Abstract
Endothelial cell migration is an essential step in vasculogenesis and angiogenesis, in which receptor tyrosine kinases play a pivotal role. We investigated the mechanism by which ephrin-B1 promotes membrane ruffling in human aortic endothelial cells, because membrane ruffling heralds cell body migration. We especially focused on the role of Crk adaptor protein in EphB-mediated signaling. Using DsRed-tagged Crk and a fluorescent time-lapse microscope, we showed that Crk was recruited to the nascent focal complex after ephrin-B1 stimulation. Furthermore, we found that p130Cas, but not paxillin, recruited Crk to the nascent focal complex. The necessity of Crk in ephrin-B1–induced membrane ruffling was shown both by the overexpression of dominant negative Crk mutants and by the depletion of Crk by using RNA interference. Then, we examined the role of two major downstream molecules of Crk, Rac1 and Rap1. The dominant negative mutant of Rac1 completely inhibited ephrin-B1–induced membrane ruffling and focal complex assembly. In contrast, rap1GAPII, a negative regulator of Rap1, did not inhibit ephrin-B1–induced membrane ruffling. However, in rap1GAPII-expressing cells, ephrin-B1 did not induce membrane spreading, probably due to instability of the focal complex. These results indicated that Crk plays a critical role in Rac1-induced membrane ruffling and Rap1-mediated nascent focal complex stabilization contributing to ephrin-B1–induced human aortic endothelial cells migration.
INTRODUCTION
Vasculogenesis and angiogenesis are the two major processes of vascular formation in physiological and pathological conditions, including embryogenesis, ovulation, wound healing, tumorigenesis, and ischemic diseases (Yancopoulos et al., 1998; Carmeliet and Jain, 2000). Vascular endothelial cells are involved in both processes. Endothelial precursor cells proliferate and differentiate to mature endothelial cells to form the primary capillary in vasculogenesis, whereas sprouting of endothelial cells is the initial step of angiogenesis (Risau, 1997). Migration of the vascular endothelial cell is regulated by the combinations of tyrosine kinase receptors and their cognate ligands such as vascular endothelial growth factor receptor/vascular endothelial growth factor, Tie receptor/Angiopoietin, and Eph/ephrin (Yancopoulos et al., 1998, 2000).
Eph receptors consist of two subgroups: EphA receptors bind to glycosylphosphatidylinositol-anchored proteins, ephrin-A, whereas EphB receptors bind to transmembrane proteins, ephrin-B (Eph Nomenclature Committee, 1997). Knowledge of the distribution of the members of Eph/ephrin is still limited. Although arterial endothelial cells have been marked by ephrin-B2, venous endothelial cells have been marked by EphB4 (Wang et al., 1998; Gale et al., 2001). However, Shin et al. (2001) have recently reported that EphB4 is expressed in the aorta and other arteries.
EphB receptor and ephrin-B ligand work reciprocally. Their engagement activates downstream signaling of both EphB and ephrin-B (Brückner et al., 1997; Dodelet and Pasquale, 2000). Recently, two ephrin-B1–binding proteins, PDZ-RGS3 (Lu et al., 2001) and Grb4 (Cowan and Henkemeyer, 2001), have been shown to regulate cell migration. EphB receptors, on the other hand, provide autophosphorylated tyrosine residues as docking sites for the Src homology (SH)2-containing molecules such as p120-RasGAP, Fyn (Hock et al., 1998), Abl (Yu et al., 2001), Grb2, Grb10 (Stein et al., 1996), Src, Crk (Zisch et al., 2000), and SHEP1 (Dodelet et al., 1999). However, involvement of these SH2-containing molecules in EphB-dependent cellular migration has not been demonstrated to date.
Crk is an adaptor protein that links phosphotyrosine-containing molecules and SH3-binding molecules (Kiyokawa et al., 1997). Alternative splicing of the human crk gene generates two Crk proteins: CrkII consists of an SH2 and two SH3 domains, whereas CrkI lacks the carboxy-terminal SH3 of CrkII (Matsuda et al., 1992) (Figure 1A). The SH2 domain of Crk binds several phosphotyrosine-containing proteins, including p130Cas (Sakai et al., 1994) and paxillin (Birge et al., 1993; Schaller and Parsons, 1995), whereas the SH3 domain of CrkII and CrkI binds to proteins, including C3G and DOCK180 (reviewed in Kiyokawa et al., 1997). C3G and DOCK180 have been shown to activate the low-molecular-weight G proteins Rap1 and Rac1, respectively; therefore, Crk functions as an adaptor protein connecting tyrosine kinases to G proteins.
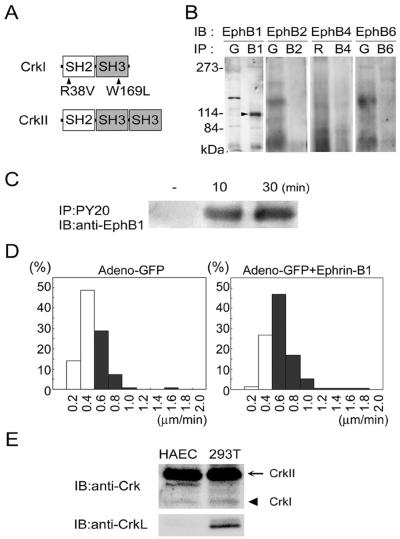
Figure 1.
HAECs express EphB1 and respond to ephrin-B1. (A) Schematic illustration of CrkI, CrkII, and mutants. Arrowheads indicate the mutation in CrkI-R38V (Arg at 38 replaced with Val) and CrkI-W169L (Trp at 169 replaced with Leu). (B) HAEC lysates were incubated with antibodies as indicated (IP), followed by SDS-PAGE, and immunoblotting with antibodies as indicated (IB). The position of EphB1 is indicated by the arrowhead. For the immunoprecipitation, species-matched normal serum, goat (G), or rabbit (R) was used. B1, anti-EphB1; B2, anti-EphB2; B4, anti-EphB4; and B6, anti-EphB6. (C) Lysates of HAECs stimulated with preclustered ephrin-B1/Fc for time as indicated at the top were immunoprecipitated with anti-phosphotyrosine antibody (PY20). Precipitates were subjected to SDS-PAGE and immuboblotted with anti-EphB1. (D) Ephrin-B1–induced HAECs migration was examined for the migratory velocity of GFP-expressing HAECs. HAECs (150) infected with a recombinant adenovirus expressing GFP were left unstimulated (left) or were stimulated (right) with preclustered 1 μg/ml soluble ephrin-B1/Fc. Cells were tracked by MetaMorph 4.6 software and analyzed for their mean migratory velocity. The details of the analysis are described in MATERIALS AND METHODS. Cells moving >0.6 μm/min are shown in the black column. (E) Cell lysates of 293T cells and HAECs were subjected to SDS-PAGE and immunoblotted with antibodies as indicated at the left. Arrow and arrowhead indicated CrkII and CrkI, respectively.
