIII, the expression of nonglycosylated UPIb (26 kDa; lanes 3–6) increased with increasing amounts of UPIb cDNA. Only at the highest amount of UPIb cDNA (0.5 and 1.0 μg; lanes 5 and 6) the glycosylated form of UPIb was detected. When in a similar manner a constant amount of UPIII was coexpressed with increasing amounts of UPIa, Western blots developed by using antibodies against UPIII (C) or UPIa (D) showed no conversion of UPIII into the mature form and its steady-state level decreased with increasing levels of UPIa. Purified AUM (lane 1 in A–D) and total cell lysates from single transfections (lane 2 in A–D) were used as controls.
Figure 5.
Only coexpression of UPIII with UPIb results in an Endo-H–resistant form of UPIII. UPIII was coexpressed with UPIa (lanes 5–8) or UPIb (lanes 9–12). Total cell lysates were treated with Endo-H (lanes 6 and 10) or N-glycanase (N-glycan*) (lanes 8 and 12). As control, samples were incubated under the same condition omitting Endo-H (lanes 5 and 9) or N-glycanase (lanes 7 and 11). All samples were then subjected to SDS-PAGE and Western blotting was carried out using an antibody directed against UPIII. AUM was subjected to the same treatment (lanes 1–4).
Only Specific Uroplakin Pairs Are Expressed at Cell Surface
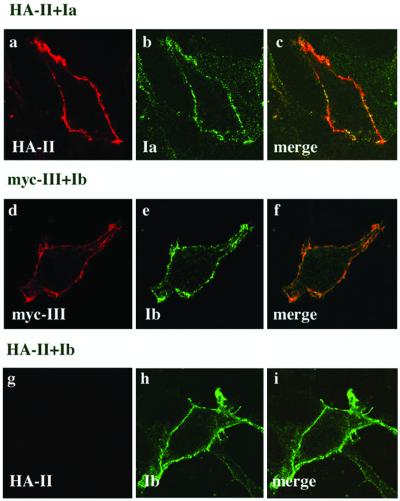
The location of uroplakin subunits expressed in 293T cells in a pairwise manner was also investigated by double immunofluorescence microscopy. Because available antibodies stained UPII and UPIII weakly in immunostaining experiments, we tagged these two uroplakins with HA or myc. Western blot analysis showed that these tags did not affect the proper pairing of UP subunits (our unpublished data). As shown in Figure 6, when HA-UPII and UPIa were coexpressed, they could both be detected at the plasma membrane (a–c). Similarly, coexpressed myc-UPIII and UPIb were both detected at the cell surface (d–f). The subunit specificity of these dimeric interactions was indicated by the fact that coexpressed UPIa and myc-UPIII failed to reach the cell surface (our unpublished data). We also coexpressed HA-UPII with UPIb (g–i) and found that, consistent with the single transfection data (Figure 2A, b), only UPIb was transported to the cell surface.
Figure 6.
Coexpression of UPIa/UPII and UPIb/UPIII pairs results in their colocalization at the plasma membrane. Cotransfection of 293T cells was carried out as described in Figures 3 and 4. Cells were fixed and incubated with the first antibodies against the UP subunits and the HA or myc tag, followed by incubation with the secondary antibodies used in Figure 2A. Immunostaining was performed on nonpermeabilized cells that were analyzed by confocal microscopy. When UPIa (b) and HA-UPII (a) were coexpressed, both subunits colocalized at the plasma membrane. This was not the case when UPIa was coexpressed with myc-UPIII, but ER staining was observed in permeabilized cells (our unpublished data). On the other hand, when UPIb (e) was coexpressed with myc-UPIII (d), both UP subunits were expressed at the plasma membrane (f), but HA-UPII cannot be detected at the plasma membrane of cells expressing UPIb and HA-UPII (g). As may be expected from our single-transfection experiments, UPIb reached the cell surface (h) when coexpressed with HA-UPII.
