Abstract
The 260-kDa heterodimeric Gal/GalNAc-specific Lectin (Gal-lectin) of Entamoeba histolytica dissociates under reducing conditions into a heavy (hgl, 170 kDa) and a light subunit (lgl, 35 kDa). We have previously shown that inhibition of expression of the 35-kDa subunit by antisense RNA causes a decrease in virulence. To further understand the role of the light subunit of the Gal-lectin in pathogenesis, amoebae were transfected with plasmids encoding intact, mutated, and truncated forms of the light subunit lgl1 gene. A transfectant in which the 55 N-terminal amino acids of the lgl were removed, overproduced an N-truncated lgl protein (32 kDa), which replaced most of the native 35-kDa lgl in the formation of the Gal-lectin heterodimeric complex and exerted a dominant negative effect. Amoebae transfected with this construct showed a significant decrease in their ability to adhere to and kill mammalian cells as well as in their capacity to form rosettes with and to phagocytose erythrocytes. In addition, immunofluorescence confocal microscopy of this transfectant with anti–Gal-lectin antibodies showed an impaired ability to cap. These results indicate that the light subunit has a role in enabling the clustering of Gal-lectin complexes and that its N-truncation affects this function, which is required for virulence.
INTRODUCTION
The ability of the human intestinal parasite Entamoeba histolytica to destroy its target cells can be separated into two steps: Recognition and adhesion to target cells followed by killing and phagocytosis (Gilchrist and Petri, 1999; Espinosa-Cantellano and Martinez-Palomo, 2000). Among the amoebic molecules that were shown to specifically participate in this process are the following: 1, Galactose/N-Acetyl-Galactosamine Specific Lectin (Gal-lectin; Kain and Ravdin, 1995; Petri and Schnaar, 1995); 2, Cysteine Proteinases (Bruchhaus et al., 1996; Que and Reed, 2000) and; 3, Amoebapores (Leippe et al., 1994; Leippe, 1997; Bracha et al., 1999).
The Gal-lectin is a 260-kDa disulfide-linked heterodimer that upon reduction dissociates into a heavy subunit (hgl) and a light subunit (lgl) (McCoy et al., 1993a). So far, five genes encoding the heavy subunit have been characterized (hgl1-hgl5) as well as three genes encoding the light subunit (lgl1-lgl3) (Ramakrishnan et al., 1996).
The heavy subunit (170 kDa) has in its extracellular part the carbohydrate-binding domain (Dodson et al., 1999; Pillai et al., 1999). It has also a transmembrane segment and a short cytoplasmic tail (Tannich et al., 1991), which was suggested to be involved in signal transduction (Vines et al., 1998). The light subunit resolves in SDS-PAGE into a triplet of bands at 35 kDa and into an additional band at 31 kDa, all of which react on Western blot with polyclonal antibodies raised against the light subunit (Ankri et al., 1999). Light subunit molecules contain five conserved cysteine residues (amino acids 58, 61, 62, 228, and 261 of the mature light subunit; McCoy et al., 1993b; Ramakrishnan et al., 1996) and appear to have a glycosyl-phosphatidyl-inositol (GPI) anchor at its C-terminal end (McCoy et al., 1993a).
No function was originally ascribed to the light subunit in the Gal-lectin complex. This notion changed when we found out by a cDNA representational difference analysis that avirulent strain Rahman has low transcription levels of the lgl1 gene (Ankri et al., 1999). An antisense transfectant for the lgl1 gene, which inhibited by 60% the expression of the light subunit in the virulent strain HM1:IMSS without affecting the levels of expression of hgl, demonstrated a significant decrease in virulence, supporting a specific and separate function for the light subunit (Ankri et al., 1999). In addition, E. histolytica that underwent long-term monoxenic cultivation with Escherichia coli serotype 055 that bears galactose residues on its surface (but not with other E. coli) were found to undergo a reduction in their virulence in vitro. The expression of the light subunit genes was found to be reduced, lending further support to the hypothesis that this subunit has a specific role in virulence (Padilla-Vaca et al., 1999). In another study (Ramakrishnan et al., 2000), a construct based on the reported sequence of the lgl2 gene (McCoy et al., 1993b), in which the 15 C-terminal residues were deleted, showed that the C-terminal truncated protein was unable to form an heterodimer with the 170-kDa heavy subunit, in contrast with the native lgl2 product in which heterodimer formation occurred. The authors claimed that this effect is due to the deletion of the GPI addition site that is presumed to be present in these 15 C-terminal residues.
To find out what is the role and which are the important domains of the light subunit of the Gal-lectin for amoebic virulence, we overexpressed intact, mutated, and truncated forms of the light subunit aimed to compete, through a dominant negative effect, with the native light subunit in the formation of the 260-kDa Gal-lectin complex. We based these constructions on the lgl1 gene, which we found to be the most abundantly transcribed gene among the lgl family of genes.
MATERIALS AND METHODS
E. histolytica Strains
Trophozoites of E. histolytica strain HM1:IMSS were cultured under axenic conditions in Diamond's TYI-S-33 medium (Diamond et al., 1978).
Determination of the Relative Abundance of Transcripts of the lgl Gene Family
A probe that cross-reacts with both lgl1 and lgl2 sequences was used to screen a cDNA library of the virulent strain HM1:IMSS (a gift from Dr. T. Nozaki, National Institute of Infectious Diseases, Tokyo, Japan). The positive plaques were amplified by PCR using primers suitable for lgl1, lgl2, and lgl3, and their identity was established by RFLP using HinfI.
Construction of Hybrid Plasmids
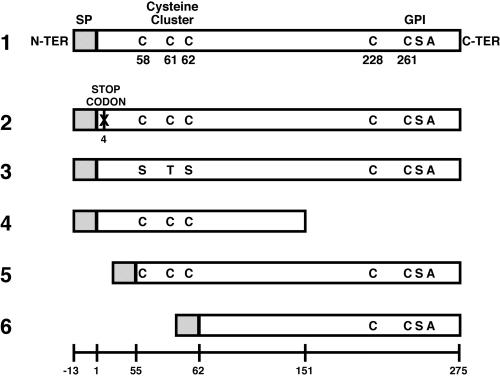
Primers are described in Table 1, constructs in Table 2, and the predicted protein products in Figure 1.
Table 1.