Involvement of Crk in cellular migration has been extensively studied using Caenorhabditis elegans, Drosophila melanogaster, and mammalian cells. We have shown that a Crk SH3-binding protein, DOCK180, induces membrane ruffling via Rac1 (Kiyokawa et al., 1998). In D. melanogaster, nonfunctional mutation in myoblast city, a homolog of DOCK180, results in the failure of dorsal closure due to abnormal cell migration (Erickson et al., 1997). Ced-2, Ced-5, and Ced-10 of C. elegans have been isolated as genes essential for the phagocytosis of apoptotic bodies and subsequently identified as homologues of mammalian crk, DOCK180, and rac, respectively (Wu and Horvitz, 1998; Reddien and Horvitz, 2000). Therefore, the Crk-DOCK180-Rac1 pathway is conserved from nematodes to humans and plays a critical role in cell migration and phagocytosis.
Another SH3-binding partner, C3G, has been shown to participate in cell adhesion to extracellular matrix (ECM) via Rap1 activation (Ohba et al., 2001; Li et al., 2002). We have demonstrated that C3G-deficient mouse embryonic fibroblasts show impaired cell adhesion and delayed cell spreading (Ohba et al., 2001). Li et al. (2002) have shown that Src, Crk, C3G, and Rap1 regulate the stability of focal adhesions.
Cell migration requires membrane extension, assembly of cell contacts to ECM in the leading edge, destabilization of those in the rear of the cell, and increased locomotive forces (Lauffenburger and Horwitz, 1996). Membrane extension, which includes filopodia and lamellipodia, is regulated by Rho-family GTPases (Bar-Sagi and Hall, 2000). Cell contacts to ECM are largely classified into three subgroups by their morphology. Focal complexes are dot-like structures at the leading edge of the lamellipodia. Focal adhesions are adhesions larger than focal complexes mostly found in the peripheral region of cells. Fibrillar adhesions are elongated structures located predominantly in the central region of cells (Rottner et al., 1999; Geiger et al., 2001). The latter two structures are connected to actin stress fibers.
In this study, we investigated the role of Crk in ephrin-B1–induced endothelial cell migration. We demonstrate that the ephrin-B1–induced membrane ruffling is mediated by the Crk-Rac1 pathway and that the assembly and maturation of focal complexes is regulated by the Crk-Rap1 pathway.
MATERIALS AND METHODS
Reagents and Antibodies
Recombinant soluble mouse ephrin-B1-human Fc chimeric protein (ephrin-B1/Fc) (Stein et al., 1996) was purchased from R & D Systems (Minneapolis, MN). Ephrin-B1/Fc was preclusterd by goat anti-human Fc before the stimulation and used at 1 μg/ml throughout this study. Protein A- and G-Sepharose were from Calbiochem (La Jolla, CA). Type-I collagen used for coating glass-base dishes was purchased from Nitta Gelatin (Osaka, Japan). Anti-EphB1, anti-EphB2, anti-EphB4, anti-EphB6, and anti-CrkL were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-human Fc was from R & D Systems; antipaxillin was from Zymed Laboratories (South San Francisco, CA); anti-β-tubulin and anti-actin were from Sigma-Aldrich (St. Louis, MO); and anti-phosphotyrosine (PY20), anti-Crk, and anti-p130Cas were from Transduction Laboratories (Lexington, KY).
Plasmids
pCA-DsRed-CrkI was derived from pCAGGS eukaryotic expression vector and expressed DsRed-tagged CrkI (Niwa et al., 1991). pIRM21 and pCXN2-FLAG-IRES (internal ribosomal entry site)-enhanced green fluorescent protein (EGFP) (Ichiba et al., 1999) were expression vectors derived from pCAGGS and contained internal ribosome entry site (IRES) and the coding region of dsFP593 and EGFP, respectively, at the 3′ side of the multiple cloning site. Synthesized cDNA encoding dsFP593 (Fradkov et al., 2000) was obtained from A. Miyawaki (RIKEN, Wako-shi, Japan). The DNA fragments encoding CrkI substituted at Arg38 for Val in the SH2 domain, or CrkI substituted at Trp169 for Leu in the SH3 domain, hereafter R38V or W169L, respectively, were amplified by polymerase chain reaction and subcloned into pIRM21 or pCXN2-FLAG-IRES-EGFP, respectively. pCXN2-FLAG-Rac1N17-IRES-EGFP expressed both FLAG-tagged Rac1 at Ser17 substituted for Asn and EGFP. pCXN2-FLAG-rap1GAPII-IRES-EGFP expressed both FLAG-tagged rap1GAPII and EGFP. pEGFP-actin was purchased from CLONTECH (Palo Alto, CA). All of the DNA fragments amplified by polymerase chain reaction were ligated into pCR-BluntII-TOPO vector (Invitrogen, Carlsbad, CA), and the sequence was confirmed with an ABI Prism 3700 (Applied Biosystems Japan, Tokyo, Japan).
Virus
We have previously developed the in vivo Ras or Rap1 activation monitoring probes Raichu-Ras or Raichu-Rap1, respectively. Briefly, Raichu-Ras consists of yellow fluorescent protein (YFP), H-Ras, Ras-binding domain of Raf, cyan fluorescent protein (CFP), and the CAAX box of Ki-Ras, whereas Ras is replaced by Rap1 in Raichu-Rap1 (Mochizuki et al., 2001) (Figure 7A). We produced recombinant adenoviruses, Adeno-Raichu-Ras, and -Rap1 by Adeno-X system according to the manufacturer's protocol (CLONTECH). DNA encoding Raichu-Ras was ligated into pShuttle vector. An expression cassette was cleaved out from pShuttle-derived vector and ligated into pAdeno-X. The adenovirus genome was cut out from pAdeno-X-derived vector and transfected into human embryonic kidney (HEK)293 cells. A recombinant adenovirus expressing Raichu-Ras was produced during 2 wk. Adenovirus expressing Raichu-Rap1 was produced in a similar manner to Raichu-Ras. A recombinant adenovirus for green fluorescent protein (GFP) expression was obtained from H. Kurose (Kyushu University, Kyushu, Japan). Adenovirus expressing CrkI-W169L and EGFP, Adeno-CrkI-W169L, was produced as reported previously (Endo et al., 2002). Briefly, DNA encoding CrkI-W169L, IRES, and EGFP from pCXN2-FLAG-CrkI-W169L-IRES-EGFP was ligated into pShuttle. A recombinant virus was produced in a similar manner to Adeno-Raichu-Ras. pIRM21-CrkI-R38V was used to produce Adeno-CrkI-R38V expressing CrkI-R38V and dsFP593.
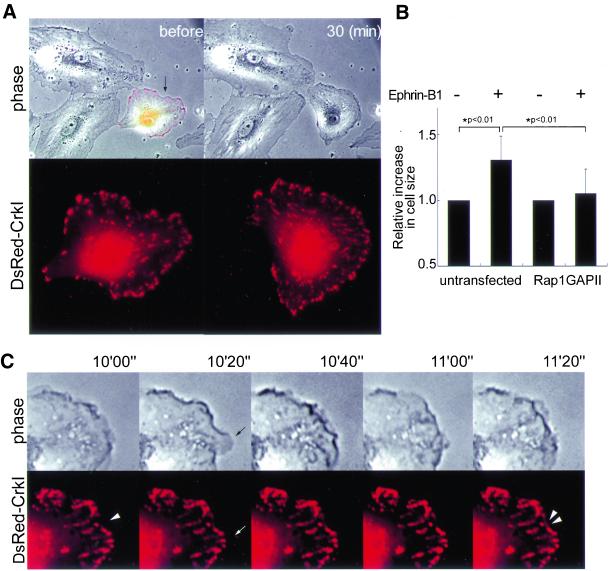
Figure 7.
