Abstract
The multipotent cytokine granulocyte macrophage-colony stimulating factor (GM-CSF) is involved in particular in the physiological response to infection and in inflammatory responses. GM-CSF is produced by many cell types, including T lymphocytes responding to T-cell receptor activation and mantle zone B lymphocytes. B-cell receptor and T-cell receptor activation generates two major signals: an increase in intracellular Ca2+ concentration and a protein kinase cascade. Previous studies have shown that the Ca2+/calmodulin-dependent phosphatase calcineurin mediates stimulation of GM-CSF transcription in response to Ca2+. In this study, we show that Ca2+ signaling also regulates GM-CSF transcription negatively through Ca2+/calmodulin-dependent kinase II (CaMK II) phosphorylation of serines in the autoinhibitory domain for DNA binding of the transcription factor Ets1. Wild-type Ets1 negatively affects GM-CSF transcription on Ca2+ stimulation in the presence of cyclosporin A, which inhibits calcineurin. Conversely, Ets1 with mutated CaMK II target serines showed an increase in transactivation of the GM-CSF promoter/enhancer. Moreover, constitutively active CaMK II inhibited transactivation of GM-CSF by wild-type Ets1 but not by Ets1 with mutated CaMK II sites. Mutation of CaMK II target serines in Ets1 also relieves inhibition of cooperative transactivation of GM-CSF with the Runx1/AML1 transcription factor. In addition, the Ca2+-dependent phosphorylation of Ets1 reduces the binding of Ets1 to the GM-CSF promoter in vivo.
INTRODUCTION
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a multipotent cytokine involved in the production and function of hematopoietic cells. In particular, GM-CSF plays a major role in the physiological response to infection and in inflammatory responses. GM-CSF and related cytokines can augment the functional antimicrobial activities of macrophages, monocytes, and neutrophils (Liles, 2001). A broad range of cells, including T cells of both the Th1 and Th2 phenotype, mantle zone B lymphocytes, macrophages, mast cells, endothelial cells, fibroblasts, and epithelial cells, are all capable of GM-CSF production in response to different immune-activating and inflammatory stimuli (Gasson, 1991; Pistoia and Corcione, 1995). Moreover, GM-CSF production can be constitutive in certain mature B-cell acute lymphoblastic leukemia cell lines (Estrov et al., 1996–1998). T cells responding to T-cell receptor (TCR) activation is a major source of GM-CSF. TCR activation generates two major signals: an increase in intracellular calcium that can be mimicked by treatment with a Ca2+ ionophore and a protein kinase cascade that can be mimicked by treatment with a protein kinase C–activating phorbol ester. Changes at the transcriptional level are important for the control of GM-CSF expression. The GM-CSF promoter region is highly conserved between the mouse and human genes. Both genes also contain similar powerful enhancers that are located ∼2–3 kb upstream from the transcription start. The promoter and the enhancer are both involved in the response of GM-CSF to TCR activation. A number of conserved cis-acting elements identified in the promoter are important for its activity. These elements include the conserved lymphokine element 0 (CLE0), which contains binding sites for Ets and AP-1 transcription factors and is located ∼40 base pairs (bp) upstream from the transcription start. Nearby on the upstream side is a binding site for Runx1 (also denoted AML1), and farther upstream are binding sites for Sp1 and nuclear factor (NF)-κB located ∼70–90 bp from the start site. Stimulation of GM-CSF transcription by Ca2+ is mediated by the calmodulin-dependent phosphatase calcineurin, and the transcription factors NF-AT, AP-1, and NF-κB have been implicated in this activation (for review and references, see Shannon et al., 1997; see also Shang et al., 1999).