Primers used in plasmid constructs
| Primer | Primer sequence | Underlined restriction site |
|---|---|---|
| A | catgccatggttatattagtcttattgata | NcoI |
| B | gcgcgtcgacttatgcaaacacaggaataa | SalI |
| C | gctagagctcatgatgctatattttgttc | SacI |
| D | catgccatggttattgaatgttcaattcag | NcoI |
| E | gcgactagttgttttactcttagcacgaac | SpeI |
| F | gcgactagtagagttatttttgcatct | SpeI |
| G | ggagatctgaattgcataacttgtgtacat | BamHI |
| H | gcgcccatggttatataagtcttattgata | NcoI |
| I | aaagacttagcaccaaatgaataacttatc | DdeI |
| J | aaggtctcctaagtgtaaaacatgttgtag | BsaI and DdeI |
| K | aaggtctcaactctaccaaatgaataacttatc | BsaI |
| L | aaggtctcagagttatttttgcatctgatt | BsaI |
Table 2.
List of genes/constructs cloned into pEhAct-Neo
| Plasmid | Encoded gene/construct |
|---|---|
| pOV-LGL1 | Native lgl1 ORF. |
| pCYS-MUT | Mutated lgl1 ORF: Codons encoding C58, C61 and C62 were exchanged for codons encoding S58, T61 and S62. |
| pC-TRUNC | 3′-truncated lgl1 ORF: Codons encoding amino acids 151–267 were removed. Therefore C228, C261, and the GPI addition site are absent. |
| pN-TRUNC | 5′-truncated lgl1 ORF: Codons encoding amino acids 1–55 were removed. Codons for signal peptide (from −13 to −1) were preserved. |
| pDEEP-N | 5′-truncated lgl1 ORF: Codons encoding amino acids 1–62 were removed. Therefore codons for C58, C61, and C62 are absent. Codons for signal peptide (from −13 to −1) were preserved. |
| pCONTROL | Mutated lgl1 ORF: an early stop codon was introduced. |
Figure 1.
Schematic representations of the predicted translation products of the transfected constructs. 1, pOV-LGL1 encoding the native lgl1 ORF; 2, pCONTROL with an early stop codon instead of the codon encoding amino acid number four; 3, pCYS-MUT; 4, pC-TRUNC; 5, pN-TRUNC; and 6, pDEEP-N. The sequential numbers of the amino acids of the native product of lgl1 ORF are shown in the bottom. GPI, glycosyl-phosphatidyl-inositol anchor; SP, signal peptide; N-ter, N-terminal end; C-ter, C-terminal end.
I. Preparation of the Cassettes
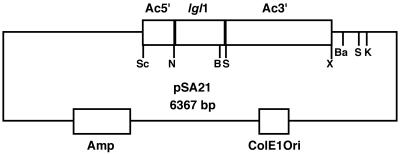
1. pOV-LGL1: This intermediate plasmid construct (pSA21) is detailed in Figure 2. Primers A and B were used to amplify by PCR the ORF of lgl1 from plasmid gEh-35/1 (accession number M96024; a gift from Dr. E. Tannich, Hamburg, Germany).
Figure 2.
Plasmid construct pSA21 based on pBluescript II KS. Ac5′, actin 5′ flanking region (500 base pairs); Ac3′, actin 3′ flanking region (2000 base pairs); lgl1, ORF of lgl1 (867 base pairs); Amp, ampicillin resistance cassette; ColE1Ori, origin of replication; Sc, SacI; N, NcoI; B, BglII; S, SalI; X, XbaI; Ba, BamHI; K, KpnI. The cassettes constructed in this plasmid are subsequently transferred to amoebic vector pEhAct-Neo (Moshitch-Moshkovitch et al., 1996).
E. histolytica 5′ actin flanking region was amplified from pEhAct-Neo (Moshitch-Moshkovitch et al., 1996) using PCR with primers C and D. Both PCR products were digested by NcoI and ligated by T4 DNA ligase. PCR with primers C and B was performed using the ligation product as template. Plasmid pEhAct-Neo was digested using SalI and XbaI to recover the 3′ actin flanking region. Triple ligation was performed between a) the digested plasmid pBluescript II KS (Stratagene, La Jolla, CA; SacI/XbaI); b) the digested PCR product containing the 5′ actin flanking region ligated to the lgl1 ORF (SacI/SalI); and c) the digested 3 ′actin flanking region recovered from plasmid pEhAct-Neo (SalI/XbaI).
2. pCYS-MUT: PCR amplification of a 5′ segment of the lgl1 ORF (225 bp) was made using pSA21 as template and primers A and E. Another PCR amplification of a 3′ segment of the lgl1 ORF (648 bp) was made using pSA21 as template and primers F and B. Both PCR products were digested by SpeI and ligated by T4 DNA ligase. PCR amplification of the ligation product was performed with primers A and B. The product of this PCR amplification was digested with NcoI and BglII and cloned into pSA21 opened with NcoI and BglII.
3. pC-TRUNC: PCR amplification of a 5′ segment of lgl1 ORF was made using pSA21 as template and primers A and G. The product of this PCR amplification was digested with NcoI and BglII and cloned into pSA21 opened with NcoI and BglII.
4. pCONTROL: PCR amplification of lgl1 ORF was made using pSA21 as template and primers H and B. The product of this PCR amplification was digested with NcoI and BglII and cloned into pSA21 opened with NcoI and BglII.
5. pN-TRUNC: PCR amplification of the 5′ actin flanking region and the codons encoding the signal peptide of the lgl1 ORF (539 bp) was made using pSA21 as template and primers C and I. This PCR product was digested by DdeI. Another PCR amplification of a 3′ segment of the lgl1 ORF (660 bp) was made using pSA21 as template and primers J and B. This PCR product was digested by BsaI, which left a DdeI-compatible overhang. Both digested PCR products were ligated by T4 DNA ligase. PCR amplification of the ligation product was performed with primers A and B. The product of this PCR amplification was digested with NcoI and BglII and cloned into pSA21 opened with NcoI and BglII.
6. pDEEP-N: PCR amplification of the 5′ actin flanking region and the codons encoding the signal peptide of the lgl1 ORF (539 bp) was made using pSA21 as template and primers C and K. This PCR product was digested by BsaI. Another PCR amplification of a 3′ segment of the lgl1 ORF (642 bp) was made using pSA21 as template and primers L and B. This PCR product was also digested by BsaI. Both digested PCR products were ligated by T4 DNA ligase. PCR amplification of the ligation product was performed with primers A and B. The product of this PCR amplification was digested with NcoI and BglII and cloned into pSA21 opened with NcoI and BglII.
II. Transfer of the Cassettes to Amoebic Vector pEhAct-Neo
Cassette-containing constructs for pOV-LGL1, pCYS-MUT, pC-TRUNC, pCONTROL, pN-TRUNC, and pDEEP-N were digested with SacI and BamHI to recover their respective intact or mutated lgl1 ORF flanked by actin flanking regions, which were subsequently cloned into pEhAct-Neo opened with SacI and BamHI to form the final constructs. The accuracy of each of the constructs was verified by DNA sequencing, and no mismatches were found.