Inhibition of Rap1 by overexpression of rap1GAPII suppresses focal complex assembly and maturation. (A) HAECs transfected with pCXN2-FLAG-rap1GAPII-IRES-EGFP and pCA-DsRed-CrkI were stimulated with preclustered ephrin-B1/Fc. Epifluorescent images of EGFP and DsRed were overlaid on the phase-contrast image before the stimulation (top left). Representative images before and 30 min after ephrin-B1 stimulation are shown. DsRed image of the cell expressing both rap1GAPII and DsRed-CrkI (indicated by arrow) was enlarged to show focal complex formation (bottom). (B) Effect of rap1GAPII on membrane extension was analyzed as described in the legend of Figure 4. A significant difference between two groups determined by t test is indicated with an asterisk (P < 0.01). (C) A series of closer views of phase contrast and DsRed images of the cell shown in A. Images were obtained at the time after stimulation, as indicated at the top. The arrows indicate ruffled membrane without focal complex formation. Note labile focal complexes indicated by the double arrowheads. The single arrowhead indicates where labile focal complex are generated (also see Video 6B).
Cells, Transfection, and Infection
Human aortic endothelial cells (HAECs) were purchased from Cascade Biologics (Portland, OR). HEK293 cells were from American Type Culture Collection (Manassas, VA). 293T cells were provided by B.J. Mayer (University of Connecticut, Storrs, CT). HAECs were maintained in HuMedia-EG2 (Kurabo, Kurashiki, Japan) supplemented with a growth additive set containing 2% fetal bovine serum (FBS), 10 ng/ml human epidermal growth factor, 1 μg/ml hydrocortisone, 50 μg/ml gentamicin, 50 ng/ml amphotericin B, 5 ng/ml human fibroblast growth factor, and 10 μg/ml heparin. HAECs were used before passage 7. HEK293 cells and 293T cells were cultured in DMEM (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine. HEK293 cells were transfected by calcium phosphate. HAECs cultured on a collagen-coated 35-mm-diameter glass-base dish (Asahi Techno Glass, Tokyo, Japan) were transfected with 3 μg of plasmid DNA by using LipofectAMINE PLUS reagent (Invitrogen) or infected with adenovirus at the appropriate multiplicity of infection for the time periods as indicated in the figure legends. HAECs were starved for >8 h before the stimulation in DMEM/F-12 (Invitrogen) without phenol red supplemented with 2 mM l-glutamine, 10 mM HEPES, 1.2 g/l NaHCO3, and 0.5% bovine serum albumin fraction V.
RNA interference
Small interfering RNAs (siRNA) corresponding to the bases 264–284 of human CrkII coding sequence 5′-AAUAGGAGAUCAAGAGUUUGA-3′ and 5′-UCAAACUCUUGTUCUCCUTUU-3′ (Dharmacon Research, Lafayette, CO) were annealed and transfected into 293T cells or HAECs by using OligofectAMINE (Invitrogen).
Immunoprecipitation, Immunoblotting, and Cell Staining
HAECs were washed with Tris-buffered saline containing 1 mM Na3VO4 and lysed in lysis buffer (150 mM NaCl, 20 mM Tris hydrocloride, pH 7.5, 1.5 mM MgCl2, 1 mM Na3VO4, 1% Triton X-100, 10 mM NaF, and protease inhibitor cocktail; Roche Applied Science, Basel, Switzerland). Lysates were precleard by centrifugation at 15,000 × g for 10 min, followed by immunoprecipitation by using antibodies indicated in the figure and protein A- or G-Sepharose (Calbiochem). Immunoprecipitates were subjected to SDS-PAGE and immunoblotting with antibodies as indicated in the figure. Proteins reacting with primary antibodies were visualized by an enhanced chemiluminescence system (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom) for detecting peroxidase-conjugated secondary antibodies and analyzed with an LAS-1000 system (Fuji Film, Tokyo, Japan). Quantitative analyses of immunoblots were performed using Image Gauge version 3.4X software included in LAS-1000 system. HAECs transfected with pCA-DsRed-CrkI cultured on a collagen-coated glass-base dish were stimulated with preclustered ephrin-B1/Fc for 20 min. Cells washed with phosphate-buffered saline were fixed by 4% paraformaldehyde at room temperature, followed by permeabilization with 0.1% Triton X-100. Permeabilized cells were incubated with anti-p130Cas or anti-paxillin antibody. Proteins reacting with antibodies were detected with Alexa 488 goat anti-mouse IgG (Molecular Probes, Eugene, OR). Confocal images for DsRed-CrkI, p130Cas, and paxillin were obtained by a BX50WI microscope controlled by Fluoview (Olympus, Tokyo, Japan).
Time-Lapse Imaging
HAECs transfected with plasmids expressing fluorescence-tagged proteins or IRES-driven fluorescence or infected with adenovirus expressing IRES-driven fluorescence were imaged on an IX-70 inverted microscope (Olympus). The microscope with a 75-W xenon arc lamp was equipped with a cooled charge-coupled device camera, CoolSNAP-HQ (Roper Scientific, Trenton, NJ), and two filter changers, controlled by MetaMorph 4.6 software (Roper Scientific). Both the EGFP image and the DsRed/dsFP593 image were obtained through an XF2043 dichroic filter (Omega Optical, Brattleboro, VT) and a set of an S484/15 excitation filter and an S515/30 emission filter (Chroma Technology, Brattleboro, VT) for EGFP and a set of an S555/25 excitation filter and an S630/60 emission filter for DsRed and dsFP593. To monitor cell shape and localization of fluorescence-tagged proteins, a phase-contrast image and a fluorescence image were obtained every 20 s. A series of time-lapse images were converted to video format by using MetaMorph 4.6 software.
Cell Motility Analysis and Quantitative Analysis of Membrane Ruffling
The motility of HAECs was analyzed as described previously (Ohba et al., 2001). Briefly, HAECs infected with recombinant GFP expressing adenovirus were spread on a collagen-coated glass-base dish and cultured in DMEM supplemented with 1% FBS for 8 h before exposure to preclustered ephrin-B1/Fc. GFP-expressing HAECs were tracked for 6 h after the stimulation by using a fluorescent microscopy in a series of time-lapse images collected using MetaMorph 4.6 software. The distance between the point where HAECs attached and the end point where HAECs moved was measured by tracing cells. The mean velocity was obtained by the distance divided by the period during which cells were tracked. The migration velocity of HAECs was analyzed by a cell-tracking system included in MetaMorph 4.6. For quantitative analysis of membrane ruffling, uninfected HAECs and those infected with either Adeno-CrkI-R38V or Adeno-CrkI-W169L were cocultured on the same dish, starved for 8 h, and exposed to preclustered ephrin-B1/Fc. A phase-contrast image and a fluorescence image were recorded first, and a sequential phase-contrast image was obtained every 20 s. A series of time-lapse images were converted to a video by MetaMorph 4.6. Ephrin-B1–induced membrane extension reflecting the membrane ruffling was quantified by measuring the cell size before and after ephrin-B1/Fc stimulation. The cell size was analyzed by a region measurement tool included in MetaMorph 4.6. HAECs transfected with either pCXN2-FLAG-Rac1N17-IRES-EGFP or pCXN2-FLAG-rap1GAPII-IRES-EGFP were analyzed for ephrin-B1–induced membrane extension similarly to those infected with Adeno-CrkI mutants.
