Abstract
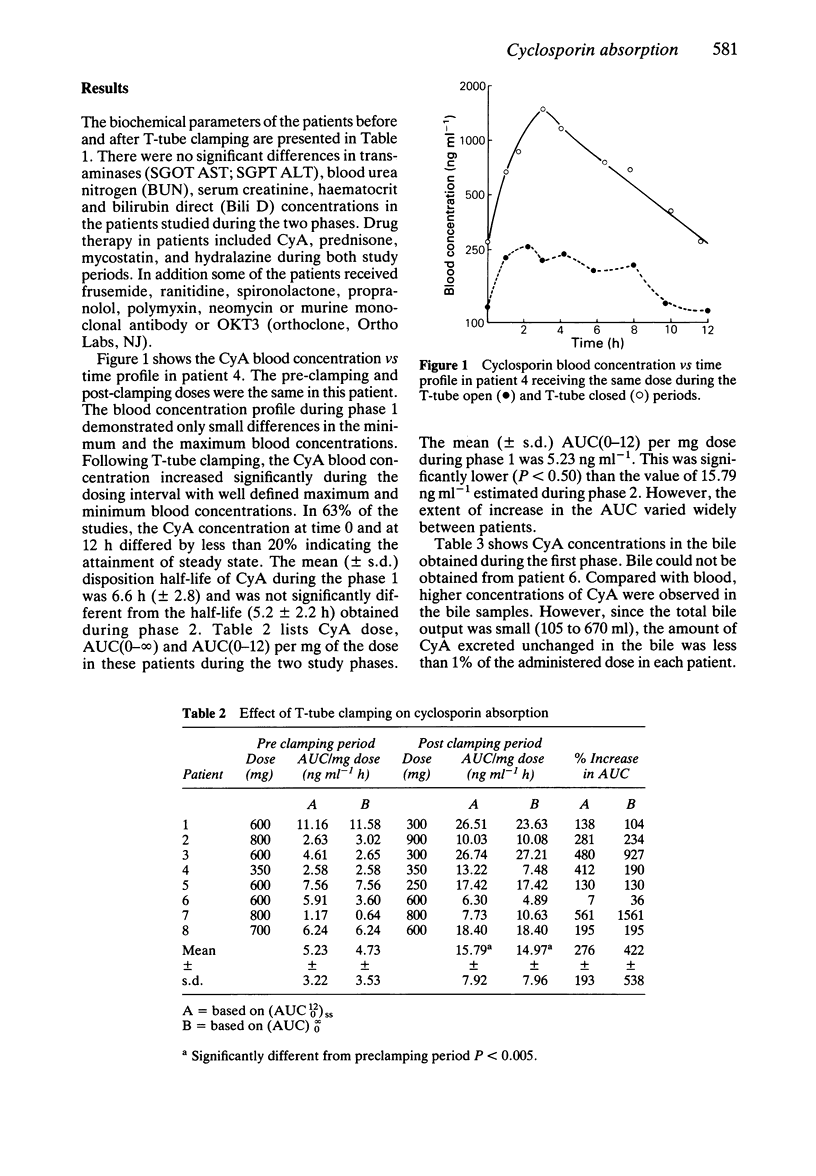
1. We quantitated the effect of biliary diversion on cyclosporin (CyA) absorption in liver transplant patients. Multiple blood samples were obtained over one dosing interval following oral CyA administration in eight liver transplant patients before and after T-tube clamping. 2. Cyclosporin concentrations were measured by a high pressure liquid chromatographic method. 3. The mean (+/- s.d.) dose-normalized area under the blood concentration vs time curve (AUC) over a dosing interval was 5.23 (+/- 3.22) ng ml-1 h during the pre clamping period and 15.79 +/- 7.92 ng ml-1 h during the post clamping period. A significant (P less than 0.05) increase (276%) in the dose normalized AUC was observed following the T-tube clamping. 4. Analysis of bile revealed less than 1 mg (less than 1% of dose) of unchanged CyA to be eliminated in bile over 12 h. Therefore the increased CyA AUC/dose following T-tube ligation cannot be explained by enterohepatic recirculation. 5. Increased bile flow secondary to clamping of the T-tube most likely increased the absorption of CyA. Increase in CyA absorption may be due to a bile mediated increase in CyA solubility or gastrointestinal membrane permeability, or increased residence time at the site of absorption. 6. Independent of the mechanism involved, our results indicate that adjustments in CyA dosage must be made whenever external bile diversion is instituted or discontinued.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W., Iwatsuki S., Shaw B. W., Jr, Starzl T. E. Cyclosporine monitoring in liver transplant patients. Transplantation. 1985 Mar;39(3):338–338. [PubMed] [Google Scholar]
- Aukee S., Venho V. M., Jussila J., Karjalainen P. Drug absorption in patients with T-tube after cholecystectomy. Ann Clin Res. 1975 Feb;7(1):42–46. [PubMed] [Google Scholar]
- Bauer L. A., Gibaldi M. Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci. 1983 Aug;72(8):978–979. doi: 10.1002/jps.2600720843. [DOI] [PubMed] [Google Scholar]
- Burckart G. J., Venkataramanan R., Ptachcinski R. J., Starzl T. E., Gartner J. C., Jr, Zitelli B. J., Malatack J. J., Shaw B. W., Iwatsuki S., Van Thiel D. H. Cyclosporine absorption following orthotopic liver transplantation. J Clin Pharmacol. 1986 Nov-Dec;26(8):647–651. doi: 10.1002/j.1552-4604.1986.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers S. G., Dujovne C. A. Digoxin therapy during T-tube biliary drainage in man. JAMA. 1978 Dec 15;240(25):2756–2757. [PubMed] [Google Scholar]