Transfection of E. histolytica Trophozoites
Exponentially growing trophozoites of E. histolytica strain HM1:IMSS were transfected with each of the aforementioned plasmids as described previously (Vines et al., 1995). The transfectant amoebae were kept at 60 μg/ml G418.
Northern Blot Hybridization
Total RNA was prepared using Tri-Reagent RNA isolation kit (Molecular Research Center, Inc., Cincinnati, OH). RNA (5 μg) was size-fractionated on 4% polyacrylamide denaturing gel containing 8 M urea and subsequently blotted electrophoretically on to a nylon membrane. Hybridization was carried out by lgl1-ORF probes randomly labeled using Rediprime II kit (Amersham Pharmacia Biotech, Buckinghamshire, England). Membranes were washed after overnight hybridization using nonstringent (0.1% SDS, 2× SSC) and stringent conditions (0.1% SDS, 0.1× SSC). Detection was done by autoradiography.
Semiquantification of Transcripts of lgl1 and 5′-Truncated lgl1
Semiquantitative PCR was performed using the LightCycler FastStart DNA Master SYBR Green I Kit (Roche Diagnostics GmbH, Mannheim, Germany) by relative quantitation with external standards, which creates a standard curve suitable for amplifications of the same efficiency. Primer pairs (Table 3) were selected and tested in advance for specific amplification of lgl1 alone (M and N), lgl1 together with the 5′-truncated lgl1 that generates the N-truncated lgl (N and O) and actin as housekeeping gene (P and Q). These primers did not amplify lgl2 or any other sequence. Primer specificity is due to the unique 3′ end of each of the primers and was tested by PCR amplification of the right template and, as a control, also on other possible cross-reacting templates. PCR products were separated in 1% agarose gel, and the single band product obtained matched the expected molecular weight. Melting analysis of PCR product gave also a single peak. The relative quantity of lgl1 as well as that of lgl1 together with the 5′-truncated lgl1 were normalized for actin and compared among samples.
Table 3.
Primers used in semiquantitative PCR
| Primer | Primer sequence | Comment |
|---|---|---|
| M | cgcggatccaagactcaagacggaaaagat | Sense primer specific for lgl1. |
| N | tttccagtccatgttgatctac | Antisense primer suitable for both lgl1 and 5′-truncated lgl1. |
| O | atggatatggaatttgatgataaaagatc | Sense primer suitable for both lgl1 and 5′-truncated lgl1. |
| P | ggtgatgatgcaccaagagc | Sense primer specific for actin. |
| Q | gtctggtgttaatacctgg | Antisense primer specific for actin. |
The template used was cDNA from nontransfected parent strain amoeba HM1:IMSS and from transfectants pN-TRUNC and pCONTROL.
SDS-PAGE and Western Immunoblotting
E. histolytica trophozoites were solubilized with 1% NP-40 in PBS in the presence of 50 μM protease inhibitor E-64. Proteins were resolved for 35 min at 200 V on 10% or 12% polyacrylamide gels (25 μg per lane) under reducing conditions (with β-mercaptoethanol) or under nonreducing conditions (without; Laemmli, 1970). Proteins were transferred electrophoretically to nitrocellulose membranes, and blots were incubated with a suspension of rabbit polyclonal antibodies anti–Gal–lectin heavy subunit (1:5000; a gift from Dr. S. L. Stanley, Washington University, St. Louis, MO) or with a suspension of rabbit polyclonal antibodies anti–Gal-lectin light subunit (1:1000; Ankri et al., 1999), then subjected to interaction with an HRP-conjugated goat anti-rabbit antibody (1:5000), and finally developed by enhanced chemiluminescence.
Affinity Chromatography Purification of Gal-lectin
Trophozoites, 1.5 × 107, were lysed in octyl-beta-d-glucopyranoside (1.5 ml, 1% in water). A lactosyl-Sepharose column (1 ml packed-volume; a gift from Dr. N. Sharon, Weizmann Institute, Israel) was used in order to bind the Gal-lectin contained in the amoebic lysate. After several washes with PBS, the lectin was eluted using 0.5 M galactose in PBS. Eluted fractions and the last washing of the column before elution were dialyzed against water and concentrated by lyophylization. Finally all the samples were analyzed by Western blot.
Immunoaffinity Chromatography Purification of Gal-lectin
We cross-linked mouse monoclonal antibodies against the heavy subunit of the Gal-lectin (0.6 μg of 3F4 and 0.6 μg of 7F4; a gift from Dr. Richard Vines, TechLab, Blacksburg, VA) to a 0.2-ml column of immobilized protein G (Seize-X Protein G immunoprecipitation kit; Pierce, Rockford, IL).
Amoebic trophozoites (4 × 106) were lysed in octyl-beta-d-glucopyranoside (0.4 ml, 1% in water). Binding to the column, washing, and elution were performed in accordance to the manufacturer's instructions. The eluted fractions were concentrated using vacuum and analyzed by Western blot.
Fluorescence-activated Cell Sorting Analysis
Trophozoites (0.25 × 106) were washed twice in PBS and incubated at 4°C in the presence of rabbit antiheavy subunit antibody (dilution 1:500 in PBS) for 30 min. After washing, amoebae were incubated with goat anti-rabbit whole IgG FITC-conjugated second antibody (dilution 1:80 in PBS; Sigma-Aldrich, St Louis, MO) at 4°C for 30 min. After washing, fixation with 3% paraformaldehyde was performed for 30 min. Amoebae were finally washed and resuspended in 500 μl of PBS for fluorescence-activated cell sorting (FACS) analysis.
Virulence and Adherence Assays
The destruction rate of tissue-cultured monolayers of baby hamster kidney (BHK) cells by transfected and untransfected viable trophozoites (2 × 105) was performed as described previously (Bracha and Mirelman, 1984). Each experiment was performed in duplicate five times.
Adhesion assay to BHK cells was performed four times in duplicate as previously described (Padilla-Vaca et al., 1999).
Erythrophagocytosis assay was performed twice in duplicate as previously described (Mora-Galindo et al., 1997).
Rosette formation assay was performed in duplicate as previously described (Ravdin and Guerrant, 1981) by incubating trophozoites with HRBCs at 4°C for 120 min.
Gal-lectin Capping Assay
Capping was induced in freshly harvested trophozoites (5 × 106) by incubation with mouse monoclonal antibodies against the heavy subunit of the Gal-lectin (1:30 of 3F4 and 1:30 of 7F4; a gift from Dr. Richard Vines, TechLab, Blacksburg, VA) or PBS alone for noninduced amoebae, at 37°C for 30 min. Fixation was performed with the addition of paraformaldehyde to a final concentration of 3.7%. Fixed trophozoites were prepared for confocal microscopy as previously described (Leippe et al., 1994) without permeabilization. Trophozoites that underwent capping induction and those who did not were incubated with the same mix of 3F4 and 7F4 antibodies and followed by incubation with FITC-labeled goat anti-mouse antibodies. Finally, samples were observed under confocal microscope (Fluoview FV500; Olympus, Tokyo, Japan).