Fluorescent Resonance Energy Transfer (FRET) Imaging
HAECs cultured on a collagen-coated 35-mm-diameter glass-base dish were infected with adenovirus as indicated in the figure legends and stimulated with preclustered ephrin-B1/Fc. Cells were imaged on an IX-70 inverted microscope (Olympus) in a method similar to time-lapse imaging. CFP and YFP images were obtained through a filter set with a XF1071 excitation filter, a XF2034 dichroic mirror, and a XF3075 emission filter for CFP and a XF3079 for YFP (Omega Optical), respectively. The emission ratio of YFP/CFP and the intensity of CFP were used for imaging of FRET in the intensity modulated display mode controlled by MetaMorph 4.6. A series of time-lapse images obtained every 20 s were converted to a video by using MetaMorph 4.6.
RESULTS
Ephrin-B1 Accelerates the Motility of HAECs
We first examined the expression of EphB-family receptors in HAECs by using antibodies against EphB1, EphB2, EphB4, and EphB6 (Figure 1B). Among them, only EphB1 was detected in HAECs (Figure 1B). To test whether EphB1 was indeed activated upon ephrin-B1, a ligand for EphB1, HAECs were stimulated with preclustered ephrin-B1/Fc. Ephrin-B1-dependent phosphorylation of EphB1 was observed for 30 min (Figure 1C). We then proceeded to test the effect of ephrin-B1 on the motility of HAECs. For the migration assay, HAECs were marked with GFP-carrying adenovirus and stimulated with preclustered ephrin-B1/Fc. Cells were tracked under a fluorescent time-lapse microscope, and the velocity of each cell was calculated. Approximately 35% of unstimulated HAECs moved >0.6 mm/min as shown in the black column (Figure 1D, left), whereas ∼70% of those stimulated with ephrin-B1 did similarly (Figure 1D, right), demonstrating that ephrin-B1 significantly accelerated cell migration. HAECs stimulated with preclustered Fc did not show any acceleration in the cell migration (our unpublished data). In the following experiments, the involvement of Crk in the ephrinB1-induced signaling was examined. Therefore, we examined the expression of CrkII and CrkI, which are alternatively spliced products of Crk gene, and that of CrkL, a Crk-related protein consisting of a SH2 and two SH3 domains similar to CrkII. CrkII was predominantly expressed in 293T cells and HAECs, although CrkI was detected in both cells, whereas CrkL was detected in 293T cells, but not in HAECs (Figure 1E).
Crk Is Translocated to the Nascent Focal Complexes upon Ephrin-B1 Stimulation in HAECs
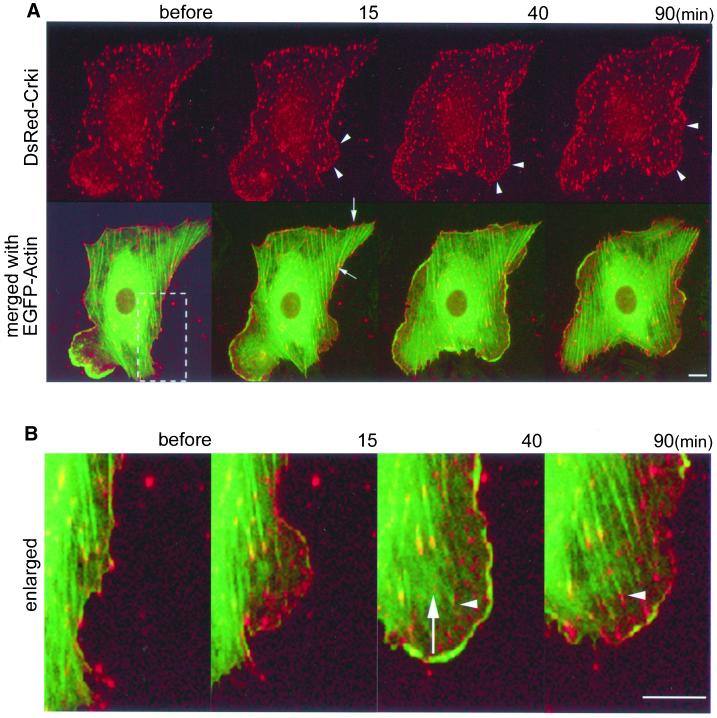
In ephrin-B1–stimulated cells, prominent membrane ruffling always preceded the cell body migration. Therefore, in the following experiments, we focused on the role of Crk, which is known to regulate cell migration in fibroblasts, in the ephrin-B1–induced membrane ruffling. Localization of Crk was monitored by the expression of DsRed-tagged CrkI with a time-lapse fluorescent microscope (Figure 2A, top). By monitoring the coexpression of EGFP-actin, we simultaneously observed the organization of actin stress fiber. Before ephrin-B1 stimulation, Crk was localized at focal or fibrillar adhesions both in the center and periphery of the cells. In the overlay images of Crk and actin, actin stress fibers were shown to bridge the accumulated Crk in the center and the periphery of the cells, indicating that Crk was localized to focal and fibrillar adhesions (Figure 2A, bottom, and Video 2A2). On ephrin-B1 stimulation, Crk was translocated to the leading edge of spreading membrane ruffles. The accumulation of Crk in membrane ruffles formed dots smaller than those found in the center of quiescent cells (Figure 2A, top, and Video 2A1). These nascent accumulations of Crk were not yet connected to actin stress fiber, indicating that these were focal complexes (Figure 2B). Next, we observed the course of the nascent focal complexes in consecutive images. Some focal complexes became larger and connected with actin stress fiber, showing the development of focal complexes to focal adhesions (Figure 2B, white arrowhead, and Video 2B). On the other hand, some focal complexes detached from the substratum (Figure 2B, white arrow, and Video 2B) and disappeared or moved toward the center of the cell. These results indicated that Crk was involved in the assembly and maturation of the focal complexes in ephrin-B1–stimulated HAECs.
Figure 2.
CrkI is translocated to nascent focal complexes upon ephrin-B1 stimulation and is involved in the development of focal adhesion. HAECs cultured on a collagen-coated glass-base dish were transfected with pCA-DsRed-CrkI, starved for 8 h, and stimulated with preclustered 1 μg/ml soluble ephrin-B1/Fc. EGFP-actin and DsRed-CrkI were imaged on an IX-70 inverted microscope (Olympus). A series of DsRed and EGFP images were collected by MetaMorph 4.6 software. Before, before the stimulation; 15, 40, and 90 min, time for stimulation. Bar, 10 μm. (A) Arrowheads indicate typical accumulation of DsRed-CrkI to nascent focal complexes (top). The GFP image of the same cell transfected with pEGFP-actin was merged with the DsRed-CrkI image (bottom). Arrows indicate an example of focal adhesions connecting actin stress fiber (also see Video 2A2). (B) The boxed region in the lower panel of A was enlarged. An arrow indicates a focal complex detached from the substratum and floating in membrane ruffles. The arrowhead indicates the development of a focal complex to a focal adhesion (also see Video 2B).
