Abstract
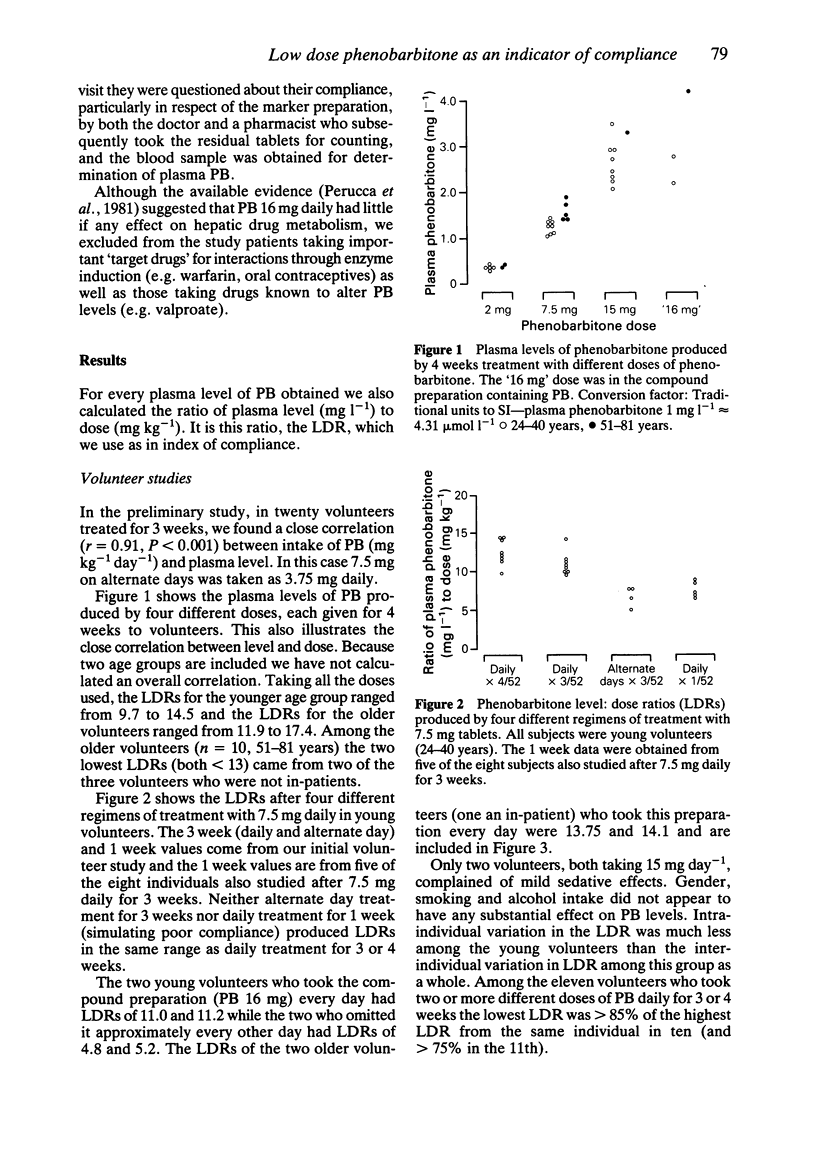
1 To assess the potential value of low-dose phenobarbitone (PB) as a marker of compliance we studied the relationship between plasma level of PB and dose (2-16 mg daily) following 3 or 4 weeks treatment in healthy volunteers (n = 26) and in-patient volunteers (n = 7). 2 Also, to simulate poor compliance, PB levels were measured in some volunteers following alternate-day (n = 6) or short-term (n = 5) treatment with similar doses. These levels, expressed as the level: dose ratios (LDRs), did not overlap with those obtained following 3 or 4 weeks of daily PB intake. 3 To evaluate the efficacy of this marker in patients taking other drugs we gave a group of out-patients (n = 24) compound tablets containing B vitamins and a small dose (16 mg) of PB; their compliance over 2-5 weeks was assessed both by measuring plasma levels of PB and residual tablet counting. 4 In the latter study, as well as providing absolute evidence of good compliance by many patients, the plasma levels of PB proved particularly valuable when non-compliant individuals 'forgot' to bring their residual tablets. 5 We suggest that phenobarbitone, in doses low enough to be non-sedative and non-enzyme inducing, is potentially useful as a pharmacological indicator of compliance with drug therapy.