RESULTS
Determination of the Relative Abundance of Transcripts of the lgl Gene Family
Analysis of 84 positive plaques containing lgl family cDNA using RFLP with HinfI revealed that 71 were lgl1 and only 13 were lgl2. No lgl3-containing plaques were found.
Transcription and Translation of Transfected Plasmid Constructs
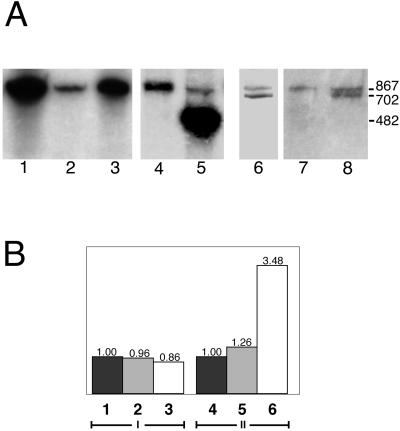
Transfection of E. histolytica trophozoites with pN-TRUNC resulted in the production of a transcript, which separated from the endogenous one as shown by Northern blot hybridization (Figure 3A). Transcripts of other transfectants were also clearly detected (Figure 3A). No significant difference was observed in the relative quantity of the transcript of lgl1 among nontransfected amoeba HM1:IMSS, transfectant pCONTROL, and transfectant pN-TRUNC as analyzed by semiquantitative RT-PCR. In addition, the relative quantity of lgl2 was also not affected in these transfectants (unpublished data). This rules out any effect due to the manipulation of the lgl1 gene on the other lgl gene family members. Transcripts, however, increased more than threefold in pN-TRUNC when lgl1 together with the 5′-truncated lgl1 were amplified (Figure 3B). This shows that the plasmid coded 5′-truncated lgl1 is responsible for this increment, outnumbering the endogenous chromosomal lgl1 transcript by more than twice.
Figure 3.
Transcription analysis. (A) Northern blot of a 4% polyacrylamide-urea denaturing gel. A probe that hybridizes with lgl1 was used. Approximately 5 μg of the correspondent RNA was loaded in each well. The size of the transcripts is 867 bases, and the G418 concentration at which the transfected trophozoites were kept before lysis is 60 μg/ml unless otherwise stated. 1, pOV-LGL1; 2, strain HM1:IMSS; 3, pCYS-MUT; 4, pCONTROL; 5, pC-TRUNC (482 bases); 6, pDEEP-N (681 bases, 6 μg/ml G418); 7, strain HM1: IMSS; and 8, pN-TRUNC (702 bases, 6 μg/ml G418). (B) Relative quantity of transcripts as obtained by RT-PCR using the LightCycler. I, lgl1 and; II, lgl1 together with the 5′-truncated lgl1. 1 and 4, Nontransfected strain HM1:IMSS; 2 and 5, pCONTROL transfectant; 3 and 6, pN-TRUNC transfectant. Results represent the average of two independent experiments that gave very similar values.
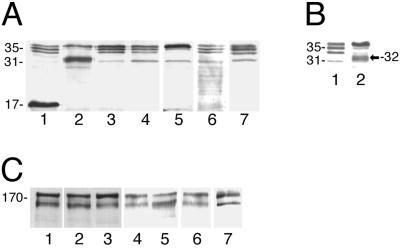
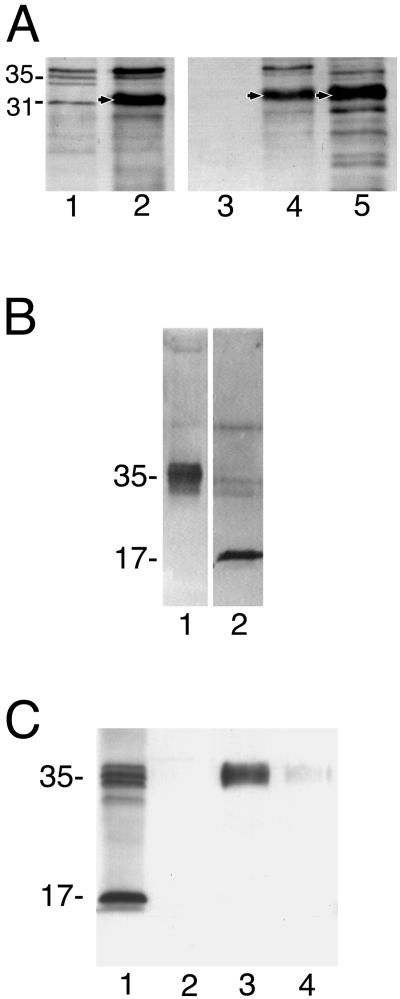
Western blot analysis of reducing SDS-PAGE gels with antilight subunit antibodies revealed a strong band at 32-kDa in transfectant pN-TRUNC that matched the expected molecular size of the N-truncated lgl (Figures 4, A and B). Western blots of reducing and nonreducing SDS-PAGE gels of transfectants' lysates probed with antiheavy subunit-specific antibodies showed only minor changes in the relative abundance of the bands of the heavy subunit (Figure 4C). Unfortunately, heavy subunit isoform variations cannot be assessed due to the lack of a set of antibodies specific to each isoform of the heavy subunit, which are 89–95% identical at the amino acid level (Petri et al., 2002).
Figure 4.
Expression analysis. (A) Western blot of a 12% reducing SDS-PAGE probed with antilight subunit antibodies shows the expression of the light subunit genes/constructs in each of the transfectants. 1, pC-TRUNC; 2, pN-TRUNC; 3, pCONTROL; 4, Nontransfected HM1: IMSS strain; 5, pOV-LGL1; 6, pCYS-MUT; and 7, pDEEP-N. (B) Western blot of an 8% reducing SDS-PAGE probed with antilight subunit antibodies. 1, lysate of nontransfected HM1:IMSS; 2, lysate of transfectant pN-TRUNC showing the additional band at 32 kDa, which is detected above the 31-kDa band of nontransfected HM1:IMSS. (C) Western blot of a 10% reducing SDS-PAGE probed with antiheavy subunit antibodies showing the expression levels of the heavy subunit of the Gal-lectin in each of the transfectants. 1, Nontransfected HM1:IMSS; 2, pN-TRUNC; 3, pCONTROL; 4, pOV-LGL1; 5, pC-TRUNC; 6, pCYS-MUT; and 7, pDEEP-N.
