Abstract
Microtubule polymerization dynamics at kinetochores is coupled to chromosome movements, but its regulation there is poorly understood. The plus end tracking protein EB1 is required both for regulating microtubule dynamics and for maintaining a euploid genome. To address the role of EB1 in aneuploidy, we visualized its targeting in mitotic PtK1 cells. Fluorescent EB1, which localized to polymerizing ends of astral and spindle microtubules, was used to track their polymerization. EB1 also associated with a subset of attached kinetochores in late prometaphase and metaphase, and rarely in anaphase. Localization occurred in a narrow crescent, concave toward the centromere, consistent with targeting to the microtubule plus end–kinetochore interface. EB1 did not localize to kinetochores lacking attached kinetochore microtubules in prophase or early prometaphase, or upon nocodazole treatment. By time lapse, EB1 specifically targeted to kinetochores moving antipoleward, coupled to microtubule plus end polymerization, and not during plus end depolymerization. It localized independently of spindle bipolarity, the spindle checkpoint, and dynein/dynactin function. EB1 is the first protein whose targeting reflects kinetochore directionality, unlike other plus end tracking proteins that show enhanced kinetochore binding in the absence of microtubules. Our results suggest EB1 may modulate kinetochore microtubule polymerization and/or attachment.
INTRODUCTION
In mammalian cells, kinetochore microtubules are recruited to sites in the kinetochore outer plate by dynein and CENP-E (Rieder and Salmon, 1998). Factors yet to be characterized are critical for subsequent attachment and force generation, both at kinetochores and on chromosome arms (Howell et al., 2001; McEwen et al., 2001). Further regulation occurs upon biorientation of sister chromatids, to facilitate directional instability across the metaphase plate. Polymerization dynamics of the embedded kinetochore microtubule plus ends is coupled to chromosome movement, although how this relates to the dynamic instability of free microtubule plus ends is incompletely understood. For example, polymerization and depolymerization rates within the kinetochore microtubule bundle are slower than for free plus ends, and rescues and catastrophes are synchronized among a population of plus ends in a kinetochore microtubule bundle, and between corresponding kinetochore microtubule bundles of a sister pair, whereas free plus ends interconvert between polymerization and depolymerization more stochastically (for discussions, see Mitchison, 1988; Rieder and Salmon, 1994; Rieder and Salmon, 1998).
Microtubule plus end tracking proteins such as EB1 are potential candidates to contribute to kinetochore microtubule dynamics and/or attachment. EB1 promotes microtubule polymerization in vertebrate cells, while its highest affinity binding to microtubules depends on their polymerization (Tirnauer et al., 2002). The property of associating with polymerizing microtubule ends (plus end tracking) is shared by dynein/dynactin, Lis1, Clip-170, CLASPs, and adenomatous polyposis coli (APC) (reviewed in (Schuyler and Pellman, 2001; Tirnauer and Bierer, 2000). Kinetochore association has also been shown for most of these proteins, yet dynein/dynactin, Lis1, and Clip-170 associate with kinetochores in the absence of microtubules, implicating a microtubule-independent binding site (Dujardin et al., 1998; Faulkner et al., 2000; Hoffman et al., 2001; Howell et al., 2001; Kaplan et al., 2001). How (and whether) these proteins contribute to the coordinated capture, attachment, dynamic instability, and force generation of kinetochore (and nonkinetochore) microtubules remains a complex problem.
EB1 was cloned by its association with the carboxy-terminus of APC (Su et al., 1995). EB1 targets to microtubule plus ends independently of APC (Berrueta et al., 1998; Morrison et al., 1998), but APC targeting to microtubule plus ends requires EB1 (Askham et al., 2000; Mimori-Kiyosue et al., 2000a). The APC carboxy-terminus cooperates with EB1 functionally to stabilize microtubules (Nakamura et al., 2001). Two recent reports showed localization of APC to kinetochores in fixed cells (Fodde et al., 2001; Kaplan et al., 2001). Embryonic stem cells lacking APC were aneuploid, implicating APC in kinetochore-microtubule attachment. This was proposed to occur via EB1, as EB1-coated microtubule ends failed to localize to kinetochores in APC-null cells (Fodde et al., 2001; Kaplan et al., 2001). The finding that fission yeast lacking the gene for the EB1 homologue Mal3 become aneuploid is also consistent with a role for EB1 in chromosome attachment (Beinhauer et al., 1997). While attractive, a role for EB1 in chromosome attachment implies stable association of EB1 with kinetochores or kinetochore microtubules. To address this issue, we used high resolution spinning disk confocal microscopy to image EB1 in living mitotic PtK1 cells.