Crk Preferentially Colocalizes with p130Cas at the Focal Complexes in HAECs upon Ephrin-B1 Stimulation
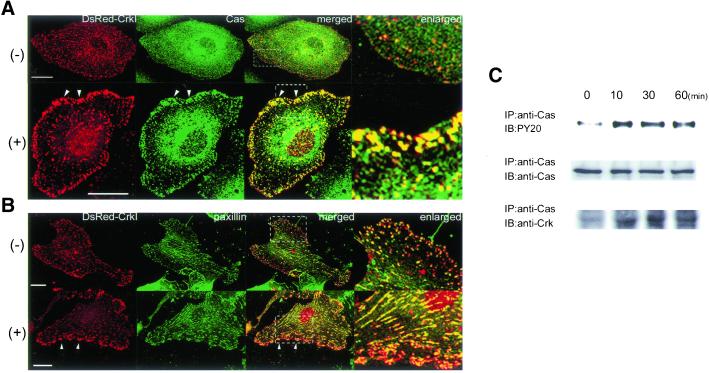
The two major SH2-binding partners of Crk in cell adhesion have been identified as p130Cas and paxillin (Schaller and Parsons, 1995; Vuori et al., 1996). To understand the role of Crk at the focal complexes, we examined the colocalization of Crk and the two SH2-binding proteins of Crk, p130Cas and paxillin (Figure 3). In unstimulated HAECs, p130Cas was condensed at the perinuclear region. Colocalization of Crk with p130Cas was not obvious. After ephrin-B1 stimulation, p130Cas was accumulated at the focal complexes, where DsRed-CrkI was also translocated (Figure 3A). We next examined the localization of paxillin (Figure 3B). In unstimulated cells, paxillin was localized at the focal adhesions and fibrillar adhesions. Colocalization of Crk with paxillin was observed throughout the cell, but its presence was more profound at the peripheral focal adhesion. The ephrin-B1 stimulation induced the accumulation of Crk at the focal complex and peripheral focal adhesion; however, paxillin was not recruited to these nascent focal adhesions. These results suggested that the accumulation of phosphorylated p130Cas recruited Crk to the focal complex in ephrin-B1–stimulated HAECs. We further confirmed that ephrinB1-induced p130Cas phosphorylation, which is indispensable for its association with Crk (Kiyokawa et al., 1997). HAECs were stimulated with preclustered ephrin-B1/Fc for time period indicated at the top (Figure 3C). p130Cas was phosphorylated (Figure 3C, top) and associated with endogeneous Crk (Figure 3C, bottom) upon ephrin-B1 stimulation.
Figure 3.
CrkI colocalizes with p130Cas at the focal complexes upon ephrin-B1 stimulation. (A) HAECs expressing DsRed-CrkI plated on the glass-base dish were starved for 8 h and stimulated with 1 μg/ml preclustered ephrin-B1/Fc for 20 min. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated with antip130Cas antibody. Immunoreactive protein was visualized by Alexa 488 goat anti-mouse IgG. Confocal images for DsRed-CrkI and p130Cas were obtained by a BX50WI microscope controlled by Fluoview (Olympus). An image for p130Cas (green) and an image for DsRed-CrkI (red) were superimposed (merged). Arrowheads indicate focal complexes. The boxed region was enlarged and is shown in the right panel (enlarged). Note that DsRed-CrkI colocalizes with p130Cas at the focal complex after the stimulation, as indicated by the yellow spots. (B) Similar experiments were performed with anti-paxillin antibody. Arrowheads indicate focal complexes. The boxed region was enlarged. Note that DsRed-CrkI (red) and paxillin (green) colocalizes mostly at the fibrillar adhesions and focal adhesions of the center of the cell. −, before ephrin-B1 stimulation; +, 20 min after the stimulation; Bar, 20 μm. (C) Lysates of HAECs stimulated with preclustered ephrin-B1/Fc for time period as indicated at the top were immunoprecipitated as indicated at the left. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with antibodies as indicated at the left.
Crk Is Responsible for Ephrin-B1–induced Membrane Ruffling in HAECs
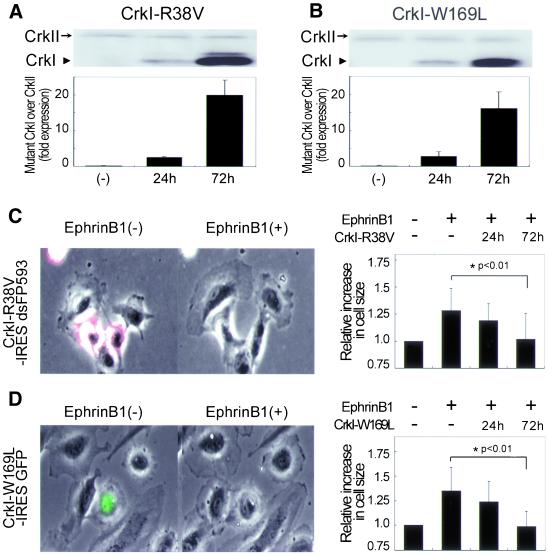
To assess the physiological role of Crk in the ephrin-B1–induced membrane ruffling, we used the adenovirus-mediated overexpression of dominant negative mutants of Crk (Figure 1A). CrkI-R38V consists of a nonfunctioning SH2 and an intact SH3, thus serving to sequester SH3-binding proteins. Similarly, CrkI-W169L, which consists of an intact SH2 and a nonfunctioning SH3, blocks the phosphotyrosine residues. We have previously shown that CrkI-W169L and CrkI-R38V work as dominant negative mutants for CrkI/II (Ichiba et al., 1997, 1999). The expression of dominant negative CrkI in HAECs infected with adenovirus carrying CrkI mutant for 24 h was comparable to the endogeneous CrkII. After 72-h infection, HAECs expressed dominant negative CrkI ∼16–20 times more than endogeneous CrkII (Figure 4, A and B). We quantified the inhibitory effect of CrkI-R38V on membrane extension by measuring the cell size upon ephrin-B1 stimulation (Figure 4C), because membrane ruffling is associated with membrane extension and/or spreading (Lauffenburger and Horwitz, 1996). Uninfected HAECs without fluorescence showed remarkable membrane extension, whereas those infected with Adeno-CrkI-R38V for 72 h and marked by IRES-driven dsFP593 did not increase their cell size (Figure 4C and Video 4A). HAECs infected for only 24 h with Adeno-CrkI-R38V responded to ephrin-B1 stimulation (Figure 4C, right). Thus, the greater the expression of CrkI-R38V, the more ephrin-B1–induced membrane extension was inhibited by CrkI-R38V. The similar inhibitory effect of CrkI-W169L on ephrin-B1–induced membrane extension was observed with CrkI-R38V (Figure 4D and Video 4B). HAECs infected with Adeno-GFP used as a negative control responded to ephrin-B1 and showed membrane ruffling, as did uninfected HAECs (our unpublished data). These results indicated that Crk SH2-binding proteins and Crk SH3-binding effectors were involved in ephrin-B1–induced membrane ruffling in HAECs.
Figure 4.