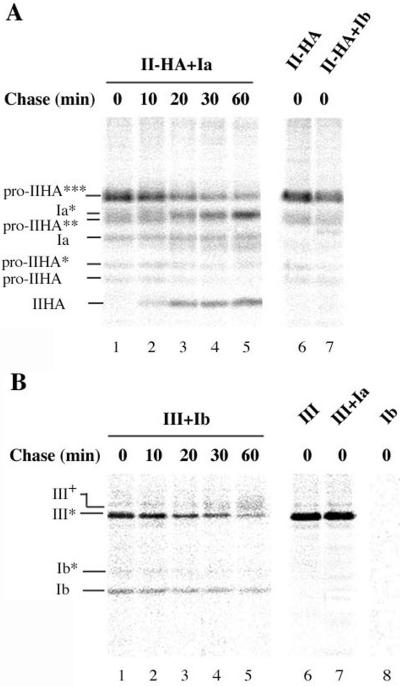
To confirm that the formation of specific uroplakin heterodimers takes place in the ER, we performed pulse-chase experiments on 293T cells coexpressing uroplakin pairs, followed by immunoprecipitation. When UPII-HA–transfected 293T cells were subjected to immunoprecipitation immediately after the pulse period, several radioactively labeled bands were detected in the autoradiograph (Figure 7A, lane 6). They corresponded to the fully glycosylated pro-UPII-HA (pro-IIHA***) as well as to partially glycosylated (pro-IIHA** and pro-IIHA*) or nonglycosylated (pro-IIHA) forms (Lin et al., 1994). When UPII-HA was cotransfected with UPIa, two additional bands could be recognized after the pulse period (lane 1) that correspond to the glycosylated (Ia*) and nonglycosylated (Ia) forms of UPIa. At chase periods of ≥10 min (lane 2–5), a band corresponding to the mature UPII-HA was observed, the intensity of which increased with increasing chase time, whereas the intensities of all precursor forms of UPII-HA decreased. At the same time, the intensity of the Ia* band increased. These results indicated that UPIa was associated with pro-UPII-HA forms before UPII-HA was proteolytically processed, which occurred in a late Golgi compartment. Combined with the evidence that UPIa and UPII remained trapped in the ER when individually expressed, this result indicated that heterodimer formation occurred in the ER. The increasing intensities of the Ia* band coimmunoprecipitated with UPII-HA at late chase time periods suggested that the interaction between UPIa and UPII-HA was strengthened after the removal of the UPII prosequence.
Figure 7.
UPIa/UPII and UPIb/UPIII heterodimers are formed in the ER. Transfected 293T cells were pulsed with [35S]methionine for 10 min and chased for the indicated time. Immunoprecipitation was then performed under nondenaturing conditions. (A) 293T cells were transfected with UPII-HA (lane 6) or cotransfected with UPII-HA and UPIa (lanes 1–5) or UPIb (lane 7). An HA antibody was used for immunoprecipitation. Immediately after the pulse period, formation of the UPIa/UPII-HA heterodimer was detected before the maturation of UPII-HA took place (lane 1). Note that pro-UPII has three potential N-glycosylation sites in the prosequence. The number of stars next to “pro-IIHA” indicates the number of N-linked high-mannose oligosaccharides attached to the propeptide. A glycosylated (Ia*) and a nonglycosylated (Ia) form of UPIa were detected. (B) 293T cells were transfected with UPIII or cotransfected with UPIII and UPIa or UPIb. Immunoprecipitation was performed using an anti-AUM antibody that precipitates only UPIII (lane 6), but not UPIb (lane 8). The electrophoretic mobility of the ER form of UPIII (III*) and the Endo-H–resistant form (III+) are indicated. UPIb was synthesized as a nonglycosylated (Ib) or glycosylated (Ib*) form. The UPIb/UPIII heterodimer can be detected immediately after the pulse period (lane 1). The Endo-H–resistant form of UPIII was detected only at chase times of >20 min. The identities of the uroplakin glycoforms were confirmed by Western blotting of control and Endo-H–treated samples (our unpublished data).