Ets1 is the founding member of the Ets family of transcription factors. It plays an important role in regulation of critical genes involved in cell proliferation, differentiation, development, transformation, angiogenesis, and apoptosis. Ets1 is highly expressed in cells of the T and B lymphoid lineages and is important for their normal differentiation, homeostasis, and activation (Bories et al., 1995; Muthusamy et al., 1995). Regulation of the GM-CSF promoter by Ets1 has been studied extensively. In addition to the Ets1 binding site in the CLE0 element, weaker Ets1 sites are also observed farther upstream in the promoter. Furthermore, Ets1 can transactivate the human GM-CSF promoter in Jurkat T cells stimulated with the Ca2+ ionophore ionomycin and the phorbol ester phorbol 12-myristate 13-acetate (PMA) (Thomas et al., 1995). Ets1 has also been reported to upregulate GM-CSF expression in mast cells (McKinlay et al., 1998). Ets1 stimulates the GM-CSF promoter in a synergistic relationship with NF-κB and AP-1 (Shannon et al., 1997; Thomas et al., 1997).
Ets1 becomes rapidly phosphorylated on antigenic stimulation of T or B lymphocytes or on treatment with ionomycin. These phosphorylations are transient and dependent on the increase in intracellular calcium concentration (Pognonec et al., 1990; Fisher et al., 1991; Rabault and Ghysdael, 1994). A major site of Ca2+-dependent phosphorylation of Ets1 is a serine cluster in exon VII adjacent to the Ets DNA binding domain (Fisher et al., 1994; Rabault and Ghysdael, 1994; Cowley and Graves, 2000). Ets1 has been identified as a calmodulin-dependent kinase II (CaMK II) target in vitro (Fisher et al., 1994). Moreover, phosphorylation of the serines adjacent to the DNA binding domain by CaMK II can specifically inhibit the DNA binding of Ets1 in vitro through a mechanism of enforcing and stabilizing an autoinhibitory conformation (Cowley and Graves, 2000). However, it is not known whether Ca2+-dependent phosphorylation of Ets1 affects the transcription of any gene in vivo.
In this study, we show that Ca2+ signaling not only positively regulates transcription from the GM-CSF promoter/enhancer through calcineurin but also negatively regulates it through CaMK II phosphorylation of serines in the autoinhibitory domain for DNA binding of Ets1. Moreover, this Ca2+-dependent phosphorylation of Ets1 reduces the DNA binding of Ets1 to the GM-CSF promoter in vivo.
MATERIALS AND METHODS
Expression and Reporter Plasmids
The wild-type human CaMK IIγβ eukaryotic expression plasmid, the inactive T286A and constitutively active T286D derivatives, and the parental expression plasmid pSRα.BKS have been described previously (Nghiem et al., 1993). The Runx1 expression plasmid pBJ9AML1b has also been described previously (Xie et al., 1999). The pCDNA-hEts1 plasmid encoding full-length human Ets1 cDNA was a kind gift from Dr. Sven Pettersson, Karolinska Institute. The Escherichia coli Ets1 expression plasmid pET20bEts1-wt was constructed by PCR with PfuTurbo DNA polymerase (Stratagene, La Jolla, CA). NdeI and HindIII sites were incorporated at the ends of the cDNA using the upstream primer 5′-GGGAATTCCATATGAAGGCGGCCGTCGAT-3′ and the downstream primer 5′-CCCAAGCTTCTCGTCGGCATCTGGCTTG-3′. The amplified DNA fragment was subcloned with NdeI and HindIII into the E. coli expression plasmid pET20b+ (Novagen, Madison, WI).
The eukaryotic Ets1 expression plasmid pBJ9Ets1-wt was constructed by subcloning the Ets1 cDNA into the pBJ9Ω vector, a kind gift from Dr. H. Land, with HindIII and BglII. These sites were introduced at the ends of the Ets1 cDNA by PCR with PfuTurbo DNA polymerase by use of the primers 5′-CCCAAGCTTCATATGAAGGCGGCCGTCGAT-3′ and 5′-GGAAGATCTTCACTCGTCGGCA-TCTGG-3′.