Crk is required for ephrin-B1–induced membrane ruffling. (A) Endogeneous CrkII and mutants of CrkI expression in HAECs infected with Adeno-CrkI-R38V for time as indicated at bottom were analyzed by immunoblotting with anti-Crk antibody (top). The quantitative analysis was performed by comparing the amount of CrkI-R38V with endogenous CrkII (bottom). (B) HAECs infected with Adeno-CrkI-W169L were similarly analyzed to CrkI-R38V. (C) Uninfected HAECs without fluorescence and HAECs infected for 72 h with Adeno-CrkI-R38V and marked by dsFP593 were cocultured, starved for 8 h, and stimulated with preclustered ephrin-B1/Fc for 30 min. Cells before and 30 min after the stimulation were imaged on an IX-70 inverted microscope (Olympus) (left). A series of time-lapse images were converted to a video. Quantitative analysis of ephrin-B1–induced membrane extension was performed by measuring the cell size of uninfected cells, those infected for 24 h, and those infected for 72 h before and after ephrin-B1 stimulation by using MetaMorph 4.6 software (right). More than 20 cells were examined in each experiment, and the results from three independent experiments have been summarized. The data are expressed as averages with the SD. A significant difference between two groups determined by t test is indicated with an asterisk (P < 0.01). (D) HAECs infected with Adeno-CrkI-W169L were similarly analyzed as shown in C. HAECs infected with Adeno-CrkI-W169L were marked by EGFP.
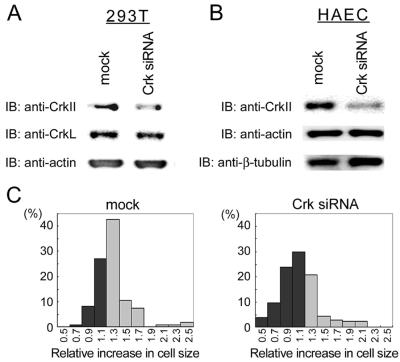
To further confirm the requirement of Crk in ephrin-B1–induced membrane ruffling, we used RNA interference. Recently, RNA interference has become an effective strategy to knock down a target protein, not only in C. elegans but also in mammalian cells (Harborth et al., 2001). 293T cells transfected with Crk siRNA for 72 h were depleted of Crk. Crk siRNA did not affect CrkL or actin, which were examined for the specificity of Crk siRNA (Figure 5A). Crk siRNA did inhibit the expression of CrkII in HAECs without affecting actin and β-tubulin expression (Figure 5B). The effect of Crk siRNA on ephrin-B1–induced membrane extension was examined in HAECs transfected with Crk siRNA. Because we could not distinguish HAECs that were depleted of Crk by siRNA from those untransfected, the cell number and the increase in cell size of Crk siRNA-transfected cells were compared with mock-transfected cells after ephrin-B1 stimulation. Approximately 70% of mock-treated HAECs increased their cell size >1.3 times in response to ephrin-B1 stimulation (Figure 5C, left), whereas only 30% of Crk siRNA-transfected HAECs increased (Figure 5C, right). Both the overexpression of dominant negative Crk and the depletion of Crk indicated that Crk was required for ephrin-B1–induced membrane ruffling in HAECs.
Figure 5.
Depletion of Crk leads to the inhibition of ephrin-B1–induced membrane extension. (A) Mock-treated HAECs and HAECs transfected with 200 nM Crk siRNA by using OligofectAMINE for 72 h were lysed, subjected to SDS-PAGE, and immunoblotted with antibodies as indicated at the left. (B) HAECs transfected with 200 nM Crk siRNA were similarly analyzed to 293T cells. (C) Mock-treated HAECs (left) and HAECs transfected with 200 nM Crk siRNA for 72 h were analyzed for their membrane extension in response to ephrin-B1. The increase in cell size of 100 HAECs before and after ephrin-B1 stimulation was calculated as described in the legend of Figure 4.
Rac1 Activation Is Required for Ephrin-B1–induced Membrane Ruffling and Subsequent Focal Complex Assembly
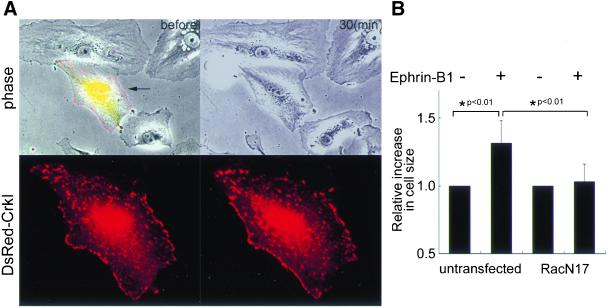
To examine whether Rac1, a downstream molecule of the Crk, is required for ephrin-B1–induced membrane ruffling, we transfected HAECs with dominant negative Rac1 (Rac1N17). Untransfected HAECs without fluorescence showed remarkable membrane ruffling, whereas HAEC expressing Rac1N17 marked by dsFP593 showed impaired membrane ruffling (Figure 6A and Video 6A). The inhibitory effect of Rac1N17 on membrane ruffling was again confirmed quantitatively by measuring the cell size (Figure 6B). Interestingly, we noticed that Rac1N17 also impaired the focal complex assembly shown as the accumulation of Crk (Figure 6A, bottom and Video 6B), although this may be simply because the membrane extension must precede the focal complex assembly.
Figure 6.
Rac1 activation is essential for ephrin-B1–induced membrane ruffling. (A) HAECs transfected with pCXN2-FLAG-Rac1N17-IRES-EGFP and pCA-DsRed-CrkI were stimulated with preclustered ephrin-B1/Fc. Epifluorescent images of both EGFP and DsRed were overlaid on phase-contrast view before the stimulation (top left). The representative phase-contrast images before and after stimulation are shown (top). DsRed image of the cell expressing Rac1N17 and DsRed-CrkI indicated by the arrow was enlarged before (bottom left) and after (bottom right) the stimulation. (B) Effect of Rac1N17 on ephrin-B1–induced membrane extension was analyzed as in Figure 4. A significant difference between two groups determined by t test is indicated with an asterisk (P < 0.01).
Inactivation of Rap1 Impairs Ephrin-B1–induced Focal Complex Formation
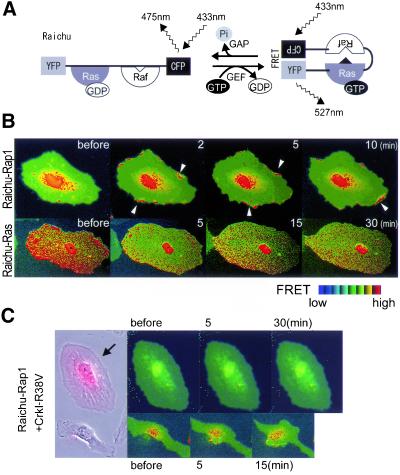
Next, we examined the role of Rap1, a downstream molecule of Crk via C3G, in ephrin-B1–induced membrane ruffling. For this, we transfected HAECs with rap1GAPII, which inactivates Rap1 by accelerating the hydrolysis of GTP (Mochizuki et al., 1999). It was noteworthy that the HAECs expressing rap1GAPII marked by dsFP593 was smaller than the untransfected cells, which were without fluorescence and accentuated in the prominent peripheral focal adhesions (Figure 7A). After ephrin-B1 stimulation, membranes began ruffling in the rap1GAPII-expressing cells; however, the membrane did not extend as efficiently as did the membranes of control cells, which were without fluorescence (Figure 7A and Video 7A). We confirmed this observation by quantifying cell size (Figure 7B). This observation clearly dissociated the effect of the Crk-Rap1 pathway from that of the Crk-Rac1 pathway.
To clarify the difference between the two pathways, we analyzed the video images of rap1GAPII-expressing HAECs upon ephrin-B1 stimulation (Figure 7C and Video 7B). After stimulation, smaller and more labile focal complexes were generated at the peripheral region. Some peripheral focal adhesions were observed to detach from the substratum and move toward the center of the cell (Video 7B). This observation suggested that Rap1 was required for the stabilization of focal complexes, which followed the membrane spreading in normal HAECs.