When UPIII was coexpressed with UPIb, the latter subunit was mainly synthesized as a nonglycosylated polypeptide (Figure 7B), whereas UPIII was initially expressed as an Endo-H–sensitive form (III*). The finding that UPIb can be coimmunoprecipitated with UPIII immediately after the pulse period (lane 1) indicated that heterodimer formation between UPIII and UPIb occurred in the ER. Although the intensities of the UPIb band remained rather constant during the chase period, the immature UPIII (III*) was converted into an Endo-H–resistant form of UPIII (III+), which appeared as a diffuse band (lane 4 and 5). The conversion reflected the transport of the heterodimer across the trans-Golgi cisternae. The specificity of the heterodimer formation was confirmed by coexpression of UPIII and UPIa (lane 7), which did not result in the coimmunoprecipitation of UPIII and UPIa.
DISCUSSION
Our long-range goal is to understand how the four uroplakin subunits assemble into crystalline arrays that cover the apical plasma membrane as well as fusiform vesicles of urothelial umbrella cells. Herein, we report on studies concerned with the initial assembly of uroplakins that occur in the ER. By expressing the uroplakin subunits ectopically in 293T cells, we have demonstrated that, except for UPIb, the individual uroplakin subunits remain trapped in the ER. Only by forming specific heterodimeric complexes are they able to exit from the ER compartment. The mechanisms that affect the assembly of the uroplakin subunits into functional oligomeric complexes is shared by a large number of plasma membrane proteins, the function of which depends on the proper assembly of several subunits (Klausner et al., 1990; Berridge, 1993; Macdonald and Olsen, 1994; Green, 1999). To ensure that the correct quaternary structure is achieved for proteins of the endomembrane system, highly specific quality control mechanisms have evolved in the ER (Green and Millar, 1995; Hammond and Helenius, 1995; Ellgaard et al., 1999). Several of these partially overlapping mechanisms affect all newly synthesized proteins translocated into the ER lumen. Chaperones and folding enzymes such as Bip, calnexin, calreticulin, GRP94, protein disulfide isomerase, or ERp57 and Erp75 may attach to incompletely folded or misfolded polypeptides, thus preventing their exit from the ER (Ellgaard et al., 1999). Except for UPIb, this is apparently the case for uroplakin subunits that are expressed in 293T cells as individual subunits, or as “incompatible” pairs that cannot assemble properly into correct heterodimeric complexes.
Our results indicate that UPIb is the only uroplakin subunit able to leave the ER when expressed by itself, whereas exit of the closely related UPIa requires the coexpression of UPII. The most obvious interpretation of these results is that UPIb can be correctly folded in the ER such that components involved in quality control functions no longer recognize and bind to this subunit, thus allowing it to exit from the ER. Alternatively, UPIb may pair with an unknown protein that is also expressed in 293T cells. Our finding that UPIb alone can exit from the ER is interesting, because UPIb is the only uroplakin that is known to be expressed in tissues other than the urothelium, namely, in the cornea, conjunctiva and possibly lung (Kallin et al., 1991; Adachi et al., 2000). Support for the notion that UPIb can exit by itself from the ER also came from experiments where the UPIII gene was deleted in knockout mice (Hu et al., 2000). As was the case in our transfection experiments, where UPIb was either expressed by itself or together with UPIII (Figure 4), the glycosylation pattern of UPIb changed significantly in the UPIII-deficient urothelium (Hu et al., 2000). Furthermore, instead of being expressed only at the apical domain of umbrella cells, UPIII knockout led to the mistargeting of UPIb to the basolateral cell surface. It seems that UPIb alone does not carry targeting information for a specific plasma membrane domain in polarized urothelium and that it acquires this targeting signal when it forms a heterodimer with UPIII. It remains to be seen at which intracellular level the mistargeting of the “unpaired” UPIb takes place in the knockout mouse. It is conceivable that in urothelium of the UPIII knockout mouse, UPIb alone is at least partially sorted into a different class of vesicles than the UPIa/UPII pair, and only the latter is transported to the apical surface of umbrella cells. Alternatively, UPIb is packaged together with UPIa/UPII complexes into post-Golgi vesicles. After being delivered to the apical plasma membrane, UPIb may then be redistributed to the basolateral domain via transcytotic vesicles (Bartles et al., 1987). At this point, we do not know why UPIa expressed alone is retained in the ER, despite its structural similarity to UPIb. In the case of CD82, which like UPIa and UPIb belongs to the “tetraspanin” family, its first transmembrane segment is known to be essential for the molecule to exit from the ER and to reach the plasma membrane of B and T cells or macrophages (Cannon and Cresswell, 2001). Interactions of domains buried in the lipid bilayer may, therefore, play a role in the assembly and targeting of these tetraspanin membrane proteins, including UPIa and UPIb.