MATERIALS AND METHODS
Proteins and Antibodies
Human EB1 (Berrueta et al., 1998) was cloned as a BamHI-HindIII fragment into Pet28A (Novagen, Madison, WI) and confirmed by sequencing. Full-length 6-His-EB1 was expressed in BL21-PlysS cells (Novagen) and purified on nickel-agarose beads (QIAGEN, Valencia, CA) according to the manufacturer's instructions, followed by dialysis into a sodium phosphate buffer lacking imidazole and flash freezing in small aliquots. Alexa488-labeled antibody to human CENP-F was a gift from Tsahai Tafari (University of North Carolina, Chapel Hill, NC). EB1 protein and CENP-F antibodies were labeled with Alexa594 (red emission) or Alexa488 (green emission) succinimidyl esters (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. P50dynamitin was prepared as described previously (Wittmann and Hyman, 1999).
Tissue Culture and Microinjection
PtK1 cells (American Type Culture Collection, Manassas, VA) were maintained in minimal essential medium (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum, antibiotics, and antimycotics in a 37°C, 5% CO2 incubator and plated on 22-mm coverslips. To prevent EB1 adherence to glass, needles were sialinized and filled using plastic loading tips. Labeled EB1 (final concentration 0.1–0.13 mg/ml) and bovine serum albumin (final concentration 5 mg/ml) were diluted into a buffer (150 mM NaCl, 50 mM NaH2PO4, pH 8) and centrifuged for 10 min at 10,000 × g. Injections with labeled EB1 and antibodies to CENP-F were done separately. P50 (final concentration 13 mg/ml) was coinjected in the same needle as EB1. Where indicated, cells were preincubated in 10 μg/ml nocodazole (Sigma-Aldrich) or 100 μM monastrol for 1 h and maintained in the drug during injection and imaging. Coverslips were enclosed in modified Rose chambers filled with HEPES-buffered L-15 media containing antibiotics, antimycotics, 10% fetal bovine serum, and oxyrase (Oxyrase, Mansfield, OH) at 1:50 dilution (Canman et al., 2000), and imaged on a microscope stage heated to 34–36°C with an air curtain incubator (model ASI 400; Nevtek, Burnsville, VA).
Immunofluorescence
Indirect immunofluorescence was performed on cells fixed in ice-cold methanol for 5 min, and rehydrated with 0.15 M NaCl, 0.02 M Tris-Cl pH 7.4, and 0.1% Triton X-100. Blocking and primary and secondary antibody incubations were done in AbDil (2% bovine serum albumin in Tris-buffered saline with 0.1% Triton X-100 and 0.1% sodium azide) at room temperature. EB1 was detected with a monoclonal antibody (Transduction Laboratories, Lexington, KY); CREST antigens were detected with human CREST immune serum. Incubations in primary antibodies were for 2 h at room temperature, followed by washing and secondary antibodies conjugated to fluorescein isothiocyanate or rhodamine (Jackson Immunoresearch Laboratories, West Grove, PA). DNA was stained with Hoechst 33342 (Sigma-Aldrich) at 1 μg/ml in 0.15 M NaCl, 0.02 M Tris-Cl pH 7.4, and 0.1% Triton X-100 for 1 min. Coverslips were mounted in 0.5% p-phenylenediamine (Sigma-Aldrich) in 20 mM Tris, pH 8.8, with 90% glycerol.
Microscopy and Image Analysis
Images were acquired using phase contrast transillumination or epi-fluorescence illumination from a 60-mW argon/krypton laser by a Yokogawa CS10 spinning disk confocal attachment (PerkinElmer Life Sciences, Boston, MA) with a cooled ORCA ER camera (Hamamatsu Photonics, Bridgewater, NJ) mounted on a TE300 inverted microscope (Nikon, Tokyo, Japan). Image acquisition and analysis were controlled by MetaMorph software (Universal Imaging, West Chester, PA). Phase contrast images were processed using the contrast inversion feature of MetaMorph; brightness and contrast were altered linearly to highlight chromosome edges. All data analysis and interpretation were done on raw images; some time-lapse sequences were processed with the unsharp mask algorithm for presentation.
Rates of kinetochore and spindle microtubule polymerization were calculated using the track points feature of MetaMorph software on images acquired at 7- and 3-s intervals, respectively. Spindle kymographs were generated from raw images of spindles acquired at 3-s intervals by using a 10-pixel-wide line and the average intensity method.
RESULTS
EB1 Binds Polymerizing Ends of Astral and Spindle Microtubules, and a Subset of Kinetochores
We microinjected Alexa488- or Alexa594-labeled EB1 into PtK1 cells at a low level that could be visualized without causing major perturbations in microtubule dynamics or mitotic progression, as assayed by microtubule polymerization rates and chromosome movements. In interphase cells, EB1 associated with polymerizing microtubule plus ends, as was observed for green fluorescent protein (GFP)-EB1 (Mimori-Kiyosue et al., 2000b) (our unpublished data). In mitotic cells, EB1 formed comets on polymerizing ends of astral and spindle microtubules (Figure 1). Although not addressed further herein, EB1 also localized to centrosomes (Figure 1), as has been observed for dynein/dynactin and APC (Howell et al., 2001; Kaplan et al., 2001).