Rap1 Is Activated at the Membrane Ruffling upon Ephrin-B1 Stimulation
Previously, we showed that epidermal growth factor stimulated Ras at the periphery and Rap1 at the center of cells. Because the requirement of Rap1 in the membrane spreading seemed to contradict this previous observation, we examined the spatiotemporal pattern of the activation of Rap1 and Ras. The principle of the monitors for Ras-family G proteins named Raichu is illustrated in Figure 8A. These probes consisted of YFP, Ras, Raf, and CFP, and the CAAX box of Ki-Ras. In its inactive form, only fluorescence from CFP can be detected. On the activation of Ras and succeeding binding to Raf, excited CFP transfers its energy to YFP, resulting in the emanation of yellow fluorescence. By measuring the fluorescence ratio of YFP/CFP, we can measure the activity of Ras.
Figure 8.
Activation of Rap1 at the membrane ruffling and inactivation of Ras upon ephrin-B1 stimulation in HAECs. (A) Schematic representation of Raichu-Ras. FRET efficiency in a manner dependent on the bound guanine nucleotides. 433 nm, excitation for CFP; 475 nm, emission from CFP; 527 nm, emission from YFP due to FRET; Ras, H-Ras; Raf, Ras-binding domain of Raf. (B) HAECs infected with Adeno-Raichu-Rap1 (top) or Raichu-Ras (bottom) were starved for 8 h and stimulated with 1 μg/ml preclustered ephrin-B1/Fc for the time indicated at the top. Arrowheads indicate membrane ruffles. The red hue and blue hue indicate an increase (high) and a decrease (low) in the ratio of YFP to CFP, respectively, reflecting FRET efficiency. (C) HAECs infected with Adeno-Raichu-Rap1 for 48 h and Adeno-CrkI-R38V for 72 h (arrow) and those infected only with Adeno-Raichu-Rap1 were cocultured, straved, and stimulated with preclustered ephrin-B1/Fc for time period as indicated at the top. Phase contrast image before the stimulation was overlaid onto the image for dsFP593 to distinguish HAECs infected with Adeno-CrkI-R38V from those infected only with Adeno-Raichu-Rap1. FRET images were obtained every 20 s.
HAECs were infected with Adeno-Raichu-Rap1 or Ras. The basal Rap1 activity was high at the perinuclear region, as we have previously reported in COS-1 cells (Figure 8B, top, and Video 8). On ephrin-B1 stimulation, Rap1 was rapidly activated at the membrane ruffling and it continued for ∼15 min. In contrast to Rap1, Ras activity was decreased by ephrin-B1 stimulation. The Ras activity reached its lowest level within 5 min after the stimulation. The inactivation of Ras continued for >30 min (Figure 8B, bottom). These results showed for the first time Rap1 activation at the membrane ruffling. Because Rap1 is known to antagonize Ras-induced extracellular signal-regulated kinase activation, this anticorrelation of the activity may be another example of the anti-Ras function of Rap1. In addition, we tested whether the Rap1 activation upon ephrin-B1 stimulation was dependent on Crk. HAECs expressing Raichu-Rap1 showed Rap1 activation at the membrane ruffling as shown in Figure 8B, whereas HAECs expressing Raichu-Rap1 and CrkI-R38V, which sequestered Crk SH3-binding proteins, did not exhibit any Rap1 activation and membrane extension (Figure 8C). CrkI-R38V did not inhibit ephrin-B1–induced inactivation of Ras (our unpublished data). These results indicated that Crk was required for the activation of Rap1 upon ephrin-B1 stimulation in HAECs.
DISCUSSION
Eph/ephrin-induced reciprocal signaling pathway has been implicated in the repulsive force produced by the cells expressing EphB and those expressing ephrin on their cell surface. This reciprocal signal from either Eph or ephrin plays critical roles in embryogenesis, rhombomere segmentation, axonal guidance, neural crest cell migration, and vasculogenesis (Holder and Klein, 1999). Furthermore, in angiogenesis, sprouting and subsequent migration of endothelial cells is thought to be promoted by ephrin/Eph engagement-dependent repulsive force between endothelial cells and/or their surrounding mesenchymal cells. However, how the repulsion mechanism is promoted in vascular endothelial cells remains elusive.
We have shown herein that the Rac1 activation is required for ephrin-B1–induced initial membrane spreading, based on the finding that the dominant negative form of Rac1 suppressed the initial membrane spreading (Figure 6). It has been demonstrated that a Crk-binding protein, DOCK180, can bind to and activate Rac1 (Kiyokawa et al., 1998). Cheresh et al. (1999) showed that the Crk-DOCK180-Rac1 pathway plays an essential role in the cell migration of fibroblasts. The requirement of Crk for Rac1 activation, as assessed by initial membrane spreading, is evidenced by the findings that both the overexpression of CrkI-R38V and the depletion of Crk by siRNA inhibited the membrane extension upon ephrin-B1 stimulation (Figures 4C and 5). Thus, ephrin-B1 probably activates Rac1 via Crk-DOCK180.
Previous reports have demonstrated that Rap1 is required for integrin-mediated cell adhesion (Caron et al., 2000; Reedquist et al., 2000) and that C3G, another Crk-binding protein, mediates Rap1 activation in this process (Ohba et al., 2001). In this report, we have shown that Rap1 activation is essential for the assembly and maturation of focal complexes in HAECs stimulated with ephrin-B1. Li et al. (2002) have recently shown that the C3G-activated Rap1 stabilizes focal adhesions. Thus, the attachment of the ruffled membrane to ECM via integrin may require Rap1 activation. This notion is supported by the fact that overexpression of rap1GAPII inhibited the focal complex formation (Figure 7 and Video 7B) and also by the finding that Rap1 was indeed activated at the membrane ruffling, upon ephrin-B1 stimulation (Figure 8 and Video 8). In addition, Rap1 activation was inhibited by CrkI-R38V, implicating Crk SH3-binding proteins in ephrin-B1–induced Rap1 activation (Figure 8C). Among Crk SH3-binding molecules, C3G is the only guanine nucleotide exchange factor (GEF) for Rap1 (Kiyokawa et al., 1997). In accordance with this, our results suggest that the Crk-C3G-Rap1 pathway may be involved in the stability and maturation of focal complexes. Further study is needed to identify the effector molecules of Rap1 in the stabilization of focal complexes.
We revealed that Crk colocalized with p130Cas at the nascent focal complexes in ephrin-B1–stimulated HAECs. Crk-p130Cas complex plays a pivotal role in cell migration through DOCK180 and Rac1 (Klemke et al., 1998; Cheresh et al., 1999). Furthermore, the activation of Src kinase promotes p130Cas association with Crk and C3G to activate Rap1 (Xing et al., 2000). Thus, the Crk-p130Cas complex might activate both Rac1 and Rap1 at the focal complexes. Previously, paxillin, another SH2-binding protein of Crk at cell adhesions, was shown to counteract p130Cas in cell migration (Yano et al., 2000). Therefore, transition from Crk-paxillin to Crk-p130Cas upon ephrin-B1 stimulation may be preferable for cell migration.
By the use of FRET technology, we have shown for the first time that Rap1 is activated at membrane ruffles in response to ephrin-B1 stimulation, whereas Ras is inactivated in HAECs.