Structural studies have demonstrated that the planar crystals of the urothelial plaques are formed by hexagonal arrays of 16-nm particles that most likely contain all four uroplakin subunits (Walz et al., 1995; Kachar et al., 1999; Liang et al., 2001). Like many cell surface receptors, ion channels and cell junctions, the 16-nm particles are composed of multiple subunits, which must at least partially assemble in the ER to be expressed at the cell surface (Green and Millar, 1995; Gorrie et al., 1997; Nagaya and Papazian, 1997; Reddy and Corley, 1998; Dietrich et al., 1999; George et al., 1999). Our pulse-chase experiments have demonstrated that uroplakin subunits assemble into UPIa/UPII and UPIb/UPIII heterodimers in the ER, suggesting that these two subcomplexes may form higher order assemblies in a post-ER compartment. We cannot exclude, however, that in the ER of urothelial cells they may form heterotetramers or assume even higher order assemblies, such as the 16-nm particles. Our transfection studies on ectopically expressed subunits have provided evidence for a stepwise assembly of uroplakin crystals starting with heterodimeric subcomplexes formed in the ER. A similar process was observed for the assembly of the pentameric acetylcholine receptor, where the formation of dimeric and trimeric intermediates has been demonstrated in the ER (Green, 1999). For the assembly of connexins into gap junctions, it was shown that the hexameric hemichannels are assembled in the ER from dimeric and tetrameric intermediates (Ahmad et al., 2001). On the other hand, higher order assembly of gap junctions into plaque-like structures is a post-Golgi event and is dependent on the phosphorylation of certain connexins (Musil and Goodenough, 1991, 1993). It has been suggested that this late-stage aggregation of hemi-connexons can prevent precocious, intracellular assembly of gap junctions. Perhaps for the same reasons, the assembly of 16-nm uroplakin particles into the crystalline AUM plaques is restricted to post-Golgi compartments, although we do not know what triggers the further assembly of the uroplakin subcomplexes into mature two-dimensional crystals. Another example for the assembly of subcomplexes of an oligomeric plasma membrane protein in the ER followed by the formation of a functional protein in the Golgi apparatus is provided by the stepwise assembly of the T-cell receptor (TCR) (Klausner et al., 1990; Dietrich et al., 1999). Initially, three heterodimeric subcomplexes are formed in the ER that, after assembly into a hexameric complex, can be transported to the Golgi apparatus. On the other hand, the homodimeric TCRζ2 subcomplex can be exported from the ER independently and locates to the Golgi apparatus. After it combines with the hexameric subcomplex in a final assembly step, the functional TCR can now translocate to the plasma membrane (Dietrich et al., 1999).
Both chemical cross-linking experiments on purified AUM (Wu et al., 1995) and chromatographic separation of detergent-solubilized AUM (Liang et al., 2001) support the idea of uroplakin pairs. The experiments presented herein demonstrate that the UPIa/UPII and UPIb/UPIII pairs are important intermediates toward formation of the 16-nm particles of urothelial plaques. The heterologous transfection system described herein, together with cell fractionation studies, will allow us to further analyze in which compartment these higher order assembly steps take place, and how this assembly process is regulated.
ACKNOWLEDGMENTS
This work was supported by grants DK-52206 (to G.K.) and DK-39753, 52206, and 57269 (to T.-T.S.) from the National Institutes of Health.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–04–0211. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0211.