The mutants were obtained by PCR-based mutagenesis with overlapping DNA segments by use of the PfuTurbo DNA polymerase and the same external primers as above. The complementary internal primers for the serine 251 and 257 mutation to alanine were 5′-ACGCTTTTGAAAGCATAGAGGCCTACGATAGTTGTG-3′ and 5′-AGGCCTCTATGCTTTCAAAAGCGTCCTGGCCCCGAG-3′, and for the serine 282 and 285 mutation to alanine, 5′-GTTCCCGCCTATGATGCATTCGACTCAGAGGACTATCC-3′ and 5′-GAGTCGAATGCATCATAGGCGGGAACACGCTGCAGGC-3′ (mutations are in boldface/italics). The complementary internal primers for the C-terminal deletion mutant ΔEts1 (amino acids 1–315) were 5′-ACCGTGCTGACCTCAATTAGGACAAGCCTGTCATTCC-3′ and 5′-GGAATGACAGGCTTGTCCTAATTGAGGTCAGCACGGT-3′.The full-length Ets1 mutants were cloned into the NdeI/HindIII-digested pET20b+ E. coli expression vector and into the HindIII/BglII-digested pBJ9Ω eukaryotic expression plasmid. All PCR-generated DNA sequences were confirmed by DNA sequencing.
The enhancer and promoter of GM-CSF (Cockerill et al., 1993) were obtained from genomic Jurkat DNA by PCR amplification with Pfu polymerase and cloned into the BglII and HindIII sites, respectively, of the pGL2-Basic reporter plasmid (Promega, Madison, WI). Existing BglII and HindIII sites of the 716-bp enhancer and the 0.6-kb promoter segment, respectively, were used, except at the 3′ end of the promoter, where the HindIII site was created with the 5′-GAGAAGCTTTAGCCTTTCTCTCTGTG-3′ primer. The HpaI/SmaI segment of pGL2-Basic (nucleotides 5451–5453) was deleted to remove a potential Runx1 site. Sequencing of the reporter plasmid showed no differences compared with the previously isolated enhancer and promoter in any part reported to be important for transcription or containing any protein-binding site (Cockerill et al., 1993). Compared with the reported isolates, an extra G was found at position 108 in the enhancer, and the nucleotides TC were absent at position 179–180 of the promoter.
Expression in E. coli and Purification of Ets1 Proteins
Ets1 variants were expressed from the plasmids pET20bEts1-wt and pET20bEts1-m3 in E. coli BL21(DE3) (Stratagene) as a fusion protein with a C-terminal His6 tag according to the manufacturer's instructions. Harvested cells were lysed by freeze-thawing followed by sonication and centrifugation. Supernatants were mixed with Ni-NTA agarose (Qiagen, Hilden, Germany) at 4°C for 1 h and washed according to the manufacturer's instructions. The proteins were eluted by increasing the imidazole concentration to 250 mM. The Ets1-containing fractions were further purified by HiPrep 16/10 DEAE ion-exchange chromatography using the UNICORN fast protein liquid chromatography system (Amersham Biosciences, Arlington Heights, IL). Purified Ets1 proteins were dialyzed against 25 mM Tris-HCl, pH 7.5, 5% glycerol, 10 mM NaCl, 0.1 mM EDTA at 4°C. The concentrations of proteins were determined with the BCA protein assay kit (Pierce), and protein aliquots were stored at −80°C.
Cell Lines and Transient Transfections
The human malignant cell lines DG75, an Epstein-Barr virus–negative Burkitt's lymphoma; Jurkat, a human T-cell line; Raji, a human Epstein-Barr virus–positive Burkitt's lymphoma; and K562, an early erythroleukemia cell line were cultured as previously described (Lars and Paschalis, 1993; Hughes et al., 1998). Transient transfections were performed as previously described (Lars and Paschalis, 1993) with 2 μg hCMV-β-gal plasmid (reference plasmid for normalization), 4 μg reporter plasmid, and 5 μg of each expression plasmid indicated. Where necessary, the corresponding empty expression vector was added to a total of 10 μg expression plasmids. Cells (1 × 107) were electroporated, followed by incubation in 10 ml of medium. Where indicated, the culture was divided 30 min after electroporation, and 5 ml of the cells were stimulated with ionomycin (1 μg/ml, Calbiochem, La Jolla, CA), PMA (25 μg/ml, Sigma, St. Louis, MO), and/or cyclosporin A (CsA) (20 nM, Sigma) (final concentrations), and 5 ml was kept unstimulated. The cells were harvested after 20 h incubation.