Previously, we demonstrated that epidermal growth factor induces Ras activation in the membrane ruffling and Rap1 activation in the perinuclear region of COS-1 cells (Mochizuki et al., 2001). Rap1 and Ras antagonize each other in cell transformation and extracellular signal-regulated kinase activation (Kitayama et al., 1989; Cook et al., 1993). This notion is consistent with the different spatial activation of Rap1 and Ras in COS-1 cells and the activation of Rap1 and the inactivation of Ras in ephrin-B1–stimulated HAECs.
Ras is inactivated upon EphA2-activation by ephrin-A1 in human prostatic epithelial cells, and bovine aortic endothelial cells (Miao et al., 2001). p120-RasGAP, a GTPase-activating protein (GAP) for Ras, binds to EphB2 in neuronal NG108 cells and participates in the down-regulation of Ras upon EphB2 activation by ephrin-B1 (Elowe et al., 2001). Thus, p120-RasGAP may be involved in ephrin-B1–induced inactivation of Ras in HAECs.
We have demonstrated that Crk is required for Rap1 activation (Figure 8C). SHEP1 is considered to be a signaling intermediator that binds EphB2 via its SH2 and Rap1 via the GEF domain (Dodelet et al., 1999), although its function has not been clearly demonstrated yet. Chat, a shorter splicing variant of SHEP, is isolated as a p130Cas/HEF1-associated adaptor (Sakakibara and Hattori, 2000). Thus, Chat may connect EphB to p130Cas-Crk and its SH3-binding proteins, C3G, to activate Rap1. Accordingly, stimulation-dependent translocation of GEFs and GAPs regulated by adaptor and/or docking proteins, including Crk, p130Cas, and p120-RasGAP may account for exclusive activation of Ras and Rap1.
In conclusion, we have demonstrated that Crk is required for cooperative activation of Rac1 and Rap1 to promote cell migration by inducing membrane ruffling and stabilizing focal complexes.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Miyawaki for the plasmids; H. Kuorse for the virus; B. J. Mayer for the cells; M. Sone, and H. Shimamoto for their technical assistance. This work was supported in part by grants from the Ministry of Health, Labor and Welfare Foundation of Japan, from the Promotion of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan, from the Human Science Foundation of Japan, and from Yamanouchi Foundation for Research on Metabolic Disorders, as well as by an Astra Zeneca Research Grant.
Abbreviations used:
- CFP
cyan fluorescent protein
- ECM
extracellular matrix
- EGFP
enhanced green fluorescent protein
- FBS
fetal bovine serum
- FRET
fluorescent resonance energy transfer
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- HAEC
human aortic endothelial cell
- IRES
internal ribosomal entry site
- SH
Src homology
- siRNA
small interfering RNA
- YFP
yellow fluorescent protein
Footnotes
Online version of this article contains video material for some figures. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0181. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0181.
REFERENCES
- Bar-Sagi D, Hall A. Ras, and Rho GTPases. a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Birge RB, Fajardo JE, Reichman C, Shoelson SE, Songyang Z, Cantley LC, Hanafusa H. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13:4648–4656. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Leng J, Klemke RL. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J Cell Biol. 1999;146:1107–1116. doi: 10.1083/jcb.146.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SJ, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- Dodelet VC, Pazzagli C, Zisch AH, Hauser CA, Pasquale EB. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J Biol Chem. 1999;274:31941–31946. doi: 10.1074/jbc.274.45.31941. [DOI] [PubMed] [Google Scholar]
- Elowe S, Holland SJ, Kulkarni S, Pawson T. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol. 2001;21:7429–7441. doi: 10.1128/MCB.21.21.7429-7441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A, Nagashima K, Kurose H, Mochizuki S, Matsuda M, Mochizuki N. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endothelial cells via vascular endothelial growth factor receptor and CrkII. J Biol Chem. 2002;277:23747–23754. doi: 10.1074/jbc.M111794200. [DOI] [PubMed] [Google Scholar]
- Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee letter. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkov AF, Chen Y, Ding L, Barsova EV, Matz MV, Lukyanov SA. Novel fluorescent protein from Discosoma coral and its mutants possesses a unique far-red fluorescence. FEBS Lett. 2000;479:127–130. doi: 10.1016/s0014-5793(00)01895-0. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- Hock B, Böhme B, Karn T, Feller S, Rübsamen-Waigmann H, Strebhardt K. Tyrosine-614, the major autophosphorylation site of the receptor tyrosine kinase HEK2, functions as multi-docking site for SH2-domain mediated interactions. Oncogene. 1998;17:255–260. doi: 10.1038/sj.onc.1201907. [DOI] [PubMed] [Google Scholar]
- Holder N, Klein R. Eph receptors and ephrins: effectors of morphogenesis. Development. 1999;126:2033–2044. doi: 10.1242/dev.126.10.2033. [DOI] [PubMed] [Google Scholar]
- Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, Mochizuki N, Matsuda M. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem. 1999;274:14376–14381. doi: 10.1074/jbc.274.20.14376. [DOI] [PubMed] [Google Scholar]
- Ichiba T, Kuraishi Y, Sakai O, Nagata S, Groffen J, Kurata T, Hattori S, Matsuda M. Enhancement of guanine-nucleotide exchange activity of C3G for Rap1 by the expression of Crk, CrkL, and Grb2. J BiolChem. 1997;272:22215–22220. doi: 10.1074/jbc.272.35.22215. [DOI] [PubMed] [Google Scholar]
- Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa E, Mochizuki N, Kurata T, Matsuda M. Role of Crk oncogene product in physiologic signaling. Crit Rev Oncog. 1997;8:329–342. doi: 10.1615/critrevoncog.v8.i4.30. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Li L, Okura M, Imamoto A. Focal adhesions require catalytic activity of SRC family kinases to mediate integrin-matrix adhesion. Mol Cell Biol. 2002;22:1203–1217. doi: 10.1128/MCB.22.4.1203-1217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T, Shibuya M. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol Cell Biol. 1992;12:3482–3489. doi: 10.1128/mcb.12.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei B, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Activation of the ERK/MAPK pathway by an isoform of rap1GAP associated with Gαi. Nature. 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–1068. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ohba Y, et al. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Hattori S. Chat, a Cas/HEF-associated adaptor protein that integrates multiple signaling pathways. J Biol Chem. 2000;275:6404–6410. doi: 10.1074/jbc.275.9.6404. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr, Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Stein E, Cerretti DP, Daniel TO. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–23593. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- Vuori K, Hirai H, Aizawa S, Ruoslahti E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- Xing L, Ge C, Zeltser R, Maskevitch G, Mayer BJ, Alexandropoulos K. c-Src signaling induced by the adapters Sin, and Cas is mediated by Rap1 GTPase. Mol Cell Biol. 2000;20:7363–7377. doi: 10.1128/mcb.20.19.7363-7377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- Yano H, Uchida H, Iwasaki T, Mukai M, Akedo H, Nakamura K, Hashimoto S, Sabe H. Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc Natl Acad Sci USA. 2000;97:9076–9081. doi: 10.1073/pnas.97.16.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Zisch AH, Dodelet VC, Pasquale EB. Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene. 2001;20:3995–4006. doi: 10.1038/sj.onc.1204524. [DOI] [PubMed] [Google Scholar]
- Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E, Pasquale EB. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19:177–187. doi: 10.1038/sj.onc.1203304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.