REFERENCES
- Adachi W, Okubo K, Kinoshita S. Human uroplakin Ib in ocular surface epithelium. Invest Ophthalmol Vis Sci. 2000;41:2900–2905. [PubMed] [Google Scholar]
- Ahmad S, Martin PE, Evans WH. Assembly of gap junction channels: mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur J Biochem. 2001;268:4544–4552. doi: 10.1046/j.1432-1327.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- Alroy J, Weinstein RS. Intraepithelial asymmetric-unit-membrane plaques in mammalian urinary bladder. Anat Rec. 1980;197:75–83. doi: 10.1002/ar.1091970107. [DOI] [PubMed] [Google Scholar]
- Bartles JR, Feracci HM, Stieger B, Hubbard AL. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987;105:1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The AM and FM of calcium signaling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- Bresnahan PA, Leduc R, Thomas L, Thorner J, Gibson HL, Brake AJ, Barr PJ, Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990;111:2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson A, Wade RH. Three-dimensional structure of luminal plasma membrane protein from urinary bladder. J Mol Biol. 1983;166:21–36. doi: 10.1016/s0022-2836(83)80048-5. [DOI] [PubMed] [Google Scholar]
- Cannon KS, Cresswell P. Quality control of transmembrane domain assembly in the tetraspanin CD82. EMBO J. 2001;20:2443–2453. doi: 10.1093/emboj/20.10.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Kastrup J, Lauritsen JP, Menne C, von Bulow F, Geisler C. TCRzeta is transported to and retained in the Golgi apparatus independently of other TCR chains: implications for TCR assembly. Eur J Immunol. 1999;29:1719–1728. doi: 10.1002/(SICI)1521-4141(199905)29:05<1719::AID-IMMU1719>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Doms RW, Lamb RA, Rose JK, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Dong JF, Gao S, Lopez JA. Synthesis, assembly, and intracellular transport of the platelet glycoprotein Ib-IX-V complex. J Biol Chem. 1998;273:31449–31454. doi: 10.1074/jbc.273.47.31449. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- George CH, Kendall JM, Evans WH. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J Biol Chem. 1999;274:8678–8685. doi: 10.1074/jbc.274.13.8678. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Gorrie GH, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart TG, Moss SJ. Assembly of GABAA receptors composed of alpha1 and beta2 subunits in both cultured neurons and fibroblasts. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WN. Ion channel assembly: creating structures that function. J Gen Physiol. 1999;113:163–170. doi: 10.1085/jgp.113.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WN, Millar NS. Ion-channel assembly. Trends Neurosci. 1995;18:280–287. [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Specific tetraspanin functions. J Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RM. The mammalian urinary bladder: an accommodating organ. Biol Rev. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol. 2000;151:961–972. doi: 10.1083/jcb.151.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kachar B, Liang F, Lins U, Ding M, Wu XR, Stoffler D, Aebi U, Sun TT. Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol. 1999;285:595–608. doi: 10.1006/jmbi.1998.2304. [DOI] [PubMed] [Google Scholar]
- Kallin B, de Martin R, Etzold T, Sorrentino V, Philipson L. Cloning of a growth arrest-specific and transforming growth factor beta-regulated gene, TI 1, from an epithelial cell line. Mol Cell Biol. 1991;11:5338–5345. doi: 10.1128/mcb.11.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Lippincott-Schwartz J, Bonifacino JS. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang FX, Riedel I, Deng FM, Zhou G, Xu C, Wu XR, Kong XP, Moll R, Sun TT. Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem J. 2001;355:13–18. doi: 10.1042/0264-6021:3550013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Wu XR, Kreibich G, Sun TT. Precursor sequence, processing, and urothelium-specific expression of a major 15-kDa protein subunit of asymmetric unit membrane. J Biol Chem. 1994;269:1775–1784. [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Min GW, Stolz M, Zhou G, Liang FX, Sebbel P, Glockshuber R, Sun TT, Aebi U, Kong XP. Localization of uroplakin Ia, the bacterial FimH receptor, on the six inner domains of the 16-nm mouse urothelial plaque particle. J Mol Biol. 2002;317:697–706. doi: 10.1006/jmbi.2002.5442. [DOI] [PubMed] [Google Scholar]