Full text
PDF

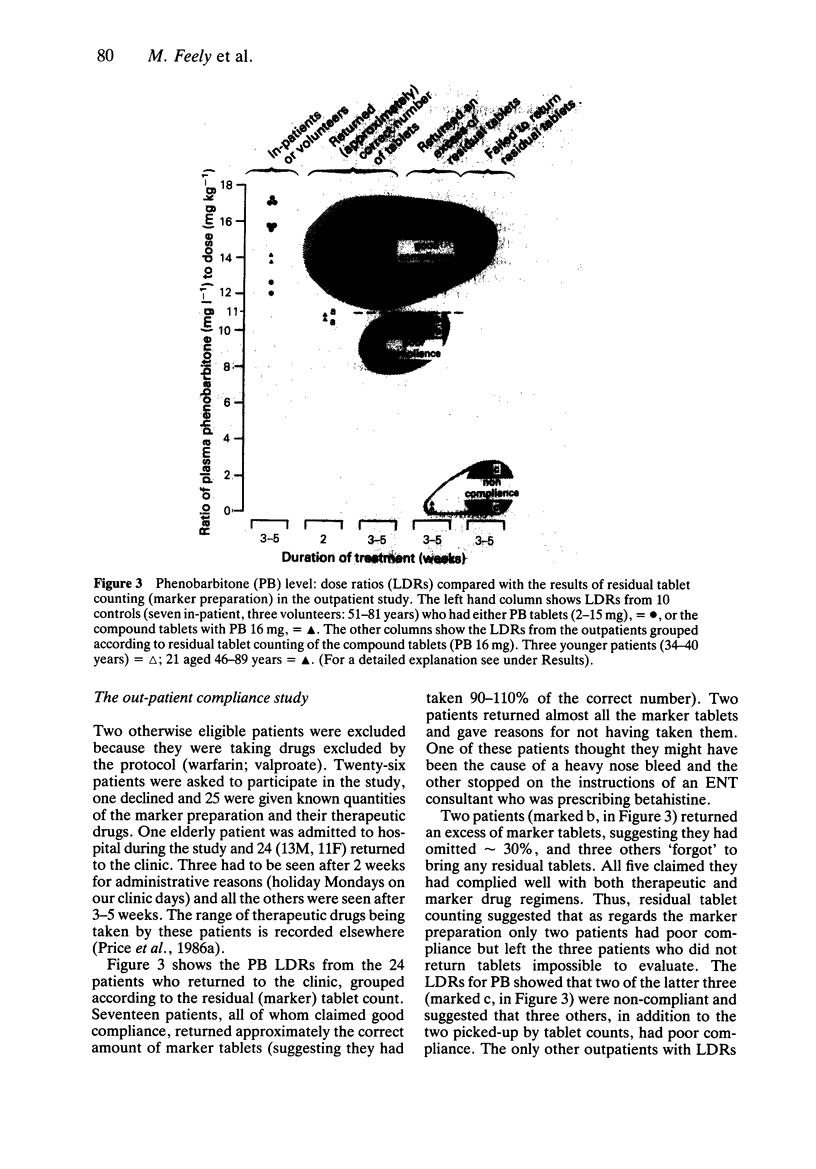



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eadie M. J., Lander C. M., Hooper W. D., Tyrer J. H. Factors influencing plasma phenobarbitone levels in epileptic patients. Br J Clin Pharmacol. 1977 Oct;4(5):541–547. doi: 10.1111/j.1365-2125.1977.tb00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraker S. A., Kirscht J. P., Becker M. H. Understanding and improving patient compliance. Ann Intern Med. 1984 Feb;100(2):258–268. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- Feely M., O'Callagan M., Duggan B., Callaghan N. Phenobarbitone in previously untreated epilepsy. J Neurol Neurosurg Psychiatry. 1980 Apr;43(4):365–368. doi: 10.1136/jnnp.43.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R., Lopes A. A., Moffat A. C. Analysis of barbiturates in blood by high-performance liquid chromatography. J Chromatogr. 1981 Nov 13;226(1):117–123. doi: 10.1016/s0378-4347(00)84212-7. [DOI] [PubMed] [Google Scholar]
- O'Hanrahan M., O'Malley K. Compliance with drug treatment. Br Med J (Clin Res Ed) 1981 Jul 25;283(6286):298–300. doi: 10.1136/bmj.283.6286.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. M. Who is taking their tablets? Br Med J (Clin Res Ed) 1982 Sep 18;285(6344):757–758. doi: 10.1136/bmj.285.6344.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E., Ruprah M., Richens A., Park B. K., Betteridge D. J., Hedges A. M. Effect of low-dose phenobarbitone on five indirect indices of hepatic microsomal enzyme induction and plasma lipoproteins in normal subjects. Br J Clin Pharmacol. 1981 Oct;12(4):592–596. doi: 10.1111/j.1365-2125.1981.tb01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. E., Mehta A., Park B. K., Hay A., Feely M. P. The effect of low-dose phenobarbitone on three indices of hepatic microsomal enzyme induction. Br J Clin Pharmacol. 1986 Dec;22(6):744–747. doi: 10.1111/j.1365-2125.1986.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D., Cooke J., Singleton S., Feely M. Doctors' unawareness of the drugs their patients are taking: a major cause of overprescribing? Br Med J (Clin Res Ed) 1986 Jan 11;292(6513):99–100. doi: 10.1136/bmj.292.6513.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENSMARK O., BUCHTHAL F. DIPHENYLHYDANTOIN AND PHENOBARBITAL. SERUM LEVELS IN CHILDREN. Am J Dis Child. 1964 Jul;108:82–87. doi: 10.1001/archpedi.1964.02090010084011. [DOI] [PubMed] [Google Scholar]
- Takaki S., Kurokawa T., Aoyama T. Monitoring drug noncompliance in epileptic patients: assessing phenobarbital plasma levels. Ther Drug Monit. 1985;7(1):87–91. doi: 10.1097/00007691-198503000-00015. [DOI] [PubMed] [Google Scholar]