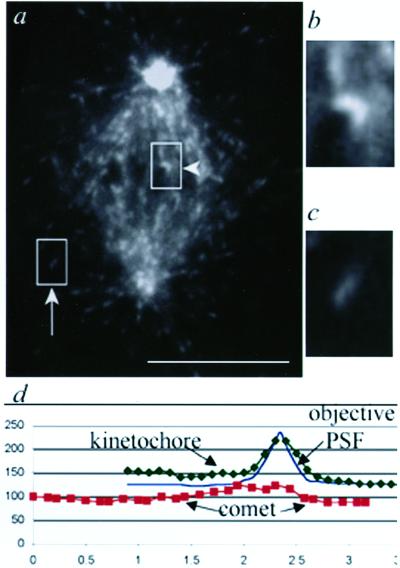
Figure 1.
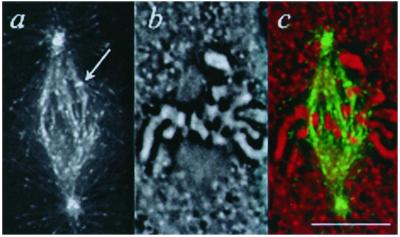
Labeled EB1 binds polymerizing ends of astral and spindle microtubules and a narrow region within the kinetochore attachment site. (a) Mitotic PtK1 cell microinjected with Alexa594-labeled EB1. One image from a time-lapse series is shown. Arrow, polymerizing astral microtubule with EB1 comet. Arrowhead, antipoleward moving kinetochore with EB1 crescent. Bar, 10 μm. (time-lapse at http://www.molbiolcell.org). (b) Enlargement of the indicated kinetochore crescent. (c) Enlargement of the indicated astral microtubule comet. (d) Fluorescence intensity line scans of the kinetochore and comet from b and c compared with a line scan through a 100-nm bead, to illustrate the point spread function of the optics (objective PSF). The intensities of the bead line scan were normalized to the maximum and minimum values for the kinetochore line scan.
The formation of EB1 comets on spindle microtubules suggested that labeled EB1 fluorescence could also be used to track the trajectories of individual microtubule plus ends within the spindle, a measurement that would be difficult to make using tubulin fluorescence. Kymographs (compressed montages of position vs. time) along the long spindle axis were constructed from time-lapse series of EB1 fluorescence in metaphase and anaphase spindles. These reproducibly showed EB1 targeting to spindle microtubules undergoing polymerization away from the associated spindle pole, but not to depolymerizing microtubules. Polymerizing microtubules sometimes crossed the spindle midline in metaphase (Figure 2, a and a′) and often extended past separated anaphase chromosomes into the central region of the spindle (Figure 2, b and b′). The mean spindle microtubule polymerization rate calculated by this kymograph method was ∼8 μm/min (for metaphase spindles, 8.1 ± 2.3 μm/min, n = 80 microtubules/5 spindles; and for anaphase spindles, 7.7 ± 2.1 μm/min, n = 79 microtubules/4 spindles). Notably, this is close to the mean astral microtubule polymerization rate (for metaphase spindles, 10.8 ± 3.1 μm/min, n = 107 microtubules/7 spindles; and for anaphase spindles, 8.0 μm/min ± 2.5, n = 79 microtubules/4 spindles). These astral microtubule polymerization rates were similar to the rate of 12.8 ± 5.7 μm/min measured using GFP-α tubulin in mitotic LLCPK cells (Rusan et al., 2001); rates of microtubule polymerization within the spindle have not been previously reported. The slight discrepancy between astral and spindle microtubule polymerization rates could represent a falsely elevated rate for astral microtubules, due to a small component of microtubule transport, or a falsely reduced rate for spindle microtubules, due to poleward flux. Our system did not allow direct testing of microtubule transport or flux because only polymerizing microtubule ends were visualized. Thus, our main finding is that microtubule polymerization was not greatly affected by the local spindle environment, and there was no evidence for a locally increased microtubule polymerization rate around chromatin. A different assay will be required to test whether spindle microtubules showed increased growth persistence or altered depolymerization rates within the spindle.
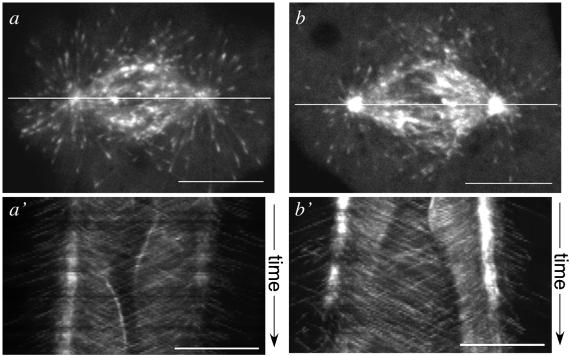
Figure 2.