- Griffith B. P., Hardesty R. L., Deeb G. M., Starzl T. E., Bahnson H. T. Cardiac transplantation with cyclosporin A and prednisone. Ann Surg. 1982 Sep;196(3):324–329. doi: 10.1097/00000658-198209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer G., Loosli H. R., Schreier E., Keller B. Disposition of cyclosporine in several animal species and man. I. Structural elucidation of its metabolites. Drug Metab Dispos. 1984 Jan-Feb;12(1):120–126. [PubMed] [Google Scholar]
- Mayersohn M., Feldman S., Gibaldi M. Bile salt enhancement of riboflavin and flavin mononucleotide absorption in man. J Nutr. 1969 Jul;98(3):288–296. doi: 10.1093/jn/98.3.288. [DOI] [PubMed] [Google Scholar]
- PEKANMAKI K., SALMI H. A. The absorption and excretion of phenolphthalein and its glucuronide by the cat. Acta Pharmacol Toxicol (Copenh) 1961;18:133–140. doi: 10.1111/j.1600-0773.1961.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Burckart G. J. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986 Mar-Apr;11(2):107–132. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Rosenthal J. T., Burckart G. J., Taylor R. J., Hakala T. R. Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther. 1985 Sep;38(3):296–300. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Iwatsuki S., Van Thiel D. H., Gartner J. C., Zitelli B. J., Malatack J. J., Schade R. R., Shaw B. W., Jr, Hakala T. R., Rosenthal J. T. Evolution of liver transplantation. Hepatology. 1982 Sep-Oct;2(5):614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Porter K. A., Iwatsuki S., Schröter G. P. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981 Jul 30;305(5):266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Weil R., 3rd, Porter K. A., Iwatsuki S., Schroter G. P., Fernandez-Bueno C., MacHugh N. Cyclosporin A and steroid therapy in sixty-six cadaver kidney recipients. Surg Gynecol Obstet. 1981 Oct;153(4):486–494. [PMC free article] [PubMed] [Google Scholar]
- Teo N. H., Scott J. M., Neale G., Weir D. G. Effect of bile on vitamin B12 absorption. Br Med J. 1980 Sep 27;281(6244):831–833. doi: 10.1136/bmj.281.6244.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutschka P. J., Beschorner W. E., Hess A. D., Santos G. W. Cyclosporin-A to prevent graft-versus-host disease: a pilot study in 22 patients receiving allogeneic marrow transplants. Blood. 1983 Feb;61(2):318–325. [PubMed] [Google Scholar]
- Venkataramanan R., Burckhart G. J., Ptachcinski R. J. Pharmacokinetics and monitoring of cyclosporine following orthotopic liver transplantation. Semin Liver Dis. 1985 Nov;5(4):357–368. doi: 10.1055/s-2008-1040633. [DOI] [PubMed] [Google Scholar]


