Abstract
The phosphorylated, activated cytoplasmic domains of the transforming growth factor-β (TGFβ) receptors were used as probes to screen an expression library that was prepared from a highly TGFβ-responsive intestinal epithelial cell line. One of the TGFβ receptor-interacting proteins isolated was identified to be the mammalian homologue of the LC7 family (mLC7) of dynein light chains (DLCs). This 11-kDa cytoplasmic protein interacts with the TGFβ receptor complex intracellularly and is phosphorylated on serine residues after ligand-receptor engagement. Forced expression of mLC7-1 induces specific TGFβ responses, including an activation of Jun N-terminal kinase (JNK), a phosphorylation of c-Jun, and an inhibition of cell growth. Furthermore, TGFβ induces the recruitment of mLC7-1 to the intermediate chain of dynein. A kinase-deficient form of TGFβ RII prevents both mLC7-1 phosphorylation and interaction with the dynein intermediate chain (DIC). This is the first demonstration of a link between cytoplasmic dynein and a natural growth inhibitory cytokine. Furthermore, our results suggest that TGFβ pathway components may use a motor protein light chain as a receptor for the recruitment and transport of specific cargo along microtublules.
INTRODUCTION
Transforming growth factor-β (TGFβ) is the prototype for the TGFβ superfamily of highly conserved growth regulatory polypeptides that also includes the activins, inhibins, bone morphogenetic proteins, decapentaplegic (Dpp), nodal, Lefty, and others (Roberts, 1998; Sporn and Vilcek, 2000; Yue and Mulder, 2001). Alterations in the TGFβ signaling components and pathways have been implicated in a vast array of human pathologies, including cancer (Massague et al., 2000; Sporn and Vilcek, 2000; Derynck et al., 2001).
TGFβ binds to two types of transmembrane serine/threonine kinase receptors (RI and RII) in a heterotetrameric complex, to activate downstream components (Roberts, 1998; Massague et al., 2000; Sporn and Vilcek, 2000; Yue and Mulder, 2001). The Smad family of signaling intermediates plays an important role in mediating TGFβ responses (Attisano and Wrana, 2000; ten Dijke et al., 2000; Yue and Mulder, 2001). Moreover, TGFβ has been shown to regulate Ras (Mulder and Morris, 1992; Hartsough et al., 1996; Yue et al., 1998) and several components of the mitogen-activated protein kinase (Mapk) pathways (Hartsough and Mulder, 1995; Frey and Mulder, 1997; Mulder, 2000; Sporn and Vilcek, 2000; Yue and Mulder, 2001). In addition to the Ras/Mapk and Smad pathways, several proteins have been identified based upon their interaction with the TGFβ receptors (Yue and Mulder, 2001). Furthermore, various Smad-interacting proteins have also been identified, including SARA and Dab2, which interact with both Smads and the TGFβ receptors (Tsukazaki et al., 1998; Hocevar et al., 2001; Yue and Mulder, 2001).
Despite advances in our understanding of the mechanisms by which the Smad and Ras/Mapk cascades mediate some TGFβ effects, these pathways seem to regulate primarily transcriptional events (Hocevar et al., 1999; Hu et al., 1999; Sporn and Vilcek, 2000; Yue and Mulder, 2000a, 2001). However, TGFβ is multifunctional and its biological responses are diverse. Thus, identification of additional TGFβ signaling pathways and components will assist in our understanding of the mechanisms by which alterations in these pathways contribute to human disease.
Dynein is a molecular motor protein that mediates intracellular transport by conveying cargo along polarized microtubules (MTs) toward the minus ends (Hirokawa, 1998). Cytoplasmic dynein superfamily members control various cell functions and are important for establishing epithelial polarity (Tai et al., 2001). Several different subunits of cytoplasmic dynein can bind to a variety of cargoes (Kamal and Goldstein, 2002; Karcher et al., 2002). However, little is known about the regulation of the movement that dynein motors drive. Two dynein intermediate chains (DICs) are known to be important for cargo binding. In addition, most cargoes interact with dynein through dynactin, which binds to DIC (Kamal and Goldstein, 2002; Karcher et al., 2002). Four light intermediate chains (LICs) and several dynein light chains (DLCs) also seem to be involved in imparting proper cargo selection. Finally, a variety of receptor systems and transporters have been shown to bind to molecular motors, either directly through the light chains (LCs), or through motor receptors or adaptor proteins (Klopfenstein et al., 2000; Kamal and Goldstein, 2002; Karcher et al., 2002).
Motor protein binding and transport of cargoes intracellularly sometimes utilizes a set of proteins involved in cell signaling (Bowman et al., 2000; Goldstein, 2001). For example, the Jun N-terminal kinase (JNK)-interacting proteins (JIPs) are thought to serve as scaffolding proteins for the JNK signaling pathway (Davis, 2000). These JIP proteins also bind with high affinity and specificity to the motor protein kinesin (Verhey et al., 2001). It is thought that kinesin carries the JIP scaffolding proteins, preloaded with cytoplasmic and transmembrane signaling molecules. Similarly, dynein-dependent movement of signaling molecules along MTs has been reported. For example, p53 was found to be localized to the MTs and physically associated with tubulin (Giannakakou et al., 2000). The transport of p53 along MTs was dynein dependent, suggesting that the interaction of p53 with dynein facilitated its accumulation in the nucleus after DNA damage (Giannakakou et al., 2000). Furthermore, a receptor–DLC interaction has been reported for the photoreceptor rhodopsin (Tai et al., 1999). The interaction between rhodopsin and Tctex-1 is thought to represent a novel mode of dynein–cargo interaction in which a dynein subunit directly binds to an integral membrane protein cargo molecule that serves as a dynein receptor.
Activation of a motor may occur by posttranslational modifications, local changes in the cellular environment, or chaperone binding (Hollenbeck, 2001). Because growth factors and cytokines are known to regulate such events, the receptors and signaling pathways for these polypeptides are potential mediators of motor protein activation and organelle trafficking, events that ultimately determine the collective spatial organization of the signaling pathways within the cell.
Herein, we describe a mammalian TGFβ receptor-interacting protein, termed mLC7-1, which is also a DLC. TGFβ stimulates not only the phosphorylation of mLC7-1, but also the recruitment of mLC7-1 to the DIC. Kinase-active TGFβ receptors are required for mLC7-1 phosphorylation and interaction with DIC. Recruitment of DLCs to the dynein complex is important not only for specifying the cargo that will bind (Vaughan and Vallee, 1995), but also for the regulation of intracellular transport itself (Karcher et al., 2002). Thus, mLC7-1 seems to function as a motor receptor, linking the dynein motor to specific cargo. We also demonstrate that mLC7-1 can mediate specific TGFβ responses, including JNK activation, c-Jun phosphorylation, and growth inhibition.
MATERIALS AND METHODS
Reagents
The anti-FLAG M2 (F3165) and anti-c-myc (M5546) antibodies and mouse IgG were from Sigma-Aldrich (St. Louis, MO). The anti-DIC monoclonal antibody was from Chemicon (Temecula, CA). The anti-V5 antibody (R960 25) was obtained from Invitrogen (Carlsbad, CA) and the anti-hemagglutinin (HA) antibody (1-583-816) was from Roche Applied Science (Indianapolis, IN). The TGFβ RII antibody (SC-220-G or -R), the phospho-c-Jun antibody (KM-1, SC-822), and rabbit IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Protein A or G agarose were purchased from Invitrogen. 125I-TGFβ (NEX-267), [32P]orthophosphate (NEX-053), γ-[32P]ATP (BLU002H), and [3H]thymidine (NET-027X) were from PerkinElmer Life Sciences (Boston, MA). TGFβ1 was purchased from R & D Systems (Minneapolis, MN).
Cell Culture
COS-1 cells (CRL-1650) and Mv1Lu cells (CCL-64) were obtained from American Type Culture Collection (Manassas, VA) and were grown in DMEM supplemented with 10% fetal bovine serum. 293T cells were obtained from T.-W. Wong (Bristol-Myers Squibb, Princeton, NJ) and were maintained as for COS-1 cells. Madin-Darby canine kidney (MDCK) cells (CCL-34) were grown in minimal essential medium-α, supplemented with 10% fetal bovine serum. Cultures were routinely screened for mycoplasma by using Hoechst staining.
Cloning of TGFβ Receptor Targets
Construction of TGFβ Receptor Expression Plasmids.
The intracellular domains of TGFβ RII and RI were polymerase chain reaction (PCR) amplified using the full-length human cDNA's for TGFβ RII (Lin et al., 1992) or TGFβ RI (Franzen et al., 1993), respectively, as templates. These domains were inserted into the pET15b-mod (containing N-terminal His and FLAG tags) or pET30c (containing N-terminal His and S tags) expression constructs, respectively, and the correct DNA sequences were confirmed.
Expression and Activation of Intracellular Domains.