Labeled EB1 tracks with polymerizing ends of nonkinetochore spindle microtubules in metaphase and anaphase. (a) Single focal plane image of a metaphase spindle in a PtK1 cell microinjected with Alexa594-labeled EB1. The line along the long spindle axis was used to generate a kymograph of position vs. time from the time-lapse series. (a′) Kymograph of the line in a, plotted vs. time (image acquisition interval 3 s). EB1 comets track away from their associated spindle pole, consistent with binding polymerizing, but not depolymerizing, microtubule ends. (b) Single focal plane image of a spindle undergoing anaphase chromosome separation, showing the line to generate a kymograph of position vs. time from the time lapse. (b′) Kymograph of the line in b, plotted vs. time (image acquisition interval 3 s). EB1 comets track away from their associated pole into and across the spindle midregion. In these kymographs, chromatin (not imaged) created a dark exclusion region in the mid spindle (a) or near the poles (b). Note the EB1 fluorescence alternating between paired sister kinetochores in metaphase (a′), and the brief period of EB1 fluorescence on an antipoleward-moving kinetochore in anaphase (b′). The spindle pole fluorescence in a seems less intense than in b because of a focal plane shift. Bars, 10 μm.
In addition to the fluorescence on individual microtubule plus ends, EB1 formed a clearly demarcated spot of fluorescence at a subset of kinetochores. In some images, this could be resolved as a crescent with the concavity toward the chromosome (Figure 1). The width of the EB1 crescent was slightly greater than the microscope objective point spread function measured with a 100-nm fluorescent bead (Figure 1c), but much less than the 1- to 1.5-μm comets at the tips of astral microtubules (Figure 1b).
EB1 Localization to Kinetochores Depends on Kinetochore Microtubules
APC, dynein/dynactin, Lis1, and Clip-170 are reported to bind kinetochores during prometaphase and largely to disappear during metaphase, as kinetochores acquire their full number of kinetochore microtubules (Fodde et al., 2001; Hoffman et al., 2001; Kaplan et al., 2001). To determine the microtubule dependence of EB1 localization, we imaged cells microinjected with labeled EB1 during prophase. Unlike these other proteins, fluorescent EB1 was not seen on prophase or early prometaphase kinetochores (Figure 3a, t = 0 and 5 min), although it did associate with the plus ends of spindle microtubules essentially instantaneously (Figure 3, t = 5 min). EB1 localized to kinetochores during late prometaphase, at an average of 13.4 ± 3.9 min (n = 6 cells) after nuclear envelope breakdown (Figure 3, t = 8 min, arrow), indicating that EB1 localization required kinetochore microtubule attachment and maturation.
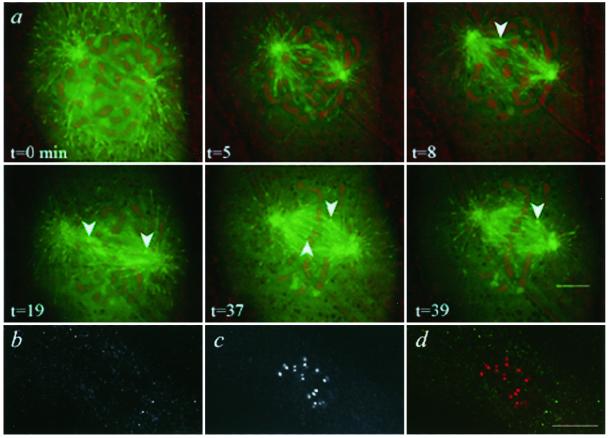
Figure 3.
EB1 is acquired at the kinetochore microtubule attachment site in late prometaphase and requires microtubule attachment to remain at kinetochores. (a) PtK1 cell microinjected with Alexa594-labeled EB1 during prophase. Images from a time-lapse series of EB1 fluorescence (green) and phase contrast (contrast inverted and red) are shown; time (minutes) is from an arbitrary start point. Before nuclear envelope breakdown and during early prometaphase, no EB1 was seen at kinetochores (t = 0 and 5 min). By late prometaphase (t = 8 min), the first kinetochore (arrow) acquired EB1 fluorescence, and additional kinetochores (t = 19 min) subsequently acquired EB1 (t = 37 and 39 min). (b–d) EB1 requires microtubule attachment to persist at kinetochores. Indirect immunofluorescence of EB1 and CREST antigens in a PtK1 cell treated with nocodazole. EB1 (b), CREST (c) and merged image of EB1 (green) and CREST (red) (d). There is no EB1 at kinetochores. Bar, 10 μm.
We tested whether kinetochore microtubule attachment was required transiently for the delivery of EB1 to kinetochores or persistently for its maintenance there. PtK1 cells growing asynchronously were treated with nocodazole for 1 h, and endogenous EB1 in mitotic cells was visualized by immunofluorescence. We used immunofluorescence for the CREST antigens (CENP-A, -B, and -C; Maney et al., 2000) to mark the kinetochores in the same cells. These images showed no evidence of EB1 localization to kinetochores upon microtubule depolymerization in all mitotic cells observed (Figure 3, b–d), consistent with the requirement for kinetochore microtubules to maintain EB1 localization.
