Abstract
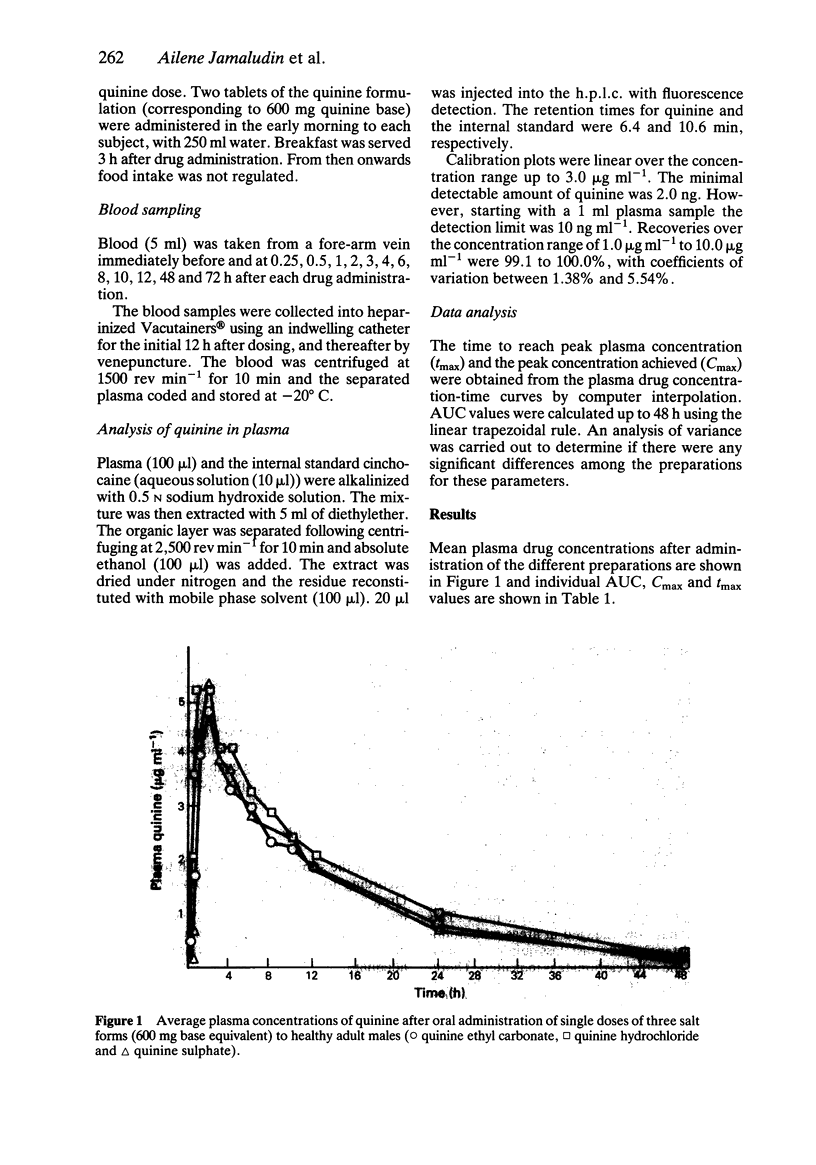
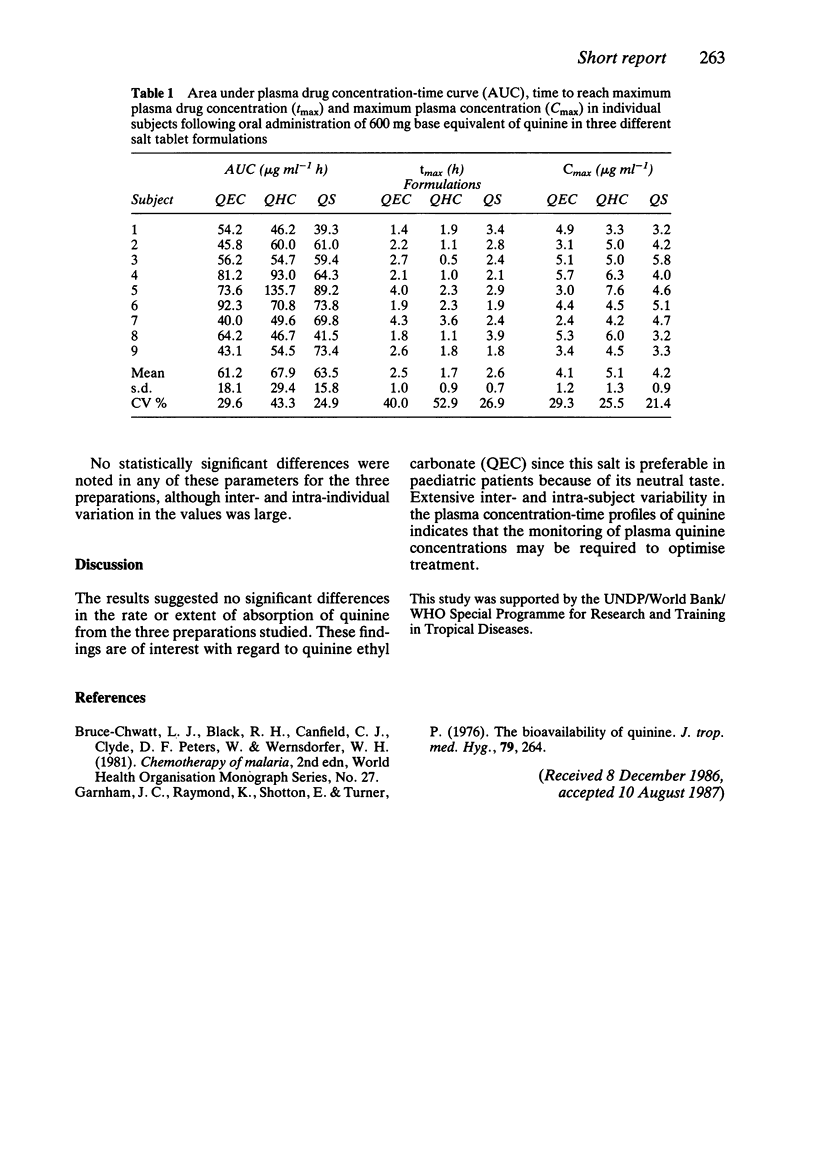
The hydrochloride, sulphate and ethylcarbonate salts of quinine were given in single oral doses (600 mg base equivalent) to nine healthy male subjects according to a cross-over design. No statistically significant differences were noted in the plasma drug concentration-time profiles although inter- and intra-subject variation in AUC, Cmax and tmax values was appreciable. The ethylcarbonate salt may be preferred for use in paediatric patients because of its neutral taste.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garnham J. C., Raymond K., Shotton E., Turner P. The bioavailability of quinine. J Trop Med Hyg. 1976 Dec;79(12):264–269. [PubMed] [Google Scholar]