The BLR (DE3) or HMS174 (Novagen) Escherichia coli strains were transformed separately with each of the TGFβ receptor-containing vectors or the corresponding empty vectors (EVs), followed by selection on kanamycin and ampicillin. Expression was induced with isopropyl β-d-thiogalactoside and verified by Western blotting using tag antibodies that differed for each receptor cytoplasmic domain. Recombinant receptor domains were affinity purified sequentially to isolate heteromeric receptors enriched for the activated complex. In vitro kinase assays (Bassing et al., 1994) were performed to phosphorylate the intracellular domains. Phosphorylation of both RI and RII was confirmed by SDS-PAGE. The higher degree of RI phosphorylation in kinase reactions performed with both receptors, as opposed to only RI, suggested that transphosphorylation of RI by RII had occurred. Supernatants derived from kinase assays with cold ATP were used to approximate the specific activity of 32P-labeled proteins.
Preparation and Screening of Expression Library from IEC 4-1 Cells.
An expression library was prepared from the rat 4-1 IEC line (Mulder et al., 1993) by using the Superscript Choice System for cDNA synthesis (Invitrogen). Double-stranded cDNA ligated to EcoRI adaptors was size selected, and relevant fractions were pooled and ligated into the TriplEx expression vector (CLONTECH, Palo Alto, CA). The ligated DNA was incorporated into phage particles (Gigapack II gold; Stratagene, La Jolla, CA) and titered by infection of E. coli strain XL1-Blue, according to the manufacturer's instructions (CLONTECH). Recombinant phage were screened using a modified CORT protocol (Skolnik et al., 1991). Briefly, the activated intracellular domains of both TGFβ receptors (prepared as described above) were incubated with filters, and interactions between phosphorylated receptors and library-expressed proteins were detected by autoradiography. Positive plaques were picked and enriched. Numerous positive clones were identified using this method, of which one will be described in detail herein. A partial cDNA of approx. 463 base pairs was originally isolated and sequenced (kathleen mulder #23 in the series, km23). This partial cDNA was then used to obtain the full-length rat km23 gene, including the 5′ and 3′ regions. A human placental cDNA library (CLONTECH) was screened to isolate human km23 (hkm23). On comparison of our sequence with human expressed sequence tags in the database, the full-length hkm23 gene was obtained. The nucleotide sequences for human (accession no. AY026513) and rat (AY026512) km23 are available at http://www.ncbi.nlm.nih.gov:80/entrez. The protein identifications are AAK18712 and AAK18711, respectively.
Transient Transfections, 125I-TGFβ Cross-Linking, Immunoprecipitation/blot, Westerns, and In Vivo Phosphorylation Assays
These assays were performed essentially as described previously (Hocevar et al., 1999; Yue et al., 1999a; Yue and Mulder, 2000a). To prepare RI-V5, the Alk-5 cDNA was digested with NotI and XhoI restriction enzymes, followed by subcloning into pcDNA3.1/V5-His (V-810-20; Invitrogen). To prepare km23-FLAG, the coding region of rat or human km23 was PCR amplified with additional suitable flanking restriction enzyme sites for BglII (5′) and SalI (3′) and inserted into pCMV5-FLAG (Sigma-Aldrich) after digestion with BglII and SalI restriction enzymes. 293T, MDCK, COS-1, or Mv1Lu cells were transiently transfected using either LipofectAMINE Plus (catalog no. 10964-013; Invitrogen) or LipofectAMINE 2000 (catalog no. 11668-027; Invitrogen), according to the manufacturer's instructions.
Phosphoamino Acid Analysis
COS-1 cells were transfected and labeled as for in vivo phosphorylation assays. After the cell lysates were normalized for radioactivity, labeled km23/mLC7-1 protein was immunoprecipitated with anti-FLAG, separated by SDS-PAGE, transferred, and visualized by autoradiography. The membrane containing 32P-labeled km23/mLC7-1 was excised, and phosphoamino acid analysis was performed as described previously (Boyle et al., 1991).
Stable Transfections
hkm23-FLAG was inserted into a pEGFP-C1 plasmid (CLONTECH) to create an N-terminal GFP tag. The resulting construct or the equivalent EV was transfected into Mv1Lu cells using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's protocol. Twenty-four hours after transfection the cells were split at a ratio of 1:5. After another 24 h, 1000 μg/ml G418 was added for a selection period of 11 d, at which time surviving colonies were pooled and maintained in the presence of 1000 μg/ml G418. Expression of km23/mLC7-1 was verified by Western blot analysis, and stably transfected pools of km23-FLAG or EV-transfected pools were used for JNK, c-Jun, and growth assays.
JNK In Vitro Kinase Assays
These assays were performed as described previously (Frey and Mulder, 1997; Yue and Mulder, 2000b), except that anti-JNK (C-17; Santa Cruz Biotechnology) was used for the immunoprecipitations (IPs) and glutathione S-transferase (GST)-c-JUN (1-79) (Santa Cruz Biotechnology) was the substrate.
Growth Assays
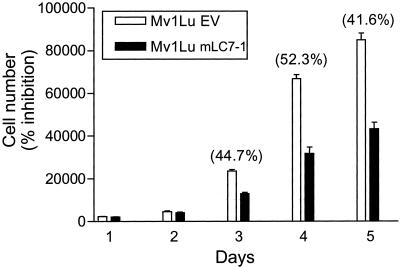
The TGFβ responsiveness of cells was verified by [3H]thymidine incorporation assays, performed as described previously (Hartsough and Mulder, 1995). For Figure 5, pools of Mv1Lu cells stably transfected with km23-FLAG or EV were plated at 2 × 103 cells per 96-well dish and were analyzed at several days thereafter using crystal violet (EMScience #1011; Fisher Scientific, Pittsburgh, PA), according to the assay protocol at http://www-ufk.med.uni-rostock.de/lablinks/protocols/e_protocols/cvassay.htm.
Figure 5.
Stable expression of mLC7-1 results in growth inhibition in the absence of TGFβ. Mv1Lu cell pools stably expressing km23/mLC7-1 or EV were plated and analyzed for cell number at several days thereafter as indicated, by using the crystal violet assay described in MATERIALS AND METHODS. The percentage of inhibition of growth is indicated in parentheses on top of the relevant bars.
GST Pull-Downs
To prepare GST-km23, the coding region of rat or human km23 was PCR amplified with additional suitable flanking restriction enzyme sites for BamHI (5′) and XhoI (3′), and inserted into pGEX-4T-1 (Amersham Biosciences, Piscataway, NJ) after digestion with BamHI and XhoI restriction enzymes. The bacterially expressed rkm23-GST was isolated according to the manufacturer's instructions (Amersham Biosciences) and used in the GST pull-downs by standard methods (Current Protocols in Molecular Biology). The products were analyzed by SDS-PAGE or immunoblotting/Coomassie staining.
RESULTS
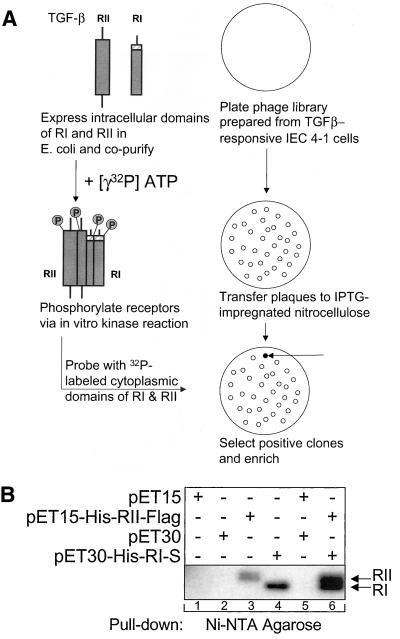
We have developed a novel method for the identification of TGFβ receptor-interacting proteins, as depicted in Figure 1A. The phosphorylated, activated cytoplasmic domains of the TGFβ receptors were used as probes to screen an expression library that was prepared from a highly TGFβ-responsive IEC line (Mulder et al., 1993). The cytoplasmic regions of both receptors were phosphorylated in vitro using a kinase assay before screening, as described in MATERIALS AND METHODS. Figure 1B illustrates the results of an vitro kinase assay performed using the cytoplasmic regions of the receptors. Lanes 3 and 4 depict the phosphorylated receptor proteins after expression of either RII or RI alone, as indicated. Autophosphorylation of both receptors is clearly visible, as described previously (Lin et al., 1992; Bassing et al., 1994; Chen and Weinberg, 1995). No phosphorylation is visible after expression of only empty vectors (pET 15/30). On expression of both receptor domains (lane 6), there is an increase in the phosphorylation level of both receptors, indicating that trans-phosphorylation was also occurring. These data indicate that the cytoplasmic domains of RI and RII can interact and become catalytically activated in vitro. These phosphorylated receptor domains were used to screen the expression library as illustrated in Figure 1A.
Figure 1.
Identification of a novel TGFβ receptor-interacting protein. (A) Method for identifying TGFβ receptor-interacting proteins. The cytoplasmic domains of both receptors were expressed, sequentially isolated, kinase-activated in vitro, and used as probes to screen an expression library. (B) In vitro kinase activation of the cytoplasmic regions of TGFβ RI and RII result in both auto- and trans-phosphorylation. Bacterially expressed TGFβ receptor proteins were precipitated with Ni2+-NTA agarose beads before performing an in vitro kinase assay. Bacterial lysates were prepared after expression of either EVs (pET15, pET30, and pET15/pET30), the intracellular domains of RII or RI alone (pET15-His-RII-FLAG and pET30-His-RI-S), or together (pET15-RII-FLAG/pET30-RI-S).