CaMK II Phosphorylation of Ets1 In Vitro
Ets1 was in vitro phosphorylated with baculovirus-produced monomers of the α-subunit of CaMK II (New England Biolabs, Beverly, MA). CaMK II phosphorylations were performed at 30°C for 1.5 h in kinase buffer (20 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 0.5 mM dithiothreitol, 0.1 mM EDTA) with 2.4 μM calmodulin, 2 mM CaCl2, 10 μg purified His-tagged Ets1 wild-type or mutant protein, 200 μM γ-[32P]-ATP, and the indicated amount of kinase. Reactions were stopped in SDS-PAGE sample buffer by boiling for 5 min and then analyzed by 10% SDS-PAGE. Gels were stained with Coomassie blue, destained, dried, and exposed to autoradiography film overnight. The level of phosphorylation was analyzed with an Image Quant phosphoimager (Molecular Dynamics, Sunnyvale, CA).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was performed as previously described (Orlando et al., 1997) with a few modifications. Briefly, 1 × 107 DG75 cells were cotransfected with 5 μg GM-CSF promoter/enhancer reporter and 5 μg pBJ9Ets1-wt or pBJ9Ets1-m3 with or without 5 μg constitutively active CaMK II. Where indicated, the transfected cells were stimulated with ionomycin in the presence of CsA 30 min after electroporation. Ten hours after transfection, the cells were cross-linked with formaldehyde (final concentration 1%, vol/vol) in RPMI medium at 4°C for 1 h, followed by the addition of glycine to a final concentration of 125 mM to inhibit further cross-linking. Cells were harvested by centrifugation and washed twice for 15 min with PBS at room temperature. The cells were lysed in RIPA buffer (1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with the complete EDTA-free protease inhibitor cocktail (Roche) and sonicated to solubilize the chromatin. The cell lysates were precleared by incubation with 30 μl protein G-Sepharose beads (Amersham Biosciences) followed by centrifugation. The supernatants were then incubated with anti-E12 antibody or C-275 anti-Ets1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. DNA–protein complexes were collected with protein G-Sepharose followed by washing 3 times with RIPA buffer. Bound DNA–protein complexes were treated with proteinase K at 56°C for 1 h and eluted from the antibodies with two incubations in 10 mM Tris-HCl, pH 8.0, 1 mM dithiothreitol, 0.5% SDS at room temperature for 30 min. Samples were then extracted twice with phenol/chloroform and precipitated with ethanol in presence of glycogen carrier. DNA fragments were recovered by centrifugation, resuspended in H2O, and used for PCR amplifications. The PCR products were fractionated on 2.0% agarose gels and stained with ethidium bromide. The primers for ChIP flanking four Ets1 binding sites and spacing 285 bp in the GM-CSF promoter were 5′-CCCATTCAGACTGCCCAG-3′ and 5′-TCTGTGTAGCTGGGCTCACTG-3′.