Immunoaffinity and lactosyl affinity chromatography-purified Gal-lectin isolated from trophozoites of transfectant pN-TRUNC revealed also a large amount of the 32-kDa protein after reduction with β-mercaptoethanol (Figure 5A).
Figure 5.
Lectin structure. (A) Affinity chromatography with lactosyl-Sepharose for lysates of pN-TRUNC transfectant. After extensive PBS washing of the column, the bound material was eluted with 0.5 M galactose. Eluted fractions were concentrated and loaded in 10% reducing SDS-PAGE for Western blot analysis with antibodies against the light subunit. 1, Nontransfected HM1:IMSS whole lysate; 2, pN-TRUNC transfectant whole lysate before column binding; 3, last washing of column before elution; 4, first Gal-eluted fraction; 5, second Gal-eluted fraction. Arrows point to the overexpressed 32-kDa N-truncated protein. (B) Western blot probed with antibodies against the light subunit of the Gal-lectin. Lane 1, Whole lysate of transfectant pC-TRUNC separated on a nonreducing 10% SDS-PAGE gel; lane 2, The band with an approximate size of 35 kDa in lane 1 was extracted, electroeluted and loaded on a reducing 10% SDS-PAGE resolving as a 17-kDa protein. (C) Immunoaffinity chromatography with antiheavy subunit antibody for lysates of pC-TRUNC transfectant. Eluted fractions were loaded in a 12% reducing SDS-PAGE for Western blot analysis with antibodies against the light subunit. 1, pC-TRUNC transfectant whole lysate before column binding; 2, last washing of column before elution; 3, first eluted fraction; 4, second eluted fraction.
The other transfectant that showed overexpression of its mutated lgl was pC-TRUNC. Western blots of nonreducing SDS-PAGE gels with antilight subunit antibodies showed a diffuse band in this transfectant approximately at the 35-kDa position (Figure 5B). After excision, electroelution and reduction with DTT, the 35-kDa band dissociated into a 17-kDa band (Figure 5B) that matched the expected size of the C-truncated lgl monomer. SDS-PAGE of lysates of transfectant pC-TRUNC under reducing conditions showed the 17-kDa band in addition to the usual bands of the light subunit (Figure 4A). No 17-kDa protein was detected, however, after reduction of either immunoaffinity or lactosyl affinity chromatography-purified Gal-lectin molecules (Figure 5C).
Transfectant pOV-LGL1 overexpressed the intact original lgl, which appeared as an enhanced upper band of the 35-kDa light subunit triplet (Figure 4A). No additional protein bands were detected in transfectants pDEEP-N, pCYS-MUT, and pCONTROL (Figure 4A).
Comparison of Surface Density of the Heavy Subunit of the Gal-lectin
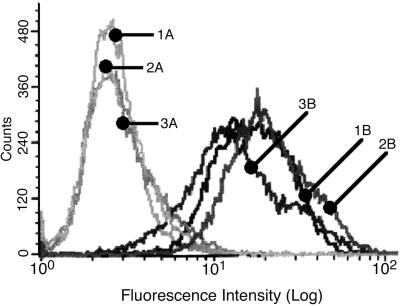
Surface density distribution of the Gal-lectin heavy subunit in transfectant pN-TRUNC, in control transfectant pCONTROL, and in nontransfected HM1:IMSS was very similar as analyzed by FACS using the 3F4 and 7F4 monoclonal antibodies against the heavy subunit of the Gal-lectin (Figure 6).
Figure 6.
FACS analysis of trophozoites incubated with an antiheavy subunit antibody. An FITC-conjugated second antibody was used. A, Controls without first antibody; B, with First antibody; 1A and 1B, nontransfected HM1:IMSS; 2A and 2B, transfectant pN-TRUNC; 3A and 3B, transfectant pCONTROL.
Effect on Cytopathic Activity
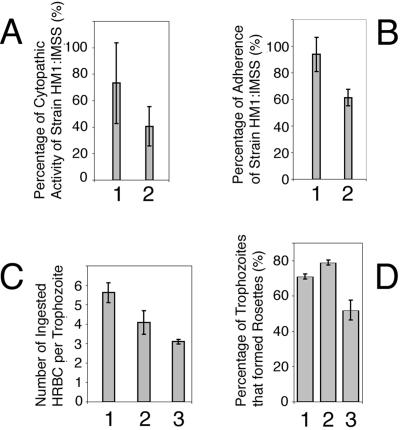
Transfectant pN-TRUNC showed a marked reduction in its cytopathic activity to almost 45% less than that of the control transfectant pCONTROL and 59% less than that of the nontransfected amoeba (single-tailed t test, p < 0.005). Average values from five independent experiments are shown in Figure 7A. These results were also validated using two independent transfections with the same constructs. No significant differences were detected for the other transfectants.
Figure 7.
Virulence and adherence assays. (A) Cytopathic activity measuring the in vitro ability of amoebae to destroy a monolayer of BHK cells. The columns show the average measurements of cytopathic activity from five independent experiments for transfectants 1, pCONTROL and 2, pN-TRUNC as a percentage of the activity of nontransfected HM1:IMSS (in the vertical axis). Bars, SEs. Statistical significance was determined by single-tailed t test (p < 0.05). (B) Adherence ability of trophozoites to a formaldehyde-fixed monolayer of BHK cells. The columns show the average measurements of adhesion from four experiments for transfectants 1, pCONTROL and 2, pN-TRUNC as a percentage of the adhesion ability exhibited by nontransfected HM1:IMSS (in the vertical axis). Bars, SEs. Statistical significance was determined by single-tailed t test (p < 0.005). (C) Erythrophagocytosis assay, measuring the in vitro ability of amoebic trophozoites to ingest human red blood cells (HRBC). The columns show the average number of HRBC internalized by trophozoites during the incubation period (in the vertical axis). Bars, SEs. Statistical significance was determined by single-tailed t test (p < 0.05). 1, Nontransfected strain HM1:IMSS; 2, transfectant pCONTROL; and 3, transfectant pN-TRUNC. (D) Percentage of trophozoites (from 100 trophozoites analyzed) that formed rosettes with HRBC. Bars, SEs. Statistical significance was determined by single-tailed t test (p < 0.05). 1, Nontransfected strain HM1:IMSS; 2, transfectant pCONTROL; and 3, transfectant pN-TRUNC.
Adherence to Monolayer of Target Cells
The percentage of pN-TRUNC transfected trophozoites that adhered to a monolayer of fixed BHK cells was 35% lower than that of the control transfectant pCONTROL and 39% lower than that of the nontransfected amoeba (average values from four independent experiments are shown in Figure 7B). Incubation in the presence of Galactose (20 mg/ml) significantly diminished the adherence ability of transfectants pN-TRUNC and pCONTROL as well as that of strain HM1:IMSS (unpublished data).