Several positive clones were isolated as described in MATERIALS AND METHODS. Among the clones isolated, km23 was pursued initially because early database searches identified the Drosophila bithoraxoid (bxd) region of the bithorax complex (BX-C) as being most closely related. The BX-C is a cluster of homeotic genes that transcribe positional information into segmental identity for specific parasegments (Morata and Kerridge, 1981; Martin et al., 1995). bxd is a 40-kb region of BX-C, immediately upstream from the Ultrabithorax (Ubx) unit, and capable of exerting cis-regulatory control over expression of this unit (Lipshitz et al., 1987). It had already been shown that the TGFβ superfamily member Dpp stimulated transcription of Ubx and that the Ubx protein was necessary but not sufficient for full activation of dpp expression (Mathies et al., 1994; Sun et al., 1995; Eresh et al., 1997). Thus, it was conceivable that a homologue of the regulatory region of Ubx might be important in TGFβ signaling. In addition, the TGFβ superfamily of secreted polypeptides is known to convey critical signals during the control of development in various contexts, and BX-C is also important in development.
Several other clones were obtained in our screen, including a previously recognized TGFβ RI-interacting protein, the alpha subunit of farnesyl protein transferase (Kawabata et al., 1995; Ventura et al., 1996). The other clones identified in our screen will be the subjects of future investigations. We would not have expected to identify Smads in our screen, because we used catalytically active TGFβ receptors as the probes. It has been proposed that activation of RSmads by RI releases them from the complex, to mediate downstream signaling. For example, Macias-Silva et al. (1996) have demonstrated that the interaction between the TGFβ receptor complex and Smad2 was increased when RI was made inactive by mutation of the kinase domain. Furthermore, Lo et al. (1998) have shown that removal of the C-terminal domain of Smad2 increased its interaction with RI, suggesting that docking was inhibited when the C-tail was phosphorylated. Therefore, in our screen, the in vitro kinase assay performed on the receptors before library screening would be expected to prevent binding of Smads to the receptor complex.
The novel TGFβ signaling intermediate we identified, initially termed km23, is a 96-amino acid protein encoded by a 291-base pair open reading frame. It is a ubiquitously expressed, cytoplasmic protein with a predicted molecular mass of 10.667 kDa and a calculated molecular mass of 11 kDa on Western blots. The rat and human km23 amino acid sequences differ by only three amino acids and are 98% similar. Additional alignments of km23 with sequences in the National Center for Biotechnology Information database indicated that km23 is the mammalian homologue of the Drosophila protein roadblock (robl), which belongs to the LC7 family of Chlamydomonas DLCs (chlLC7) (Bowman et al., 1999). robl is a light chain of the motor protein dynein that interacts with the DIC. It is involved in mitosis and axonal transport. Mutants lacking this gene display defects in intracellular transport, and an accumulation of cargoes, as well as an increase in the mitotic index.
Table 1 lists the percentage of homologies, identities, and similarities of some of the DLCs of the km23/robl/LC7 family. Differences in the number of amino acids are also shown. As indicated, there is a second mammalian member of the LC7 family in the National Center for Biotechnology Information database. This form of mLC7 (designated mLC7-2 in Table 1; AA446298) displays 70% homology with the km23/mLC7-1 form we have identified. In contrast, a total of five LC7/robl-like genes have been identified in Drosophila, yet Caenorhabditis elegans seems to have only a single km23/robl-like gene (National Center for Biotechnology Information database T24H10.6; Bowman et al., 1999). There does not seem to be a family member in Saccharomyces cerevisiae. There are also other DLC families that bind to DIC, including Tctex-1/LC14, Tctex-2/LC2, LC6, and LC8/PIN (Bowman et al., 1999; King, 2000; Makokha et al., 2002). Of these other DLCs that bind to the DIC, Tctex-1 and LC8 have been shown to function as motor receptors to link cargo to the motor machinery (Almenar-Queralt and Goldstein, 2001). Although Tctex-1 and LC8 share limited sequence identity, both bind a number of unrelated cargo in a similar manner (Mok et al., 2001; Makokha et al., 2002). Similarly, these DLCs are only 8 and 14% identical to mLC7-1, respectively. It is conceivable that mLC7-1 also mediates motor complex assembly and connection to the transported cellular cargo.
Table 1.
Comparison of km23/mLC7-1 to some other family members
| Homologue | Species | % Homology | % Identity | % Similarity | Amino acids |
|---|---|---|---|---|---|
| mLC7-2 | Homo sapiens | 70 | 77 | 91 | 96 |
| robl | Drosophila melanogaster | 67 | 71 | 81 | 97 |
| ch/LC7 | Chlamydomonas | 59 | 55 | 74 | 105 |
| bxd-like | C. elegans | 56 | 47 | 76 | 95 |
| bxd | D. melanogaster | 42 | 23 | 51 | 101 |
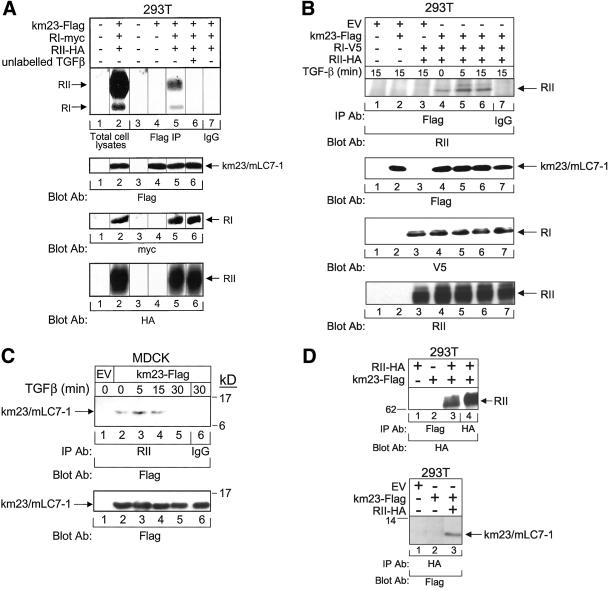
Because we had identified mLC7-1 by its ability to interact with the cytoplasmic regions of the TGFβ receptors, it was of interest to verify whether mLC7-1 was present in association with the TGFβ receptors intracellularly. Accordingly, affinity cross-linking experiments were performed using 125I-TGFβ (Yue et al., 1999a). Figure 2A indicates that both RI and RII are present in km23/mLC7-1 immunocomplexes (lane 5) from cell lysates of 293T cells, which had been transiently transfected with both TGFβ receptors and km23-FLAG. The positions of RI and RII were confirmed by analysis of total cell lysates (lane 2). Unlabeled TGFβ completely competed for binding to both receptors as shown in lane 6 (Figure 2A). Furthermore, no receptors were detectable in FLAG IPs after expression of both receptors without km23/mLC7-1 (our unpublished data). The control blots in the lower panels demonstrate that the appropriate constructs were expressed to similar levels. Thus, these results suggest that mLC7-1 is associated with the activated receptor complex.
Figure 2.
Verification of TGFβ receptor interaction with mLC7-1. (A) RI and RII TGFβ receptors are present in mLC7-1 immunocomplexes. 293T cells were transiently transfected with km23-FLAG, RI-myc, RII-HA, and/or EVs, and affinity labeling was performed. After the 4-h 125I-TGFβ labeling period (4°C), the cross-linking agent disuccinimidyl suberate was added for an additional 15 min. Top, total cell lysates (lanes 1 and 2) or lysates immunoprecipitated with an anti-FLAG M2 antibody (lanes 3–6) or with IgG (lane 7, control) were visualized by SDS-PAGE and autoradiography. No bands were visible in FLAG IPs after transfection of only RI and RII (our unpublished data). Bottom, Western blots for FLAG, myc, and HA demonstrate expression of the relevant constructs (lanes 2 and 4–6 for km23/mLC7-1; lanes 2, 5, and 6 for RI and RII). (B) Interaction between mLC7-1 and the TGFβ receptors occurs within 5 min of TGFβ addition. 293T cells were transiently transfected with km23-FLAG, RI-V5, RII-HA, and/or EVs, followed by IP/blot analyses with FLAG as the IP antibody and an RII polyclonal antibody as the blotting antibody (top). Cells were incubated in serum-free medium for 60 min before addition of TGFβ for the indicated times. Bottom, controls for expression and loading of km23/mLC7-1 (FLAG blot), RI (V5 blot), and RII (RII blot). (C) TGFβ induces a rapid association of mLC7-1 with endogenous TGFβ receptors in MDCK cells. EV or km23-FLAG constructs were expressed in MDCK cells, and TGFβ treatments and IP/blot analyses were performed as for Figure 2B. (D) mLC7-1 interacts with RII via IP/blot analyses in 293T cells. Cells were transiently transfected with km23-FLAG and RII-HA as indicated. Top, cell lysates were immunoprecipitated with anti-FLAG or HA and blotted with an HA antibody. The presence of RII in lanes 3 and 4, but not in lanes 1 and 2, demonstrates an interaction between km23-FLAG and RII-HA. Bottom, lysates were immunoprecipitated with anti-HA and blotted with anti-FLAG. The presence of km23/mLC7-1 in only lane 3 indicates that an interaction between km23-FLAG and RII-HA is detectable in this direction as well. Results are representative of two experiments for each.