RESULTS
Calcium Signaling Regulates the GM-CSF Promoter/Enhancer Positively through Calcineurin and Negatively by a Calcineurin-Independent Mechanism
To analyze the effects of an increased intracellular calcium ion concentration on GM-CSF transcription, we used a reporter plasmid containing the human GM-CSF promoter and enhancer. The Ca2+ ionophore ionomycin was used to create changes in the intracellular Ca2+ concentration in the presence and absence of the phorbol ester PMA. The effect of Ca2+ was analyzed both in the Jurkat T helper cell line and in the B-lymphocyte cell line DG75. In both cell lines, ionomycin in combination with the phorbol ester showed a strong positive effect on the GM-CSF promoter/enhancer (Figure 1). However, the GM-CSF activation was negligible in the presence of CsA, a drug that inhibits calcineurin. This is consistent with previous reports that GM-CSF activation by ionomycin in the presence of phorbol ester is calcineurin dependent (Shannon et al., 1997). It is also noteworthy that ionomycin alone had a small but significant positive effect in both cell lines (Figure 1). Interestingly, the calcineurin inhibitor CsA not only blocked this activation but also decreased the GM-CSF activity slightly below the level of nontreated Jurkat cells and to almost a 2.5-fold lower level than the nontreated DG75 cells. Thus, we conclude that in addition to the established positive calcineurin-dependent effect of Ca2+ on GM-CSF expression, there is also a negative Ca2+ signaling effect on GM-CSF that appears to be calcineurin independent. PMA had a strong activating effect in DG75 cells without ionomycin as well. To a large extent, this activation was inhibited by CsA, suggesting that the intracellular calcium ion concentration in the presence of PMA was sufficient to activate calcineurin in this cell line. This finding is not surprising, considering that a calcineurin-activated transcription factor, NF-AT, which participates in regulation of GM-CSF (Shannon et al., 1997; Feske et al., 2000), can react on smaller Ca2+ concentration increase than other Ca2+-activated transcription factors (Dolmetsch et al., 1997). Importantly, in this cell line, a further increase in Ca2+ concentration by ionomycin treatment in the presence of PMA and CsA led to a smaller GM-CSF activation than without ionomycin. Thus, Ca2+ signaling can also have a negative calcineurin-independent effect on GM-CSF in the presence of phorbol ester.
Figure 1.
Effects of the phorbol ester PMA, the Ca2+ ionophore ionomycin, and the calcineurin inhibitor CsA on the GM-CSF promoter/enhancer in (A) the T-helper cell line Jurkat and (B) the B-lymphocyte cell line DG75. Bars represent average ratio of luciferase/β-galactosidase activity in arbitrary units from three independent transfections ± SD, using β-galactosidase expression from an hCMV-β-gal plasmid for normalization.
Increased Transactivation of the GM-CSF Promoter/Enhancer by Ets1 with Mutated CaMK II Sites
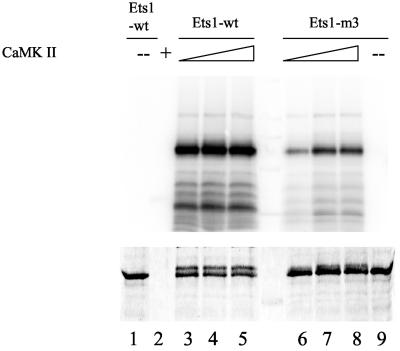
The Ets1 transcription factor makes a large contribution to the activity of the GM-CSF promoter/enhancer (Thomas et al., 1995; Shannon et al., 1997; Thomas et al., 1997; McKinlay et al., 1998) and is therefore a putative target for a calcium ion effect on GM-CSF transcription. Four internal serines, located amino-terminal to the Ets domain, are Ca2+-dependent phosphorylation targets (Rabault and Ghysdael, 1994; Cowley and Graves, 2000). Significantly, phosphorylation of Ets1 by Ca2+-dependent pathways is thought to inhibit DNA binding in vitro (Fisher et al., 1994; Rabault and Ghysdael, 1994; Cowley and Graves, 2000). To analyze the role of these four serines, S251, S257, S282, and S285, in transcription, we constructed three mutant derivatives of human Ets1 (Figure 2). The mutant Ets1-m1 contains S251A and S257A substitutions, Ets1-m2 contains S282A and S285A substitutions, and Ets1-m3 contains all these four substitutions. Treatment of Ets1 by T-cell nuclear extract or phosphorylation of these four serines by calmodulin-dependent kinase II (CaMK II) has recently been reported to decrease Ets1 DNA binding by reinforcing autoinhibition (Cowley and Graves, 2000). To confirm that these sites can be specifically and efficiently phosphorylated by CaMK II, His-tagged wild-type and Ets1-m3 proteins were produced in E. coli, purified, and used as a substrate for CaMK II in vitro (Figure 3). Wild-type Ets1 was efficiently phosphorylated by CaMK II, as evidenced by 32P incorporation and by a retarded band after separation by PAGE and staining of the gel with Coomassie blue. In contrast, the level of phosphorylation was dramatically decreased in the Ets1-m3 mutant, with all four reported CaMK II phosphorylation sites replaced with alanine, and very little of the protein had altered mobility (Figure 3). A higher concentration of CaMK II was needed to reach maximal phosphorylation of Ets1-m3, and this level was still 5.1-fold lower than for the wild type. This is in agreement with a recent report indicating that mutation of these four serines reduced CaMK II phosphorylation from approximately 5 to 1 mole of phosphate per mole of Ets1 (Cowley and Graves, 2000). These findings confirm that Ets1 can be efficiently and specifically phosphorylated by CaMK II in vitro and that the four mutated serines are major phosphorylation sites.