Immunofluorescence in fixed cells was used to investigate the localization of endogenous EB1 and its position relative to core kinetochore components. The localization of labeled EB1 to kinetochores was not an artifact of labeled protein injection, because immunofluorescence of endogenous EB1 in fixed cells showed a pattern similar to the fluorescence of injected EB1 in living cells (Figure 4). On a subset of kinetochores in fixed cells, endogenous EB1 localized just distal to CREST antigens, and in cells coinjected with Alexa488-labeled EB1 and an Alexa594-labeled antibody to CENP-F, a protein of the kinetochore outer plate (Rattner et al., 1993), EB1 localized with or distal to the CENP-F (Figure 4, c and f). Thus, EB1 targeted in the region of the outer kinetochore plate or the microtubule plus end–kinetochore interface.
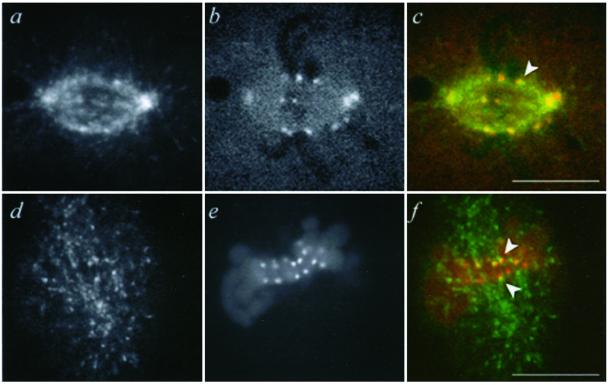
Figure 4.
Microinjected EB1 colocalizes with the kinetochore marker CENP-F and localizes similarly to endogenous EB1. (a–c) Metaphase PtK1 cell microinjected with Alexa594-labeled EB1 and Alexa488-labeled antibodies to CENP-F, imaged live. (a) EB1 fluorescence. (b) Anti-CENP-F fluorescence. (c) Merged image of EB1 (green) and CENP-F (red). (d and e) Immunofluorescence for EB1 and CREST antigens. EB1 (d), CREST (e), and merged image of EB1 (green) and CREST (red) (f). The focal plane chosen to highlight kinetochores in d–f does not contain the spindle poles, but these showed bright EB1 immunofluorescence in the original z-series of this cell. Arrows, EB1 colocalization with kinetochore marker. Bars, 10 μm.
EB1 Targeting to Kinetochores Depends on Microtubule Plus End Polymerization
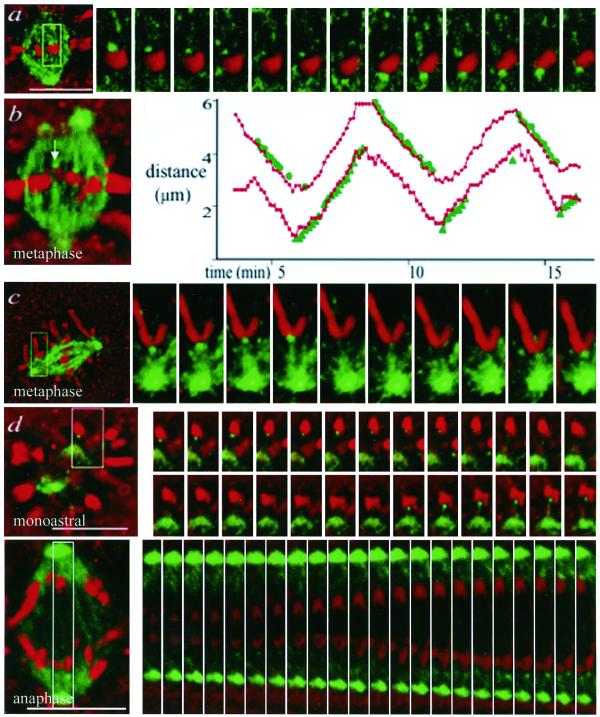
EB1 was only present on a subset of attached kinetochores (Figures 4c and 5), so we tested whether microtubule polymerization was a distinguishing factor in kinetochore targeting. We acquired paired time-lapse images of EB1 fluorescence and phase contrast morphology to make correlations for several spindle configurations. For bioriented chromosomes on bipolar spindles (Figure 5a), mono-oriented chromosomes on bipolar spindles (Figure 5c), and mono-oriented chromosomes on spindles made monopolar by monastrol treatment (Mayer et al., 1999) (Figure 5d), kinetochores became brightly labeled with EB1 during antipoleward chromosome movement, corresponding to kinetochore microtubule polymerization, and lost EB1 fluorescence during poleward movement, corresponding to depolymerization. For separating sister chromosomes in anaphase, microtubule depolymerization predominated, but during the brief episodes of antipoleward movement associated with microtubule polymerization, EB1 targeted to kinetochores as well (Figure 5e). Thus, passage beyond the mitotic checkpoint did not affect EB1 targeting. The correlation between EB1 localization and chromosome movements is illustrated graphically in Figure 5b and dynamically in the supplemental movies. The velocity of kinetochore antipoleward movements was 1.5 ± 0.6 μm/min, similar to that seen in uninjected cells (Khodjakov and Rieder, 1996). We also saw instances of EB1 localizing to kinetochores on chromosomes pausing during metaphase, where poleward microtubule flux is associated with a microtubule polymerization rate of 0.5 μm/min (Mitchison, 1989). EB1 localization is thus the first specific marker of the polymerization status of kinetochore microtubules.