To determine whether the interaction between the receptors and mLC7-1 occurred rapidly after ligand stimulation, we performed IP/blot analyses in the presence and absence of TGFβ. Coexpression of both TGFβ receptors is known to result in heteromeric complex formation and receptor activation in the absence of ligand (Ventura et al., 1994), as shown in Figure 2B (lane 4). However, Figure 2B demonstrates not only that km23/mLC7-1 interacts with RII, but also that TGFβ induces this interaction within 5 min of TGFβ addition (lanes 4–6, top). The appearance of the RII band with slightly slower mobility (lanes 5 and 6) suggests that TGFβ also induced the interaction of km23/mLC7-1 with a differentially phosphorylated/modified form of RII. No specific band was apparent after expression of only km23/mLC7-1 or the receptors alone (lanes 2 and 3, top). We were unable to assess whether RI was also present in the complex using this assay, due to the interference of the IgG bands at the RI position on such blots. However, because an RII antibody was used as the blotting antibody in these experiments, our data indicate that mLC7-1 does associate with RII.
To ensure that the interaction was not the result of overexpression of the TGFβ receptors, we performed similar IP/blot analyses in MDCK cells expressing endogenous TGFβ receptors. These cells are TGFβ responsive as revealed by a 70% inhibition of cell growth within 24 h of 10 ng/ml TGFβ addition (our unpublished data). As seen in Figure 2C, TGFβ induced a rapid interaction between km23/mLC7-1 and endogenous TGFβ receptors. The kinetics were similar to those observed for the 293T cells. Thus, TGFβ induces the interaction of mLC7-1 with the TGFβ receptors in two different cell types, and without overexpression of the receptors.
The results in Figure 2, A–C, are consistent with mLC7-1 interacting with both receptors in the complex simultaneously or with RII alone, due to the fact that RII interacts with and controls ligand binding to the complex (Wrana et al., 1992). To determine whether both receptors were required for mLC7-1 interaction with the receptor complex, we performed IP/blot analyses after expression of only RII in 293T cells. Figure 2D depicts the interaction of km23/mLC7-1 with RII, either using FLAG as the IP antibody, and the HA antibody as the blotting antibody (top), or by performing the analyses in the reverse direction (bottom). As indicated by the results in either direction, it seems that km23/mLC7-1 can interact with RII alone. In contrast, upon expression of RI alone, no detectable interaction of RI with mLC7-1 was observed (our unpublished data). However, because 293T cells do express a low level of endogenous RI receptors, overexpression of RII could cause an interaction of RII with the endogenous RI receptors. It is possible, then, that some RI is still present in the receptor complex in Figure 2D. Thus, mLC7-1 may interact with the receptor complex through the RII receptor, and RI may not be a direct binding partner. In contrast, expression of RII alone may be sufficient for TGFβ regulation of mLC7-1.
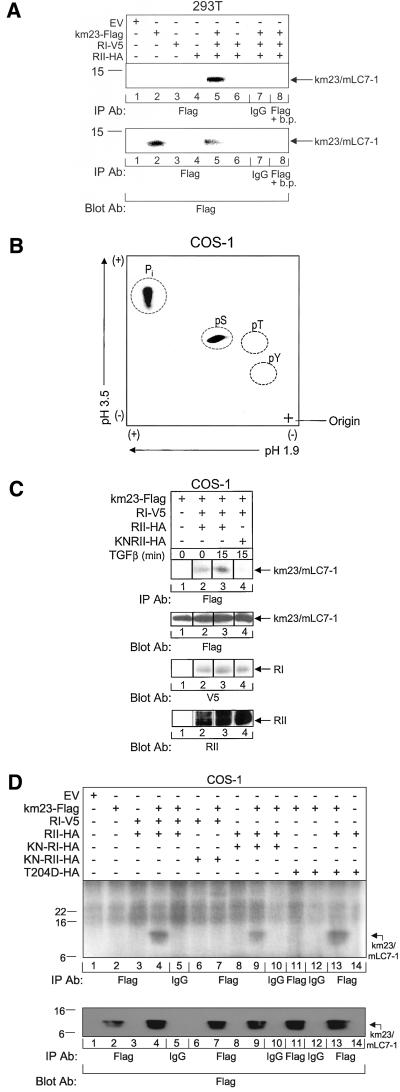
TGFβ receptors have serine/threonine kinase activity, which can mediate the phosphorylation of intracellular proteins as one mechanism for initiating TGFβ signaling events and responses. Thus, if mLC7-1 is a component of a TGFβ signaling cascade, it is conceivable that the TGFβ receptors could phosphorylate mLC7-1 as a mechanism for activation. To determine whether mLC7-1 was phosphorylated by the TGFβ receptors, we performed in vivo phosphorylation assays (Yue and Mulder, 1999a;b) after transient expression of km23/mLC7-1 and both receptors, each being detectable by distinct tag antibodies, as indicated in Figure 3A. From the results in the top panel, it is clear that the TGFβ receptor complex resulted in phosphorylation of km23/mLC7-1 (lane 5). Expression of km23/mLC7-1 alone did not result in a band at the km23/mLC7-1 position (lane 2), indicating that mLC7-1 is not constitutively phosphorylated when expressed in these cells. The IgG and FLAG binding peptide control lanes (7 and 8) indicate that the band noted is specific for km23/mLC7-1.
Figure 3.
A functional RII TGFβ receptor is required for mLC7-1 phosphorylation. (A) mLC7-1 is phosphorylated upon activation of TGFβ receptors. 293T cells were transiently transfected with RI-V5, RII-HA, and either EV or km23-FLAG. Forty-eight hours after transfection, cells were labeled for 3 h with [32Pi], lysed, and immonoprecipitated with an anti-FLAG antibody. Top, in vivo phosphorylation of km23/mLC7-1 was visualized by SDS-PAGE and autoradiography. A blocking peptide (b.p.) for the FLAG antibody was added in lane 8. Bottom, expression of transfected km23-FLAG was confirmed by immunoblot analysis. Results are representative of three experiments. B, activation of the TGFβ receptors results in phosphorylation of mLC7-1 primarily on serine residues. km23/mLC7-1 was phosphorylated in vivo as for A, and phosphoamino acid analysis was performed. 32P-labeled km23/mLC7-1 was excised from the polyvinylidene difluoride membrane and subjected to acid hydrolysis (6 M HCl, 1 h, 110°C). Phosphoserine (pS), phosphothreonine (pT), and phosphotyrosine (pY) were separated in two dimensions by using Hunter Thin Layer Peptide Mapping Electrophoresis System (CBS Scientific, Del Mar, CA), together with phosphoamino acid standards. Labeled and standard phosphoamino acids were visualized by ninhydrin spray (0.25% in acetone). 32P-labeled phosphorylated amino acids were visualized by autoradiography. (C) TGFβ cannot phosphorylate mLC7-1 when a kinase-deficient RII is expressed with RI. COS-1 cells were transiently transfected as in A, except that the KNRII was coexpressed with wild-type RI in lane 4. Cells were incubated in serum-free, phosphate-free medium for 30 min and TGFβ was added during the last 15 min of the labeling period (lanes 3 and 4). Lysates were analyzed as for A. Top, in vivo phosphorylation of km23/mLC7-1. Bottom, expression of transfected km23/mLC7-1, RI, and RII was confirmed by Western blot analysis with FLAG, V5, and a polyclonal RII antibody, respectively, as indicated. Results are representative of two experiments. (D) Kinase activity of RI does not seem to be required for phosphorylation of mLC7-1. In vivo phosphorylation assay and transfection of COS-1 cells was performed as for Figure 3C, except that no TGFβ was added and different receptor mutants were evaluated as indicated. Results are representative of two experiments.
After complex formation, the TGFβ receptors are known to become phosphorylated on specific serine and threonine residues (Souchelnytskyi et al., 1996). Moreover, TGFβ receptor activation affects the phosphorylation of specific serine residues in RSmads, which are required for TGFβ signaling (Souchelnytskyi et al., 1997). Thus, if mLC7-1 is a substrate for the TGFβ receptor kinase activity, phosphorylation of mLC7-1 on serine residues might be expected. To examine whether this was the case, we performed phosphoamino acid analysis of phosphorylated mLC7-1 obtained after coexpression of km23/mLC7-1 and both TGFβ receptors in COS-1 cells, similar to the analyses for Figure 3A in 293T cells. Figure 3B indicates that km23/mLC7-1 is phosphorylated primarily on serine residues in response to TGFβ receptor activation. These findings are consistent with mLC7-1 functioning as a substrate for the kinase activity of the TGFβ receptors. Conversely, mLC7-1 does not seem to stimulate the kinase activity of the receptors (our unpublished data).