Erythrophagocytosis Assay
The rate of ingestion of HRBC by transfectant pN-TRUNC was 44% lower than that of nontransfected strain HM1:IMSS and 24% lower than the rate of transfectant pCONTROL (Figure 7C).
The activities of pCONTROL are in most cases slightly lower than those of nontransfected strain HM1:IMSS because of the effect of the selective antibiotic.
Rosette Formation Assay
The percentage of rosette-forming amoeba in transfectant pN-TRUNC was 35% lower than that of the control transfectant pCONTROL and 28% lower than the percentage observed for the nontransfected amoeba HM1:IMSS (Figure 7D).
Capping Formation Assay
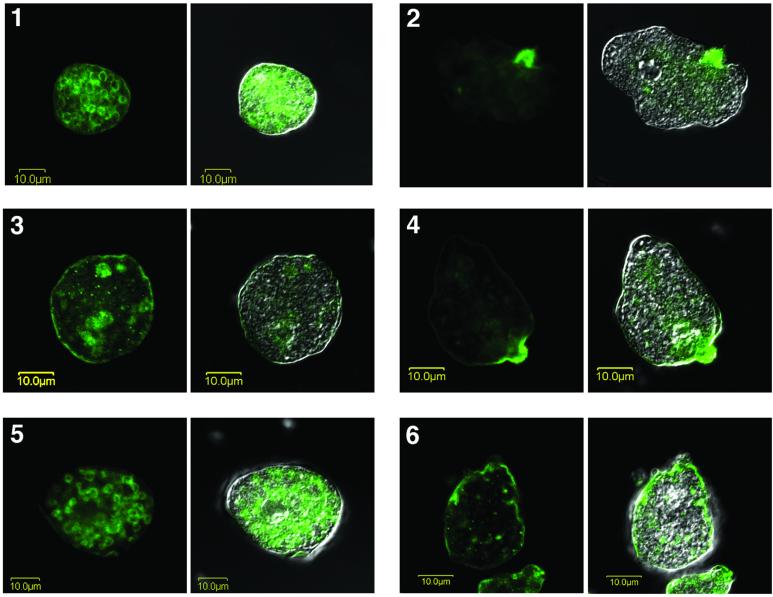
A significantly diminished ability to cap the Gal-lectin complexes was detected in transfectant pN-TRUNC after capping induction by monoclonal antibodies specific for the hgl subunit (Figure 8). Trophozoites of HM1:IMSS and pCONTROL showed large concentrations of fluorescence in the uroid region of the amoeba after capping induction for 30 min (Figure 8). Amoebae that did not undergo capping induction showed a patched membranal pattern, with few concentrations of fluorescence. No fluorescence was present in control amoebae that were not incubated with antiheavy subunit antibody.
Figure 8.
Induction of capping. Confocal microscopy of trophozoites before (panels 1, 3, and 5) and after (panels 2, 4, and 6) induction of capping for 30 min using antibodies against the heavy subunit of the Gal-lectin. Each panel shows the fluorescent Gal-lectin on the left side and the fluorescent Gal-lectin superimposed on a Nomarsky section on the right side. 1 and 2, Nontransfected strain HM1:IMSS; 3 and 4, transfectant pCONTROL; and 5 and 6, transfectant pN-TRUNC.
DISCUSSION
Numerous investigations have clearly determined that the Gal-lectin is a virulence factor of E. histolytica (Kain and Ravdin, 1995; Gilchrist and Petri, 1999). The carbohydrate-binding domain and a putative cytoplasmic signal transduction motif have been identified in the heavy 170-kDa subunit (Dodson et al., 1999; Pillai et al., 1999). Nevertheless, recent findings have raised the possibility that the light subunit also has a role in virulence (Ankri et al., 1999; Padilla-Vaca et al., 1999). To determine which domains of the light subunit might be important for virulence, a dominant negative approach was used, and a number of transfectant amoebae were prepared with different modifications in their light subunit (Table 2).
We chose to study the lgl1 gene because of 1) its transcription dominance, 85% among lgl gene family transcripts; 2) because of the fact that the lgl1 was found to be underrepresented in avirulent strain Rahman when compared with virulent HM1:IMSS (Ankri et al., 1999); and 3) because of our previous work with antisense lgl1, which gave an avirulent phenotype (Ankri et al., 1999).
Although all the transfectants showed good levels of transcription of their respective light subunit and plasmid constructs, only transfectant pN-TRUNC expressed a modified light subunit, which was able to form dominant negative heterodimers with the heavy subunit of the Gal-lectin. Transfectant pN-TRUNC expressed a 32-kDa N-truncated light subunit, which competed with the native light subunit to form Gal-lectin heterodimers with the 170-kDa heavy subunit. Purification of the Gal-lectin molecules of transfectant pN-TRUNC by either immunoaffinity chromatography or by lactosyl affinity chromatography followed by reduction of the disulfide bond revealed that the dominant component bound to the heavy subunit of the Gal-lectin was the 32-kDa N-truncated light subunit. This also correlated with the higher levels of 5′-truncated lgl1 transcripts, which were detected by semiquantitative RT-PCR in comparison to the endogenous lgl1 transcript. The purification of Gal-lectin molecules containing the N-truncated light subunit by lactosyl affinity chromatography demonstrates that the replacement of most of the native 35-kDa lgl molecules by the 32-kDa N-truncated lgl does not affect the individual Gal binding ability of the Gal-lectin heterodimers, at the molecular level. Unfortunately, an attempt to tag the N-truncated lgl1 light subunit with the FLAG epitope (DYKDDDDK) did not lead to the production of a tagged N-truncated product, perhaps because of the alteration in the protein structure by the FLAG peptide, which was placed adjacent to the signal peptide. This may have affected the ability of the amoeba to produce the mature protein by cleavage of the signal peptide.
The fact that only the N-truncated lgl became bound to the heavy subunit, whereas the modified lgl encoded in pCYS-MUT and pDEEP-N did not, suggests that the cysteine residues at positions 58, 61, and 62 of the mature lgl molecule, which are absent in pCYS-MUT and pDEEP-N, are required for the lgl molecule to be able to form a disulfide bond with the hgl 170-kDa heavy subunit. In the absence of the above mentioned cysteine residues, these mutated proteins may have been targeted to protein digestion pathways.
The only other transfectant that was found to overproduce a mutated lgl was pC-TRUNC. The C-truncated lgl did not bind to the hgl subunit and accumulated in the trophozoites, mainly as a disulfide-linked homo-oligomer, which upon reduction yielded the expected 17-kDa C-truncated lgl. Removal of the C-terminal part of the lgl1 product, which has been reported to be modified with a GPI-anchor (McCoy et al., 1993a), appears to cause a misplacement of the light subunit, abrogating its ability to bind to the hgl. A somewhat similar observation was made by Ramakrishnan et al. (2000), who showed that a C-truncated form of the lgl2 gene product was unable to bind the 170-kDa heavy subunit, in contrast with the native lgl2 product in which heterodimer formation occurred. The authors claimed that this effect is due to the deletion of the GPI addition/cleavage site that is presumed to be present in these 15 C-terminal residues.