Figure 5.
EB1 binds polymerizing kinetochore microtubule ends on bioriented, mono-oriented, and anaphase chromosomes. PtK1 cells were microinjected with Alexa594-labeled EB1; paired phase contrast and fluorescence images (phase contrast, red; EB1, green) were acquired every 7 s. (a) Metaphase cell, bioriented chromosome. EB1 localizes to kinetochores with polymerizing microtubules. (time lapse at http://www.molbiocell.org). (b) Metaphase cell with the corresponding graph of the EB1 to spindle pole distance (green) and chromosome constriction to spindle pole distance (red). EB1 localization on both sister chromatids is shown on the same graph. (c) Late prometaphase cell, mono-oriented chromosome. On this chromosome, clearly separated from the metaphase plate, EB1 is present during microtubule polymerization. (d) Monastrol-treated cell. Chromosomes are well separated and EB1 can be seen to target to kinetochores during kinetochore microtubule polymerization. Occasionally, targeting to both kinetochores of a pair is visible (time lapse at http://www.molbiolcell.org). (e) Anaphase cell. Most kinetochore microtubules are depolymerizing, but in rare cases of kinetochore microtubule polymerization during brief oscillations, EB1 is visible at the kinetochore. (time lapse series at http://www.molbiolcell.org). Images were processed to highlight the kinetochore-localized EB1. Bars, 10 μm.
To verify that loss of EB1 from poleward-moving kinetochores was not due to a focal plane shift, we acquired paired images from cells coinjected cells with Alexa488-labeled EB1 and Alexa594-labeled antibodies to CENP-F. At the concentration used, these antibodies did not inhibit chromosome oscillations, progression to anaphase, or EB1 localization (our unpublished data). These showed that the EB1 signal disappeared during microtubule depolymerization even when the CENP-F antigen remained in the focal plane (our unpublished data).
EB1 Binds Kinetochores Independently of Dynein/Dynactin
Dynein/dynactin accumulate at kinetochores in the absence of microtubules and are depleted upon microtubule capture, indicating a microtubule-independent binding site for dynein/dynactin on the kinetochore (Dujardin et al., 1998; Faulkner et al., 2000; Hoffman et al., 2001; Howell et al., 2001). EB1 interacts with dynein and the dynactin complex (Berrueta et al., 1999), binding directly to the p150glued dynactin subunit (Tirnauer et al. 2002). We asked whether localization of EB1 to kinetochores depended on dynein/dynactin by coinjecting p50dynamitin to disrupt the dynactin complex and dissociate dynein from kinetochores (Echeverri et al., 1996; Howell et al., 2001). P50dynamitin injection induced the expected metaphase arrest, spindle widening, and separation of centrosomes from the ends of spindle fibers (Howell et al., 2001). In these p50dynamitin-injected cells, EB1 localization to kinetochores was preserved, demonstrating it was independent of dynein/dynactin function (Figure 6).
Figure 6.
EB1 localization to the kinetochore microtubule attachment site is independent of dynein. PtK1 cell coinjected with Alexa594-labeled EB1 and p50dynamitin. (a) EB1 fluorescence. (b) Phase contrast image, contrast-inverted. (c) Merged image of EB1 (green) and phase contrast (red). Arrow shows EB1 targeting to a kinetochore. Bar, 10 μm.
DISCUSSION
Unlike Other Plus End Tracking Proteins, EB1 Targets to Kinetochores during Kinetochore Microtubule Polymerization
Although the microtubule polymerization requirement for EB1 at kinetochores is not surprising based on targeting to other populations of polymerizing microtubules, it contrasts with the other microtubule plus end tracking proteins characterized to date, such as CLIP-170, Lis1, and dynein (Dujardin et al., 1998; Faulkner et al., 2000; Hoffman et al., 2001). These bind kinetochores independently of microtubules; in fact, their binding is enhanced upon the microtubule loss induced by nocodazole treatment (Hoffman et al., 2001). Recently, a hierarchy of binding among dynein, Lis1, and CLIP-170 to kinetochores has been characterized, with dynein most proximal to the kinetochore, dynein heavy chain (on two sites) binding Lis1, and Lis1 recruiting CLIP-170 (Coquelle et al., 2002; Tai et al., 2002). Our finding that EB1 bound to antipoleward oscillating kinetochores in the presence of p50dynamitin is relevant to this line of investigation, because it suggests that EB1 at kinetochores is not simply part of this large dynein/dynactin-dependent complex. Although EB1 interacts with dynein/dynactin in the bulk cytosol of tissue culture cells (Berrueta et al., 1999) and Xenopus eggs (Tirnauer et al., 2002), at the kinetochore outer plate they may be functionally or spatially separated. The distinct pattern of EB1 targeting to kinetochores relative to the other plus end tracking proteins implies an alternative mechanism of association with kinetochores and possibly different functions there.