Based upon the current model for TGFβ receptor activation, RII mediates the phosphorylation of RI and the activation of downstream TGFβ components and responses (Roberts, 1998; Massague et al., 2000; Sporn and Vilcek, 2000; Yue and Mulder, 2001). Accordingly, if TGFβ activation of the receptor complex is required for phosphorylation of mLC7-1, expression of a kinase-deficient version of RII (KN-RII) would be expected to block mLC7-1 phosphorylation. Figure 3C (top) depicts the results of in vivo phosphorylation of km23/mLC7-1 after coexpression of either wild-type RII (lanes 2 and 3) or KN-RII (lane 4) with wild-type RI. As shown previously, km23/mLC7-1 alone was not constitutively phosphorylated (lane 1), and expression of both TGFβ receptors with km23/mLC7-1 resulted in km23/mLC7-1 phosphorylation (lane 2). Figure 3C indicates, furthermore, that TGFβ treatment for 15 min enhanced km23/mLC7-1 phosphorylation (lane 3). This phosphorylation of km23/mLC7-1 was completely blocked upon expression of the KN-RII (lane 4), thereby demonstrating that the kinase activity of RII is required for mLC7-1 phosphorylation.
To determine whether RI was also required for mLC7-1 phosphorylation, we performed similar in vivo phosphorylation experiments using various kinase-active and kinase-deficient versions of RI. Figure 3D confirmed that expression of both receptors with km23/mLC7-1 induced km23/mLC7-1 phosphorylation (lane 4) and that KN-RII blocked this phosphorylation (lane 7). However, in addition, this figure indicates that km23/mLC7-1 is still phosphorylated after coexpression of RII with KN-RI (lane 9). Only limited phosphorylation of Smad2 has been reported to occur under such conditions (Macias-Silva et al., 1996). Because the KN-RI would be expected to abrogate any residual activity from endogenous RI receptors present in COS-1 cells, these data suggest that the RI kinase is not required for phosphorylation of mLC7-1, although it is present in mLC7-1 immunocomplexes with RII by affinity-labeling experiments (Figure 2A). Lane 11 in Figure 3D demonstrates no detectable phosphorylation of km23/mLC7-1 after expression of a constitutively active RI mutant (T204D). However, when wild-type RII was coexpressed with this mutant, km23/mLC7-1 phosphorylation was observed (lane 13), presumably due to the kinase activity of RII. Collectively, the data suggest that although both receptors may be present in a complex with mLC7-1, the RII kinase is required for mLC7-1 phosphorylation. The data do not rule out the possibility that another kinase is also present in the complex.
The method of isolation of mLC7-1, as well as the results in Figures 2 and 3, suggest that mLC7-1 may function as a signaling intermediate for TGFβ. Thus, it was of interest to examine whether mLC7-1 could mediate any of the known TGFβ signaling events. We have previously shown that TGFβ rapidly activates the JNK family of Mapks (Frey and Mulder, 1997). Furthermore, JNK activation by TGFβ is required for such TGFβ responses as production of TGFβ1 and induction of fibronectin expression (Hocevar et al., 1999; Yue and Mulder, 2000a). JNK activation by TGFβ may also play a role in TGFβ-mediated growth inhibition, either through the amplification of TGFβ production, via cross talk with the Smads, and/or by regulation of cell cycle inhibitors (Derynck et al., 2001; Yue and Mulder, 2001).
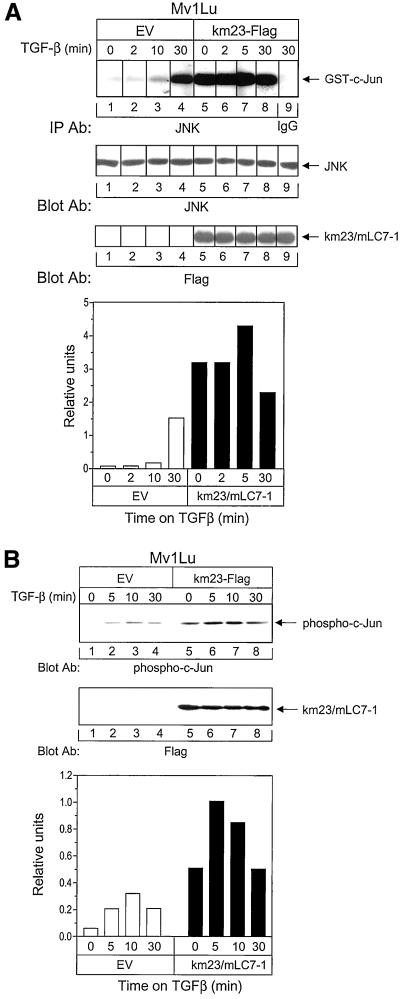
To determine the effect of forced expression of wild-type mLC7-1 on JNK activation, we stably expressed a FLAG-tagged version of km23/mLC7-1 in mink lung epithelial cells (Figure 4A, third panel) and performed in vitro kinase assays to determine the ability of JNK to phosphorylate GST-c-Jun in the absence and presence of TGFβ. As shown in Figure 4A, in the EV-expressing cells, TGFβ began activating JNK within 10 min of TGFβ addition; JNK activity increased further by 30 min posttreatment (top, left). These kinetics are similar to those obtained for other cell types (Frey and Mulder, 1997). In contrast, when km23/mLC7-1 was stably expressed in these cells, JNK was superactivated in the absence of TGFβ (top, right). JNK activity was ∼15 times greater in the km23/mLC7-1-expressing cells than in the EV-expressing cells during the 2- to 10-min period after TGFβ addition. By 30 min post-TGFβ treatment, JNK activation levels were more similar between the km23/mLC7-1– and EV-expressing cells. These findings suggest that mLC7-1 may function as a signaling intermediate for the activation of JNK by TGFβ.
Figure 4.
mLC7-1 expression can induce JNK and result in phosphorylation of the downstream target c-Jun. (A) Stable expression of mLC7-1 results in activation of JNK in the absence of TGFβ. Top, Mv1Lu cell pools, stably transfected with either empty vector (lanes 1–4) or km23-FLAG (lanes 5–8), were incubated in serum-free medium for 30 min before addition of 10 ng/ml TGFβ for the indicated times. Cell lysates were immunoprecipitated with anti-JNK (C-17; Santa Cruz Biotechnology) and subjected to in vitro kinase assays using GST-c-JUN (1-79) as the substrate. The phosphorylated proteins were resolved by SDS-PAGE and visualized by autoradiography. Normal rabbit IgG was used as the negative control. Middle, equal JNK and km23/mLC7-1 expression was confirmed by Western blotting. Bottom, plot of densitometric scan of results in top. (B) Stable expression of mLC7-1 results in phosphorylation of c-Jun in the absence of TGFβ. Top, cells were treated with TGFβ and lysates were obtained as for Figure 4A, except that they were analyzed by Western blot analysis with a phospho-c-Jun antibody (KM-1 and SC-822). Middle, Western blot demonstrating equal km23/mLC7-1 expression in the pools stably expressing km23/mLC7-1, but not in the EV-transfected pools. Bottom, plot of densitometric scan of results in top. Results are representative of two experiments for each.
Previous results have indicated that c-Jun, a downstream effector of JNK, can be phosphorylated by TGFβ (Huang et al., 2000). To determine whether this downstream effector of JNK could also be phosphorylated by stable expression of mLC7-1, we performed immunoblot analysis at various times after TGFβ treatment using a phospho-c-Jun–specific antibody. This antibody is specific for c-Jun phosphorylated at serine-63, and does not cross-react with unphosphorylated c-Jun or with the phosphorylated forms of Jun B or Jun D. These studies were performed in the same Mv1Lu cells stably expressing km23/mLC7-1 that were used for Figure 4A. The results in Figure 4B demonstrate that forced expression of km23/mLC7-1 induced the phosphorylation of c-Jun in the absence of TGFβ (comparing left and right, top). As for JNK activity, c-Jun phosphorylation was superactivated in the absence of TGFβ. Figure 4B, bottom, indicates that c-Jun phosphorylation levels were approximately 10 times greater in the km23/mLC7-1–expressing cells than in the EV-expressing cells. Collectively, the results in Figure 4 suggest that mLC7-1's cellular effects on JNK and c-Jun activation are downstream of TGFβ receptor activation.
In addition, our findings in Figure 4 suggest that overexpression of mLC7-1 may result in the constitutive activation of specific TGFβ signaling components and pathways. These intermediates may, in turn, be involved in mediating specific TGFβ responses in the absence of ligand activation of receptors. Accordingly, because one of TGFβ's most prominent biological effects is growth inhibition of epithelial cells, we examined whether overexpression of mLC7-1 in the Mv1Lu-transfected pools could result in growth inhibition in the absence of TGFβ. The results in Figure 5 indicate that, relative to EV-transfected pools, the km23/mLC7-1–expressing cells were growth inhibited by ∼50%. These data support the contention that overexpression of mLC7-1 may mediate some TGFβ responses in a constitutive manner. Alternatively, with regard to the growth inhibitory effect observed, the overexpression of mLC7-1 may have disrupted the interaction of cytoplasmic dynein with the kinetochore, thereby reducing growth. Similar results have been reported upon overexpression of dynamitin, a dynactin subunit that can disrupt the dynein/dynactin interaction (Echeverri et al., 1996).