The fact that the C-truncated molecule was able to form homo-oligomers perhaps indicates that one of the cysteine residues at positions 58, 61, and 62, which are the ones preserved in the C-truncated protein, is implicated in interprotein disulfide binding. One of these cysteine residues may be responsible for the disulfide linkage to the heavy subunit but appears to require the C-terminal end, perhaps because of the GPI anchor.
Transfectant pN-TRUNC was the only transfectant that showed a clear reduction in in vitro virulence as assayed by cytopathic activity. In addition, pN-TRUNC showed a reduced rate of phagocytosis of HRBC and an impaired ability to adhere to a monolayer of BHK cells as well as to form Rosettes with HRBC. In these assays the observed reduction of activity was between 35 and 45%. The inhibitions of cytopathic activity, adherence, rosette formation, and erythrophagocytosis seen in transfectant pN-TRUNC appear not to be due to a decrease in the number of surface Gal-lectin molecules. Analysis by FACS showed that binding of specific antiheavy subunit antibodies was very similar among pN-TRUNC, pCONTROL, and parent strain HM1:IMSS. A clear difference was detected, however, in the ability of pN-TRUNC trophozoites to cap its Gal-lectin molecules when compared with both pCONTROL and parent strain HM1:IMSS. As shown by confocal microscopy, the induction of capping of the Gal-lectin molecules in pCONTROL and in nontransfected HM1:IMSS by antiheavy subunit antibodies was quite fast, and the vast majority of lectin molecules concentrated in the posterior uroid region of the trophozoites within minutes. Capping of the Gal-lectin in the uroid has been previously shown (Arhets et al., 1995). In trophozoites of pN-TRUNC, however, this did not happen, and most of the Gal-lectin molecules remained associated in several patches in the membrane, similar to what was seen before capping induction with antiheavy subunit antibodies. The ability of a trophozoite to efficiently cap its surface components appears to be an important prerequisite for interaction with other cells and for exerting a virulent behavior. The inability of trophozoites to cap surface molecules, together with a decreased virulence, has been previously observed in dominant negative mutants overproducing a mutated form of light meromyosin (Arhets et al., 1998).
As it has been shown for other systems, the initial recognition of a ligand by a lectin molecule creates a weak bond, which is then strengthened by subsequent lectin molecules that rapidly cluster and cap the ligand, creating a stronger attachment (McCoy et al., 1994; Tavares et al., 2000). If the lectin molecules have a difficulty in lateral surface mobility, they may not be able to properly cluster, or at least not at the appropriate pace. No apparent difference in the motility of intact trophozoites of transfectant pN-TRUNC compared with those of nontransfected HM1:IMSS was observed.
Overexpression of the native lgl1 gene (pOV-LGL1 transfectant) showed no influence in virulence, ruling out that the effect seen in transfectant pN-TRUNC might be only a consequence of misbalance of the relative abundance of the molecules that form the Gal-lectin heterodimer.
In principle, there could be three possible levels in which the N-truncated light subunit may interfere with the normal function of the Gal-lectin by virtue of a dominant negative effect:
- 1.
The N-truncated protein affects the ability of the lectin heterodimer to bind galactose or N-acetyl-galactosamine residues due to conformational changes in the modified Gal-lectin heterodimeric complex. This, however, is not apparent in view of the fact that similar amounts of Gal-lectin molecules of strain HM1:IMSS and transfectant pN-TRUNC (this last one overwhelmingly consisting of the heavy subunit and the N-truncated light subunit) were recovered from the lactosyl affinity column, which indicates competence of the Gal-lectin complex in binding to Gal residues.2. The N-terminal domain of the light subunit, lacking in transfectant pN-TRUNC, has a role in the clustering of Gal-lectin molecules, which is an important mechanism that follows the initial carbohydrate binding event and provides additional strength and stability to the attachment of the trophozoite to target cells (McCoy et al., 1994). Clustering requires initial Gal-binding of the target cell through the heavy subunit. This is compatible with the fact that lectin competence for the initial carbohydrate binding was preserved in transfectant pN-TRUNC as shown by the lactosyl-sepharose affinity column. This is also in line with the observed reduction in adherence to BHK cells, in rosette formation, in erythrophagocytosis and in cytopathic activity, all of which require adherence and subsequent Gal-lectin clustering. Supporting this possibility is the impaired ability of transfectant pN-TRUNC Gal-lectin molecules to undergo capping.3. The N-truncated light subunit affects the postbinding response of the lectin complex, perhaps because of interference with a secondary binding activity that might strengthen the Gal-lectin binding or with an effector pathway for which the N-terminal domain of the light subunit might be important.
Perhaps a combination of the aforementioned hypothesis may actually be the ultimate reason for the phenotype of transfectant pN-TRUNC, but the weight of the evidence supports a defect in clustering as the principal reason for the phenotype.
It is reasonable to assume that the phenotypes seen with these constructs represent the role of the light subunit in general, due to the dominance of the lgl1 transcript on which the constructs were based.
In view of these results, we conclude that amoebae expressing Gal-lectin complexes in which the light subunit lacks the 55-amino acid N-terminal segment of the mature protein encoded in the lgl1 gene are deficient in Gal-lectin clustering, and as a consequence of this, the interaction with other cells as well as amoebic virulence are impaired.
ACKNOWLEDGMENTS
This work was partially supported by grants from The Center for Emerging Diseases, Jerusalem, Israel, and from Mr. Henry H. Meyer, Jr., United States. U.K. was supported by a Feinberg Graduate School Doctoral Fellowship.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–06–0344. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–06–0344.