EB1 Targeting to Kinetochore Microtubule Plus Ends Compared with Free Microtubule Plus Ends
Kymographs of EB1 fluorescence revealed its localization to polymerizing kinetochore, spindle, and astral microtubules, consistent with a similar mechanism of targeting in all cases. EB1 is thus a valuable marker for tracking microtubule polymerization rates at all of these sites. Although the localization pattern is similar, the crescent spot at the kinetochore is compact compared with the extended comet tail seen on the ends of nonkinetochore microtubules. The spot on kinetochore microtubules could reflect a cluster of comets with very short tails, because kinetochore microtubules polymerize approximately ninefold slower than astral microtubules. Alternatively, signaling proteins may reduce the length of the EB1 signal at kinetochore microtubules. In support of this idea, we found that EB1 comets were shorter in mitotic tissue culture cells compared with interphase cells (Tirnauer and Mitchison, unpublished data), possibly reflecting shifts in the kinase-phosphatase balance during mitosis.
Two models, not mutually exclusive, could account for microtubule polymerization-specific EB1 localization to kinetochores. EB1 might bind to polymerizing kinetochore microtubules, or it might bind to a kinetochore component(s) in a manner that is coregulated with kinetochore behavior. Currently, it is unknown whether conversion between microtubule polymerization and depolymerization at kinetochores is controlled by chemical changes (such as phosphorylation events) or whether the changes are purely structural. Detailed analysis of EB1 targeting could help address this fundamental issue of kinetochore mechanochemistry. With respect to EB1 targeting, our data suggest that polymerizing ends of kinetochore microtubules resemble polymerizing ends of free microtubules, and depolymerizing microtubule ends at kinetochores resemble depolymerizing free microtubule ends. These parallels support the hypothesis that directional instability of kinetochores is based on the dynamic instability of microtubule plus ends.
Potential EB1 Functions at the Kinetochore
We envision three potential roles for EB1 at the kinetochore. First, EB1 may affect the polymerization dynamics of microtubules in the kinetochore bundle. For example, it could reduce the frequency of catastrophes on kinetochore microtubules, as it does for cytoplasmic microtubules in yeast (Tirnauer et al., 1999) and for astral microtubules in Xenopus egg extracts (Tirnauer et al., 2002). Such an effect would explain a paradox in the literature: the drastically reduced turnover of kinetochore microtubules compared with free microtubules in vivo (Mitchison and Kirschner, 1985; Zhai et al., 1995), but the enhanced dynamic instability of microtubules that interact with kinetochores in vitro (when EB1 is not present) (Hyman and Mitchison, 1990). A recent study showed that the ability of EB1 to promote polymerization of microtubules in vitro was dependent on the APC carboxy terminus (Nakamura et al., 2001). This result raises the possibility that EB1 at microtubule plus ends might not fully stabilize microtubules until it interacts with an activator (APC or another protein) at the kinetochore. Such regulation would allow microtubules that are not captured to undergo catastrophes, while facilitating stabilization of those that do attach. In addition to reducing catastrophes for individual microtubules, regulating polymerization of kinetochore microtubules could help synchronize their dynamic instability, an inherently stochastic process that must be coordinated within the kinetochore microtubule bundle for chromosome oscillations to occur.
A second potential function for EB1 at kinetochores is in microtubule end-on attachment. EB1 has been proposed to mediate binding of kinetochore microtubules to kinetochores via APC, because APC-null cells become aneuploid, and in fixed preparations colocalization of EB1 with APC is lost (Fodde et al., 2001; Kaplan et al., 2001). This is an attractive model, because the budding yeast EB1 homologue Bim1p, in addition to promoting microtubule polymerization, links microtubule ends to the cortical protein Kar9p at the bud tip (Tirnauer et al., 1999; Korinek et al., 2000; Lee et al., 2000; Miller et al., 2000). Although they are not clear sequence homologues, there may be functional overlap between Kar9p and APC (Mimori-Kiyosue and Tsukita, 2001; Schuyler and Pellman, 2001). Based on our observations, a protein–protein interaction involving EB1 would not be able to provide continuous kinetochore-microtubule attachment, because EB1 only targets to kinetochores during microtubule polymerization. This result raises the possibility that separate anchoring complexes could associate with polymerizing vs. depolymerizing kinetochore microtubules, although it does not establish whether EB1 is a component of such a complex. If it were, EB1 might preferentially bind to newly added tubulin subunits at the tips of polymerizing kinetochore microtubules and therefore continually bias kinetochore binding to the most recently added subunits.