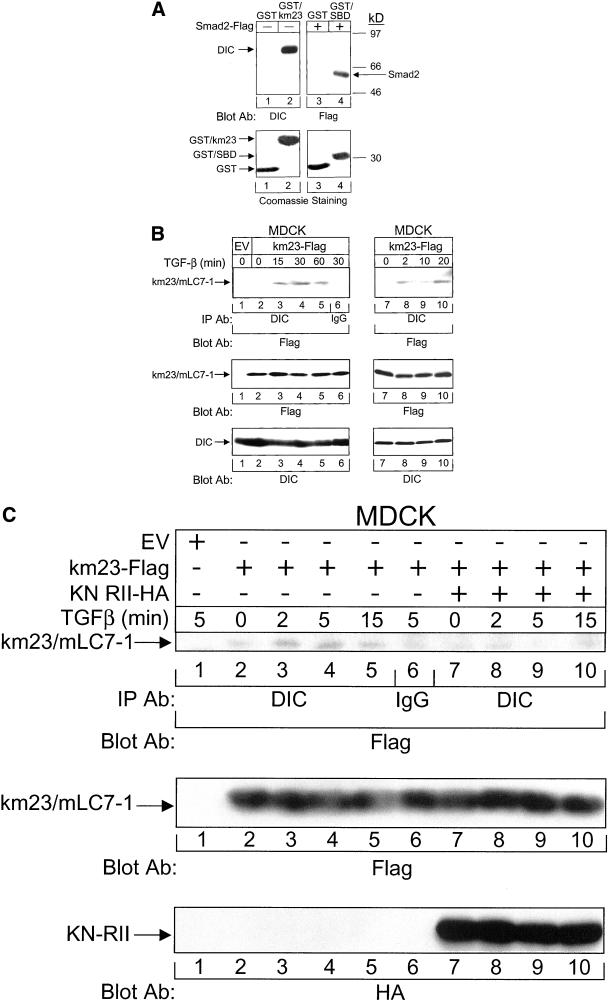
As mentioned above, mLC7-1 is the mammalian homologue of the chlLC7 and Drosophila robl proteins, which are DLCs (Bowman et al., 1999). Accordingly, it was of interest to determine whether mLC7-1 could interact with the DIC as chlLC7/robl does. As shown in Figure 6A, we performed GST pull-down assays after expressing and purifying GST-km23. An anti-DIC antibody was used as the blotting antibody to detect the presence of dynein in the GST-km23 complexes. This antibody detects a protein of ∼74 kDa. In Figure 6A, it is clear that dynein is visible in GST-km23 immunoprecipitates (lane 2), but not in immunoprecipitates from GST alone (lane 1). The interaction between the Smad binding domain (SBD) of SARA and Smad2-FLAG (Tsukazaki et al., 1998) is shown as a positive control for comparison (lane 4). The results clearly demonstrate that mLC7-1 is a dynein-associated protein.
Figure 6.
Interaction between the mLC7-1 and the DIC is regulated by TGFβ and requires RII kinase activity. (A) mLC7-1 interacts with DIC via GST pull-down assays. Top, MDCK cell lysates were incubated with Sepharose-bound bacterially expressed GST alone, GST-km23, or GST-SBD (positive control). GST-bound proteins were analyzed by SDS-PAGE (10%) and were immunoblotted with an anti-DIC antibody. Proteins were detected by enhanced chemiluminescence. Dynein interacts with GST-km23 (lane 2), but not with GST alone (lane 1). The interaction between FLAG-tagged Smad2 and GST-SBD (Tsukazaki et al., 1998) was confirmed as a positive control (lane 4). Bottom, Coomassie staining of gel in top panel, demonstrating the presence of GST and GST fusion proteins in the relevant lanes. The sizes are as expected for the different fusion proteins (approx. 37 kDa for GST-km23; approx. 35 kDa for GST-SBD) or GST alone (approx. 27 kDa). (B) TGFβ stimulates the recruitment of the mLC7-1 to the DIC within 2 min of TGFβ treatment. MDCK cells were transiently transfected with either empty vector or km23-FLAG. Thirty-six hours after transfection, cells were incubated in serum-free medium for 60 min before addition of 10 ng/ml TGFβ for the indicated times. Cell lysates were subjected to IP by using a monoclonal anti-DIC antibody, followed by immunoblot analysis with an anti-FLAG antibody (top). Western blot analysis with anti-FLAG (middle) or anti-DIC (bottom) demonstrates equal protein expression and loading. The right side shows the results at earlier time points. Results are representative of three experiments. (C) Phosphorylation of mLC7-1 is required for recruitment of mLC7-1 to the DIC. Cell treatments and IP/blot analyses were performed in MDCK cells as for B, except that cells were transfected with km23-FLAG in the absence (left, top) or presence (right, top) of KN RII. Western blot controls for expression of km23/mLC7-1 and KN RII are shown in the middle and bottom panels, respectively.
The finding that mLC7-1 associates with and is phosphorylated by activated TGFβ receptors, and that it can activate JNK and c-Jun and inhibit cell growth, suggests that mLC7-1 may function in a TGFβ signaling pathway. Furthermore, because it is thought that DLCs may be important for specifying the nature of the cargo that will be carried by the motor (Klopfenstein et al., 2000; Kamal and Goldstein, 2002), it is likely that extracellular factors (such as growth factors and cytokines) might be able to select the particular DLCs that are recruited to the motor in specific cellular contexts. Accordingly, it was of interest to determine whether TGFβ could mediate the recruitment of mLC7-1 to the DIC. For these studies, we performed IP/blot analyses by using anti-DIC as the IP antibody and anti-FLAG as the blotting antibody. Figure 6B, top, demonstrates that km23/mLC7-1 does interact with cytoplasmic DIC by IP/blot analyses. In addition, as shown in lanes 3–5 and 8–10 of this figure, 10 ng/ml TGFβ induced a rapid recruitment of km23/mLC7-1 to the DIC. Although a basal level of interaction between km23/mLC7-1 and DIC was detectable in some cases (lane 2), a threefold increase in this association was visible within 15 min of TGFβ addition to the TGFβ-responsive MDCK cells. This increase in the interaction between km23/mLC7-1 and DIC began as early as 2 min after TGFβ addition (top right) and seemed to remain relatively constant for at least 60 min (lanes 4 and 5, top). The bottom panels demonstrate roughly equal expression and loading. Thus, TGFβ rapidly induced the recruitment of the mLC7-1 to the DIC.
The results in Figure 6B indicate that TGFβ can stimulate the recruitment of mLC7-1 to the DIC, suggesting a connection between TGFβ signaling and DLC recruitment. To provide definitive evidence that TGFβ receptor activation is required for the mLC7-1–DIC interaction, we examined the interaction between mLC7-1 and DIC in the absence and presence of a kinase-deficient form of TGFβ RII. This receptor mutant can function in a dominant negative manner to block the kinase activity of endogenous RII when overexpressed in cells (Wieser et al., 1993). Furthermore, we have shown in Figure 3D that expression of KN RII with wild-type RI does not permit mLC7-1 phosphorylation. Figure 6C indicates that the TGFβ-induced interaction between km23/mLC7-1 and DIC (lanes 3–5) was blocked when KN RII was expressed (lanes 7–10). No specific band was detectable in EV and IgG control lanes. Expression of km23/mLC7-1 and KN RII in the relevant lanes was also confirmed (middle and bottom panels). Thus, mLC7-1 phosphorylation by kinase-active TGFβ receptors is necessary for the recruitment of mLC7-1 to the DIC.
DISCUSSION
Our results provide a novel method for the identification of TGFβ signaling components, based upon their ability to bind to the phosphorylated intracellular domains of the TGFβ receptors. Furthermore, we have verified the success of this method with the isolation of a unique TGFβ receptor-interacting protein, termed mLC7-1. The mLC7-1 interaction with the TGFβ receptors was confirmed by 125I-TGFβ affinity labeling and by IP/blot analysis. Furthermore, TGFβ induced the interaction of mLC7-1 with endogenous TGFβ receptors within 5 min of ligand addition in MDCK cells, and a similar kinetic profile was observed in at least one other cell type. Finally, mLC7-1 was able to transduce specific TGFβ signaling events, including an activation of JNK, a phosphorylation of c-Jun, and an inhibition of cell growth.
We have also shown that TGFβ receptor activation results in the phosphorylation of mLC7-1 primarily on serine residues, consistent with the kinase specificity for the receptors. For example, the RSmads are activated by serine phosphorylation at a C-terminal SSxS motif (Souchelnytskyi et al., 1997). Although this could suggest that mLC7-1 is a direct substrate of the TGFβ receptor kinase activity, it is also possible that another kinase is associated with the mLC7-1/TGFβ receptor complex. There are consensus phosphorylation sites for protein kinase C and casein kinase II within the mLC7-1 coding region. Perhaps, these or other serine kinases are the immediate activators of mLC7-1. However, it is clear that TGFβ does stimulate the interaction of mLC7-1 with the receptors, and that TGFβ receptor activation leads to mLC7-1 phosphorylation and recruitment of mLC7-1 to DIC.