- Misumi Y, Oda K, Fujiwara T, Takami N, Tashiro K, Ikehara Y. Functional expression of furin demonstrating its intracellular localization and endoprotease activity for processing of proalbumin and complement pro-C3. J Biol Chem. 1991;266:16954–16959. [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Papazian DM. Potassium channel alpha and beta subunits assemble in the endoplasmic reticulum. J Biol Chem. 1997;272:3022–3027. doi: 10.1074/jbc.272.5.3022. [DOI] [PubMed] [Google Scholar]
- Porter KR, Bonneville MA. An Introduction to the Fine Structure of Cells and Tissues. New York, NY: Lea and Febiger; 1963. [Google Scholar]
- Porter KR, Kenyon K, Badenhausen S. Specializations of the unit membrane. Protoplasma. 1967;63:263–274. [PubMed] [Google Scholar]
- Reddy PS, Corley RB. Assembly, sorting, and exit of oligomeric proteins from the endoplasmic reticulum. Bioessays. 1998;20:546–554. doi: 10.1002/(SICI)1521-1878(199807)20:7<546::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Riedel I, Czernobilsky B, Lifschitz-Mercer B, Roth LM, Wu XR, Sun TT, Moll R. Brenner tumors but not transitional cell carcinomas of the ovary show urothelial differentiation: immunohistochemical staining of urothelial markers, including cytokeratins and uroplakins. Virchows Arch. 2001;438:181–191. doi: 10.1007/s004280000315. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Hicks RM. Analysis of membrane structure in the transitional epithelium of rat urinary bladder: 2. The discoidal vesicles and Golgi apparatus: their role in luminal membrane biogenesis. J Ultrastruct Res. 1979;69:279–296. doi: 10.1016/s0022-5320(79)90117-5. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Robertson JD. Analysis of the three-dimensional structure of the urinary bladder epithelial cell membranes. J Ultrastruct Res. 1984;87:23–30. doi: 10.1016/s0022-5320(84)90113-8. [DOI] [PubMed] [Google Scholar]
- Trimmer JS. Regulation of ion channel expression by cytoplasmic subunits. Curr Opin Neurobiol. 1998;8:370–374. doi: 10.1016/s0959-4388(98)80063-9. [DOI] [PubMed] [Google Scholar]
- Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J, Longley W, Robertson JD. A hexagonal arrangement of subunits in membrane of mouse urinary bladder. J Mol Biol. 1969;46:593–596. doi: 10.1016/0022-2836(69)90200-9. [DOI] [PubMed] [Google Scholar]
- Walz T, Haner M, Wu XR, Henn C, Engel A, Sun TT, Aebi U. Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: a closed “twisted ribbon” structure. J Mol Biol. 1995;248:887–900. doi: 10.1006/jmbi.1995.0269. [DOI] [PubMed] [Google Scholar]
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RC, Hicks RM. Structure of the subunits in the thick luminal membrane of rat urinary bladder. Nature. 1970;227:280–281. doi: 10.1038/227280b0. [DOI] [PubMed] [Google Scholar]
- Wu XR, Lin JH, Walz T, Haner M, Yu J, Aebi U, Sun TT. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem. 1994;269:13716–13724. [PubMed] [Google Scholar]
- Wu XR, Manabe M, Yu J, Sun TT. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem. 1990;265:19170–19179. [PubMed] [Google Scholar]
- Wu XR, Medina JJ, Sun TT. Selective interactions of UPIa and UPIb, two members of the transmembrane 4 superfamily, with distinct single transmembrane-domained proteins in differentiated urothelial cells. J Biol Chem. 1995;270:29752–29759. doi: 10.1074/jbc.270.50.29752. [DOI] [PubMed] [Google Scholar]
- Wu XR, Sun TT. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J Cell Sci. 1993;106:31–43. doi: 10.1242/jcs.106.1.31. [DOI] [PubMed] [Google Scholar]
- Yu J, Lin JH, Wu XR, Sun TT. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994;125:171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]