A final potential function for EB1 at the kinetochore, distinct from microtubule stabilization and attachment, may be to tag the microtubule end, allowing other proteins to find and distinguish this unique site. For proteins that specifically need to act at attached kinetochores, binding polymerizing microtubule plus ends might be more advantageous than binding to the microtubule-independent kinetochore proteins. Our studies do not determine whether EB1 performs some, all, or none of the proposed roles at the vertebrate kinetochore. Determining EB1 function at kinetochores is likely to require EB1 mutations that differentially affect its kinetochore targeting, or perturbation of critical EB1 binding partners at the kinetochore.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tsahai Tafari for the generous gift of labeled antibodies, Paul Maddox for expert microscopy advice, and members of the Mitchison laboratory for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health grants K08 DK-02578 and R03 DK 58766 (to J.S.T.), GM-24364 (to E.D.S.), GM-39565 (to T.J.M.), and a Universal Imaging Corporation Fellowship to the Cell Division Group at the Marine Biological Laboratory, Woods Hole, MA.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0236. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0236.
REFERENCES
- Askham JM, Moncur P, Markham AF, Morrison EE. Regulation, and function of the interaction between the APC tumor suppressor protein, and EB1. Oncogene. 2000;19:1950–1958. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- Beinhauer JD, Hagan IM, Hegemann JH, Fleig U. Mal3, the fission yeast homolog of the human APC-interacting protein EB-1, is required for microtubule integrity and the maintenance of cell form. J Cell Biol. 1997;139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Kraeft S-K, Tirnauer JS, Schuyler S, Chen LB, Hill DE, Pellman D, Bierer B. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc Natl Acad Sci USA. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- Canman JC, Hoffman DB, Salmon ED. The role of pre- and post-anaphase microtubules in the cytokinesis phase of the cell cycle. Curr Biol. 2000;10:611–614. doi: 10.1016/s0960-9822(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Coquelle FM, et al. Lis1, CLIP-170's key to the dynein/dynactin pathway. Mol Cell Biol. 2002;22:3089–3102. doi: 10.1128/MCB.22.9.3089-3102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin D, Wacker UI, Moreau A, Schroer TA, Rickard JE, De Mey JR. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner NE, Dujardin DL, Tai C-Y, Vaughan KT, O'Connell CB, Wang Y-L, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Fodde R, et al. Mutations in the APC tumor suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ. Modulation of microtubule stability by kinetochores in vitro. J Cell Biol. 1990;110:1607–1616. doi: 10.1083/jcb.110.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek WS, Copeland MJ, Chaudhuri A, Chant J. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science. 2000;287:2257–2259. doi: 10.1126/science.287.5461.2257. [DOI] [PubMed] [Google Scholar]
- Lee L, Tirnauer JS, Li J, Schuyler SC, Liu JY, Pellman D. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science. 2000;287:2260–2262. doi: 10.1126/science.287.5461.2260. [DOI] [PubMed] [Google Scholar]
- Maney T, Ginkel LM, Hunter AW, Wordeman L. The kinetochore of higher eukaryotes: a molecular view. Int Rev Cytol. 2000;194:67–131. doi: 10.1016/s0074-7696(08)62395-5. [DOI] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell. 2001;12:2776–2789. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Cheng S-C, Rose MD. Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules. Mol Biol Cell. 2000;11:2949–2959. doi: 10.1091/mbc.11.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules, and concentrates at their growing ends in epithelial cells. J Cell Biol. 2000a;148:505–517. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000b;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Tsukita S. Where is APC going? J Cell Biol. 2001;154:1105–1109. doi: 10.1083/jcb.200106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ. Microtubule dynamics and kinetochore function in mitosis. Annu Rev Cell Biol. 1988;4:527–549. doi: 10.1146/annurev.cb.04.110188.002523. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW. Properties of the kinetochore in vitro. II. Microtubule capture and ATP-dependent translocation. J Cell Biol. 1985;101:766–777. doi: 10.1083/jcb.101.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumor suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Zhou XZ, Lu KP. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol. 2001;11:1062–1067. doi: 10.1016/s0960-9822(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Rattner JB, Rao A, Fritzler MJ, Valencia DW, Yen TJ. CENP-F is a ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskeleton. 1993;26:214–226. doi: 10.1002/cm.970260305. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan NM, Fagerstrom CJ, Yvon A-MC, Wadsworth P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-α tubulin. Mol Biol Cell. 2001;12:971–980. doi: 10.1091/mbc.12.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler S, Pellman D. Microtubule “plus-end-tracking proteins”: the end is just the beginning. Cell. 2001;105:421–424. doi: 10.1016/s0092-8674(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Su L-K, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Tai C-Y, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in Lis1 kinetochore function. J Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, Grego S, Salmon ED, Mitchison TJ. EB1-microtubule interactions in xenopus egg extracts: role of EB1 in microtubule stabilization and mechanisms of targeting to microtubules. Mol Biol Cell. 2002;13:3614–3626. doi: 10.1091/mbc.E02-04-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, O'Toole E, Berrueta L, Bierer B, Pellman D. Yeast Bim1p promotes the G1-specific dynamics of microtubules. J Cell Biol. 1999;145:993–1007. doi: 10.1083/jcb.145.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Hyman T. Recombinant p50/dynamitin as a tool to examine the role of dynactin in intracellular processes. Methods Cell Biol. 1999;61:137–143. doi: 10.1016/s0091-679x(08)61978-0. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics at the metaphase-anaphase transition. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.