Our results indicate, furthermore, that the kinase activity of the RII receptor is required for mLC7-1 phosphorylation and interaction with DIC, because a kinase-deficient version of RII blocked TGFβ induction of both events. TGFβ RI did not seem to be required for mLC7-1 phosphorylation, although RI was present in mLC7-1 immunoprecipitates in affinity-labeling experiments. Several pieces of evidence support the conclusion that RII is the activating receptor for mLC7-1. First, coexpression of RII with a kinase-deficient version of RI induced mLC7-1 phosphorylation to an extent equivalent to that which occurred by expression of RII alone. Second, expression of both TGFβ receptors resulted in no additional increase in km23/mLC7-1 phosphorylation compared with expression of only RII. Finally, constitutively active RI alone did not result in phosphorylation of mLC7-1, as it does for the RSmads. Similarly, previous studies have described TGFβ signaling molecules that were regulated specifically by the RII receptors. For example, the Daxx adaptor protein has been proposed to mediate TGFβ-induced apoptosis through its interaction with RII (Perlman et al., 2001).
Based upon the report describing the cloning of the Drosophila robl protein and the chlLC7 (Bowman et al., 1999), km23/mLC7-1 is the mammalian homologue of the DLC/LC7/robl. We have shown that TGFβ leads to the recruitment of mLC7-1 to the DIC in a rapid, TGFβ-inducible manner. This interaction, however, occurred within a slightly different time frame than the interaction of mLC7-1 with the TGFβ receptors. This finding suggests that the receptors themselves may not be the cargo that dynein will transport via mLC7-1. That is, the mLC7-1-receptor interaction peaks at 5 min, and seems to begin declining by 15 min after TGFβ addition (Figure 2, B and C), consistent with the receptors being released once mLC7-1 has been phosphorylated. In contrast, it is clear from Figure 6, B and C, that the interaction between mLC7-1 and DIC begins as early as 2 min after TGFβ addition, yet mLC7-1 is still bound to DIC at 60 min after TGFβ addition. Previous studies have indicated that the transport of p53 along MTs was dynein dependent, suggesting that the interaction of p53 with dynein facilitated its accumulation in the nucleus after DNA damage (Giannakakou et al., 2000). Similarly, subsequent to receptor activation, TGFβ signaling components may be transported along the MTs through the interaction of mLC7-1 with DIC.
Although evidence indicates that Smads 2/3/4 may be distributed along the MT network, the MTs seemed to sequester the Smads from the receptor before cellular stimulation by TGFβ (Dong et al., 2000). Perhaps this occurs because a motor protein light chain such as mLC7-1 is in an inactive, unphosphorylated state until TGFβ receptor activation occurs. Phosphorylation of the DLC may affect a conformational change in this protein, followed by its recruitment to a motor complex for transport of TGFβ signaling components (i.e., Smads and JNKs) along the MTs.
A link between TGFβ receptor signaling and the minus-end MT motor protein dynein has not been demonstrated previously. However, a receptor–DLC interaction has been reported for the photoreceptor rhodopsin (Tai et al., 1999). In addition, the Trk neurotrophin receptors have been shown to associate with the DLC Tctex-1, suggesting that transport of neurotrophins during vesicular trafficking may occur through this direct interaction between the Trk receptor and the dynein motor machinery (Yano et al., 2001). It has been shown that nerve growth factor remains bound to TrkA after endocytosis, thereby allowing the receptor to continue to activate signaling proteins (Grimes et al., 1996). In the case of TGFβ, however, the receptor location for either initiation or transmission of TGFβ signaling activities has not been clearly defined. It has been shown that heteromeric TGFβ receptors are internalized and down-regulated after TGFβ activation via a clathrin-dependent mechanism (Anders et al., 1997; Doréet al., 1998) and that the kinase activity of RII is required for these processes to occur optimally (Anders et al., 1998). A more recent report has indicated that Smad phosphorylation does not occur until the GTPase dynamin 2ab excises the budded vesicle from the plasma membrane to form an endocytic vesicle (Penheiter et al., 2002). This report also demonstrated that the formation and activation of the receptor complex was not sufficient for Smad signaling, and that an activity or activities downstream of dynamin 2ab function was/were required. It is possible that mLC7-1 recruitment to the DIC, and dynein motoring of TGFβ signaling components along the MTs, represent at least some of these activities.
Because vesicles derived from a donor compartment fuse with specific acceptor membranes to directionally transfer cargo molecules during trafficking (Gonzalez and Scheller, 1999), it is likely that distinct events occur in different cell compartments during TGFβ signaling. Thus, the fate of the TGFβ–receptor complex and specific signaling complexes may differ. With regard to the Drosophila TGFβ superfamily member Dpp, the rates of endocytic trafficking and degradation determine Dpp signaling range (Entchev et al., 2000). A similar situation may exist for TGFβ in mammalian cells. However, further investigation will be required for a complete understanding of how TGFβ receptor endocytosis, intracellular trafficking, and cell signaling events are integrated.
Collectively, our data are consistent with a role for mLC7-1 in both TGFβ signaling and dynein-mediated transport along MTs. It is likely that the binding of mLC7-1 to the DIC after TGFβ receptor activation is important for specifying the nature of the cargo that will be transported along the MTs. Any disruption in mLC7-1 could prevent or alter movement of specific cargo along MT's. In this way, alterations in mLC7-1 might result in a mislocalization of these proteins, with a disruption of TGFβ growth inhibitory signals. Along these lines, protein traffic direction is required for the maintenance of cell polarity, which, if lost, can result in tumor formation (Peifer, 2000; Bilder et al., 2000). Accordingly, sequence alterations at specific regions of mLC7-1 in human tumors might play a role in tumor development or progression. Future studies will address this possibility.
ACKNOWLEDGMENTS
We thank L. Liotta (National Cancer Institute, Bethesda, MD) and T.W. Wong (Bristol-Myers Squibb, Princeton, NJ) for helpful discussions. We appreciate the assistance of S. R. Hann and M.A. Gregory (Vanderbilt University, Nashville, TN) with the phosphoamino acid analysis procedure. We also thank J. Massague (Memorial Sloan-Kettering Cancer Center, New York, NY) for KNRI-HA and KNRII-HA; J. Wrana (Samuel Lundenfeld Res. Institute, Toronto, Canada) for pCMV5-HA-TGFβRII, Smad2-FLAG, and GST-SBD; R. Derynck (University of California, San Francisco) for RI-myc; K. Miyazono (University of Tokyo, Tokyo, Japan) for the Alk-5 RI cDNA; Q. Chen (Pennsylvania State University College of Medicine, Hershey, PA) for the pcDNA3.1/V5-His vector; H. Lodish (Massachusetts Institute of Technology, Cambridge, MA) for the RII cDNA; H. Moses (Vanderbilt) for the T204D RI; and T.W. Wong for pFLAG-CMV5 and for preparing the RI-pFLAG-CMV5 expression construct. This work was supported by National Institutes of Health grants CA-51452, CA-54816, CA-68444, CA-90765, and CA-92889, and Dept of Defense award DAMD17-0110592 (to K.M.M).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0245. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0245.