REFERENCES
- Ankri S, Padilla-Vaca F, Stolarsky T, Koole L, Katz U, Mirelman D. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNac lectin complex inhibits Entamoeba histolytica virulence. Mol Microbiol. 1999;33:327–337. doi: 10.1046/j.1365-2958.1999.01476.x. [DOI] [PubMed] [Google Scholar]
- Arhets P, Gounon P, Sansonetti P, Guillen N. Myosin II is involved in capping and uroid formation in the human pathogen Entamoeba histolytica. Infect Immun. 1995;63:4358–4367. doi: 10.1128/iai.63.11.4358-4367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhets P, Olivo JC, Gounon P, Sansonetti P, Guillen N. Virulence and functions of myosin II are inhibited by overexpression of light meromyosin in Entamoeba histolytica. Mol Biol Cell. 1998;9:1537–1547. doi: 10.1091/mbc.9.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha R, Mirelman D. Virulence of Entamoeba histolytica trophozoites. Effects of bacteria, microaerobic conditions, and metronidazole. J Exp Med. 1984;160:353–368. doi: 10.1084/jem.160.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha R, Nuchamowitz Y, Leippe M, Mirelman D. Antisense inhibition of amoebapore expression in Entamoeba histolytica causes a decrease in amoebic virulence. Mol Microbiol. 1999;34:463–472. doi: 10.1046/j.1365-2958.1999.01607.x. [DOI] [PubMed] [Google Scholar]
- Bruchhaus I, Jacobs T, Leippe M, Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol Microbiol. 1996;22:255–263. doi: 10.1046/j.1365-2958.1996.00111.x. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other. Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Dodson JM, Lenkowski PW, Jr, Eubanks AC, Jackson TF, Napodano J, Lyerly DM, Lockhart LA, Mann BJ, Petri WA., Jr Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J Infect Dis. 1999;179:460–466. doi: 10.1086/314610. [DOI] [PubMed] [Google Scholar]
- Espinosa-Cantellano M, Martinez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev. 2000;13:318–331. doi: 10.1128/cmr.13.2.318-331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist CA, Petri WA. Virulence factors of Entamoeba histolytica. Curr Opin Microbiol. 1999;2:433–437. doi: 10.1016/S1369-5274(99)80076-9. [DOI] [PubMed] [Google Scholar]
- Kain KC, Ravdin JI. Galactose-specific adhesion mechanisms of Entamoeba histolytica: model for study of enteric pathogens. Methods Enzymol. 1995;253:424–439. doi: 10.1016/s0076-6879(95)53037-1. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leippe M. Amoebapores. Parasitol Today. 1997;13:178–183. doi: 10.1016/s0169-4758(97)01038-7. [DOI] [PubMed] [Google Scholar]
- Leippe M, Andra J, Nickel R, Tannich E, Muller-Eberhard HJ. Amoebapores, a family of membranolytic peptides from cytoplasmic granules of Entamoeba histolytica: isolation, primary structure, and pore formation in bacterial cytoplasmic membranes. Mol Microbiol. 1994;14:895–904. doi: 10.1111/j.1365-2958.1994.tb01325.x. [DOI] [PubMed] [Google Scholar]
- McCoy JJ, Mann BJ, Petri WA., Jr Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around [published erratum appears in Infect. Immun. 1994 Dec;62(12):5707] Infect Immun. 1994;62:3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JJ, Mann BJ, Vedvick TS, Pak Y, Heimark DB, Petri WA., Jr Structural analysis of the light subunit of the Entamoeba histolytica galactose-specific adherence lectin. J Biol Chem. 1993a;268:24223–24231. [PubMed] [Google Scholar]
- McCoy JJ, Mann BJ, Vedvick TS, Petri WA., Jr Sequence analysis of genes encoding the light subunit of the Entamoeba histolytica galactose-specific adhesin. Mol Biochem Parasitol. 1993b;61:325–328. doi: 10.1016/0166-6851(93)90079-d. [DOI] [PubMed] [Google Scholar]
- Mora-Galindo J, Gutierrez-Lozano M, Anaya-Velazquez F. Entamoeba histolytica: kinetics of hemolytic activity, erythrophagocytosis and digestion of erythrocytes. Arch Med Res. 1997;28:200–201. [PubMed] [Google Scholar]
- Moshitch-Moshkovitch S, Stolarsky T, Mirelman D, Alon RN. Stable episomal transfection and gene expression in Entamoeba dispar. Mol Biochem Parasitol. 1996;83:257–261. doi: 10.1016/s0166-6851(96)02766-1. [DOI] [PubMed] [Google Scholar]
- Padilla-Vaca F, Ankri S, Bracha R, Koole LA, Mirelman D. Down regulation of Entamoeba histolytica virulence by monoxenic cultivation with Escherichia coli O55 is related to a decrease in expression of the light (35-kilodalton) subunit of the Gal/GalNAc lectin. Infect Immun. 1999;67:2096–2102. doi: 10.1128/iai.67.5.2096-2102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA, Jr, Haque R, Mann BJ. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu Rev Microbiol. 2002;15:15. doi: 10.1146/annurev.micro.56.012302.160959. [DOI] [PubMed] [Google Scholar]
- Petri WA, Jr, Schnaar RL. Purification and characterization of galactose- and N-acetylgalactosamine-specific adhesin lectin of Entamoeba histolytica. Methods Enzymol. 1995;253:98–104. doi: 10.1016/s0076-6879(95)53011-8. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Wan PS, Yau YC, Ravdin JI, Kain KC. The cysteine-rich region of the Entamoeba histolytica adherence lectin (170-kilodalton subunit) is sufficient for high-affinity Gal/GalNAc-specific binding in vitro. Infect Immun. 1999;67:3836–3841. doi: 10.1128/iai.67.8.3836-3841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que X, Reed SL. Cysteine proteinases and the pathogenesis of amebiasis. Clin Microbiol Rev. 2000;13:196–206. doi: 10.1128/cmr.13.2.196-206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G, Lee S, Mann BJ, Petri WA., Jr Entamoeba histolytica: deletion of the GPI anchor signal sequence on the Gal/GalNAc lectin light subunit prevents its assembly into the lectin heterodimer. Exp Parasitol. 2000;96:57–60. doi: 10.1006/expr.2000.4543. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G, Ragland BD, Purdy JE, Mann BJ. Physical mapping and expression of gene families encoding the N-acetyl d-galactosamine adherence lectin of Entamoeba histolytica. Mol Microbiol. 1996;19:91–100. doi: 10.1046/j.1365-2958.1996.356885.x. [DOI] [PubMed] [Google Scholar]
- Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannich E, Ebert F, Horstmann RD. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares P, Sansonetti P, Guillen N. Cell polarization and adhesion in a motile pathogenic protozoan: role and fate of the Entamoeba histolytica Gal/GalNAc lectin. Microbes Infect. 2000;2:643–649. doi: 10.1016/s1286-4579(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Vines RR, Purdy JE, Ragland BD, Samuelson J, Mann BJ, Petri WA., Jr Stable episomal transfection of Entamoeba histolytica. Mol Biochem Parasitol. 1995;71:265–267. doi: 10.1016/0166-6851(95)00057-8. [DOI] [PubMed] [Google Scholar]
- Vines RR, Ramakrishnan G, Rogers JB, Lockhart LA, Mann BJ, Petri WA., Jr Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a beta2 integrin motif. Mol Biol Cell. 1998;9:2069–2079. doi: 10.1091/mbc.9.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]