REFERENCES
- Almenar-Queralt A, Goldstein LSB. Linkers, packages and pathways: new concepts in axonal transport. Curr Opin Neurobiol. 2001;11:550–557. doi: 10.1016/s0959-4388(00)00248-8. [DOI] [PubMed] [Google Scholar]
- Anders RA, Arline SL, Doré JJE, Leof EB. Distinct endocytic responses of heteromeric and homomeric transforming growth factor receptors. Mol Biol Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Doré JJE, Jr, Arline SL, Garamszegi N, Leof EB. Differential requirement for type I and type II transforming growth factor receptor kinase activity in ligand-mediated receptor endocytosis. J Biol Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Bassing CH, Yingling JM, Howe DJ, Wang T, He WW, Gustafson ML, Shah P, Donahoe PK, Wang XF. A transforming growth factor β type I receptor that signals to activate gene expression. Science. 1994;263:87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kamal A, Ritchings BW, Philip AV, McGrail M, Gindhart JG, Goldstein LSB. Kinesin dependent axonal transports is mediated by the Sunday driver (SYD) protein. Cell. 2000;103:583–594. doi: 10.1016/s0092-8674(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Bowman B, Patel-King RS, Benashski SE, McCaffery JM, Goldstein LS, King SM. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J Cell Biol. 1999;146:165–180. [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Method Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Chen F, Weinberg RA. Biochemical evidence for the autophosphorylation and transphosphorylation of transforming growth factor β receptor kinases. Proc Natl Acad Sci USA. 1995;92:1565–1569. doi: 10.1073/pnas.92.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK kinase group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGFβ activity. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- Doré JJE, Jr, Edens M, Garamszegi N, Leof EB. Heteromeric and homomeric transforming growth factor-receptors show distinct signaling and endocytic responses in epithelial cells. J Biol Chem. 1998;273:31770–31777. doi: 10.1074/jbc.273.48.31770. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. Gradient formation of the TGF-β homolog Dpp. Cell. 2000;103:981–91. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Eresh S, Riese J, Jackson DB, Bohmann D, Bienz M. A CREB-binding site as a target for decapentaplegic signaling during Drosophilaendoderm induction. EMBO J. 1997;16:2014–2022. doi: 10.1093/emboj/16.8.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, Miyazono K. Cloning of a TGF-β type I receptor that forms a heteromeric complex with the TGF-β type II receptor. Cell. 1993;75:681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- Frey RS, Mulder KM. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor β in the negative growth control of breast cancer cells. Cancer Res. 1997;57:628–633. [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules, and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2:709–717. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- Goldstein LS. Transduction. When worlds collide–trafficking in JNK. Science. 2001;291:2102–2103. doi: 10.1126/science.1059766. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Jr, Scheller RH. Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/s0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsough MT, Frey RS, Zipfel PA, Buard A, Cook SJ, McCormick F, Mulder KM. Altered transforming growth factor-β signaling in epithelial cells when ras activation is blocked. J Biol Chem. 1996;271:22368–22375. doi: 10.1074/jbc.271.37.22368. [DOI] [PubMed] [Google Scholar]
- Hartsough ME, Mulder KM. Transforming growth factor β activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu X-X, Howe PH. The adaptor molecule disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. Kinesin delivers: identifying receptors for motor proteins. J Cell Biol. 2001;152:F25–F27. doi: 10.1083/jcb.152.5.f25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu PP, Shen X, Huang D, Liu Y, Counter C, Wang XF. The MEK pathway is required for stimulation of p21(WAF1/CIP1) by transforming growth factor-β. J Biol Chem. 1999;274:35381–35387. doi: 10.1074/jbc.274.50.35381. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hutter D, Liu Y, Wang X, Sheikh MS, Chan AM, Holbrook NJ. Transforming growth factor-β1suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun and JNK activities. J Biol Chem. 2000;275:18234–18242. doi: 10.1074/jbc.M909431199. [DOI] [PubMed] [Google Scholar]
- Kamal A, Goldstein LS. Principles of cargo attachment to cytoplasmic motor proteins. Curr Opin Cell Biol. 2002;14:63–68. doi: 10.1016/s0955-0674(01)00295-2. [DOI] [PubMed] [Google Scholar]
- Karcher RL, Deacon SW, Gelfand VI. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 2002;12:21–27. doi: 10.1016/s0962-8924(01)02184-5. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K, Engel ME, Moses HL. Interaction of the transforming growth factor-β type I receptor with farnesyl-protein transferase-alpha. J Biol Chem. 1995;270:29628–29631. doi: 10.1074/jbc.270.50.29628. [DOI] [PubMed] [Google Scholar]
- King SM. The dynein microtubule motor. Biochim Biophys Acta. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- Klopfenstein DR, Vale RD, Rogers SL. Motor protein receptors: moonlighting on other jobs. Cell. 2000;103:537–540. doi: 10.1016/s0092-8674(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Lin HY, Wang X-F, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Lipshitz HD, Peattie DA, Hogness DS. Novel transcripts from the Ultrabithorax domain of the bithorax complex. Genes Dev. 1987;1:307–322. doi: 10.1101/gad.1.3.307. [DOI] [PubMed] [Google Scholar]
- Lo RS, Chen Y-G, Shi Y, Pavletich NP, Massague J. The L3 loop: a structural motif determining specific interactions between SMAD proteins and TGFβ receptors. EMBO J. 1998;17:996–1005. doi: 10.1093/emboj/17.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Makokha M, Hare M, Li M, Hays T, Barbar E. Interactions of cytoplasmic dynein light chains Tctex-1 and LC8 with the intermediate chain IC74. Biochem. 2002;41:4302–4311. doi: 10.1021/bi011970h. [DOI] [PubMed] [Google Scholar]
- Martin CH, Mayeda CA, Davis CA, Ericsson CL, Knafels JD, Mathog DR, Celniker SE, Lewis EB, Palazzolo MJ. Complete sequence of the bithorax complex of Drosophila. Proc Natl Acad Sci USA. 1995;92:8398–8402. doi: 10.1073/pnas.92.18.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Mathies LD, Kerridge S, Scott MP. Role of the teashirt gene in Drosophilamidgut morphogenesis: secreted proteins mediate the action of homeotic genes. Development. 1994;120:2799–2809. doi: 10.1242/dev.120.10.2799. [DOI] [PubMed] [Google Scholar]
- Mok Y-K, Lo KW-H, Zhang M. Structure of Tctex-1, and its interaction with cytoplasmic dynein intermediate chain. J Biol Chem. 2001;276:14067–14074. doi: 10.1074/jbc.M011358200. [DOI] [PubMed] [Google Scholar]
- Morata G, Kerridge S. Sequential functions of the bithorax complex of Drosophila. Nature. 1981;290:778–781. doi: 10.1038/290778a0. [DOI] [PubMed] [Google Scholar]
- Mulder KM. Role of Ras and Mapks in TGFβ signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Mulder KM, Morris SL. Activation of p21ras by transforming growth factor β in epithelial cells. J Biol Chem. 1992;267:5029–5031. [PubMed] [Google Scholar]
- Mulder KM, Segarini PR, Morris SL, Ziman JM, Choi HG. Role of receptor complexes in resistance or sensitivity to growth inhibition by TGF-β in intestinal epithelial cell clones. J Cell Physiol. 1993;154:162–174. doi: 10.1002/jcp.1041540120. [DOI] [PubMed] [Google Scholar]
- Peifer M. Cell biology. Travel bulletin – traffic jams cause tumors. Science. 2000;289:67–69. doi: 10.1126/science.289.5476.67. [DOI] [PubMed] [Google Scholar]
- Penheiter SG, Mitchell H, Garamszegi N, Edens M, Doré JJE, Jr, Leof EB. Internalization-dependent and -independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Mol Cell Biol. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-β-induced apoptosis is mediated by the adaptor protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- Roberts AB. Molecular and cell biology of TGF-β. Miner Electrolyte Metab. 1998;24:111–119. doi: 10.1159/000057358. [DOI] [PubMed] [Google Scholar]
- Skolnik EY, Margolis B, Muhammadi M, Lowenstein E, Fischer R, Drepps A, Ullrich A, Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991;65:83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, Tamaki K, Engstrom U, Wernstedt C, ten Dijke P, Heldin CH. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J Biol Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, ten Dijke P, Miyazono K, Heldin C-H. Phosphorylation of Ser165 in TGF-β type I receptor modulates TGF-β-induced cellular responses. EMBO J. 1996;15:6231–6240. [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Vilcek JT, editors. Cytokine and Growth Factor Reviews - TGFβ Special Issue. 2000;11:1–168. [Google Scholar]
- Sun B, Hursh DA, Jackson D, Beachy PA. Ultrabithorax protein is necessary but not sufficient for full activation of decapentaplegic expression in the visceral mesoderm. EMBO J. 1995;14:520–535. doi: 10.1002/j.1460-2075.1995.tb07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Bode C, Wolfrum U, Sung C-H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–87. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Sung C-H. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J Cell Biol. 2001;153:1499–1509. doi: 10.1083/jcb.153.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin C-H. Signaling inputs converge on nuclear effectors in TGF-β signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGF-β receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura F, Doody J, Liu F, Wrana JL, Massague J. Reconstitution and transphosphorylation of TGF-β receptor complexes. EMBO J. 1994;13:5581–5589. doi: 10.1002/j.1460-2075.1994.tb06895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura F, Liu F, Doody J, Massague J. Interaction of transforming growth factor-β receptor I with farnesyl-protein transferase-alpha in yeast and mammalian cells. J Biol Chem. 1996;271:13931–13934. doi: 10.1074/jbc.271.24.13931. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Attisano L, Wrana JL, Massague J. Signaling activity of transforming growth factor β type II receptors lacking specific domains in the cytoplasmic region. Mol Cell Biol. 1993;13:7239–7247. doi: 10.1128/mcb.13.12.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGFβ signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Yano H, Lee FS, Kong H, Chuang J-Z, Arevalo JC, Perez P, Sung C-H, Chao MV. Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J Neurosci. 2001;21:RC125. doi: 10.1523/JNEUROSCI.21-03-j0003.2001. , 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Buard A, Mulder KM. Blockade of TGFβ3up-regulation of p27Kip1 and p21Cip1 by expression of RasN17 in epithelial cells. Oncogene. 1998;17:47–55. doi: 10.1038/sj.onc.1201903. [DOI] [PubMed] [Google Scholar]
- Yue J, Frey RS, Hartsough MT, Frielle T, Mulder KM. Cloning and expression of a rat Smad1: regulation by TGF-β and modulation by the Ras/MEK pathway. J Cell Physiol. 1999a;178:387–396. doi: 10.1002/(SICI)1097-4652(199903)178:3<387::AID-JCP13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Yue J, Frey RS, Mulder KM. Cross-talk between the Smad1 and Ras/MEK signaling pathways for TGF-β. Oncogene. 1999b;18:2033–2037. doi: 10.1038/sj.onc.1202521. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor β for transforming growth factor β-1 production in a Smad-dependent pathway. J Biol Chem. 2000a;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming Growth Factor β Protocols, vol. 142. Louisville, KY: Humana Press; 2000b. Activation of the mitogen-activated protein kinase pathways by transforming growth factor-β; pp. 125–131. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming growth factor-β signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]