Figure 2.
Schematic of the structure of the human Ets1 protein. The positions of the CaMK II target serines in exon VII are indicated by an asterisk. Three mutants were constructed in which different combinations of these serines were replaced with alanine. Amino acid sequence is shown in single-letter code.
Figure 3.
Phosphorylation of Ets1 wild-type and Ets1-m3 mutant by CaMK II in vitro. Ets1 proteins were separated by 10% SDS-PAGE, and the phosphorylated form was detected by autoradiography (top), whereas both forms were detected by Coomassie blue staining (bottom). The amounts of baculovirus-produced CaMK II (New England Biolabs) used were 125 U in lanes 3 and 6, 250 U in lanes 2, 4, and 7, and 500 U in lanes 5 and 8.
Ets1 transactivates the GM-CSF promoter in a PMA- and ionomycin-dependent manner in Jurkat T cells (Thomas et al., 1995). To analyze whether Ets1 has the corresponding effect on regulation of GM-CSF expression in DG75 cells, we used the GM-CSF promoter/enhancer reporter construct along with expression vectors for wild-type or mutant Ets1 in transient transfections of DG75 cells with or without subsequent stimulation with PMA and ionomycin (Figure 4A). In the absence of stimulation, expression of wild-type Ets1 had no positive effect on the expression of the reporter. In contrast, expression of Ets1-m1 or Ets1-m2, each with two of the phosphorylation sites replaced by alanine, increased expression of the GM-CSF reporter 1.7- and 1.5-fold, respectively. Moreover, Ets1-m3 with all four phosphorylation sites replaced by alanine could transactivate the reporter construct by 2.4-fold. In addition, each Ets1 mutant increased transactivation of the GM-CSF reporter considerably more than the wild type when the cells were treated with PMA and ionomycin. Expression of Ets1-m3 increased activation of the GM-CSF reporter 11.3-fold on treatment with PMA and ionomycin, whereas wild-type Ets1 increased activation only 3.1-fold (Figure 4A). The mutants Ets1-m1 and Ets1-m2 had intermediate effects (Figure 4A). The positive effects of the Ets1 mutations were not through increased expression of the mutated proteins, because Western blot analysis of transfected cells showed equal expression of transfected Ets1 wild-type and mutant constructs (Figure 4C). Furthermore, the increase is not a result of a changed nuclear distribution of Ets1, because mutations in the CaMK II sites of Ets1 do not affect its nuclear translocation (Rabault and Ghysdael, 1994). Taken together, these data show that mutations in the CaMK II sites of Ets1 increase transactivation of the GM-CSF reporter in DG 75 cells regardless of PMA and ionomycin stimulation.
Figure 4.
Effects of Ets1 wild-type and mutants on the GM-CSF promoter/enhancer in DG75 cells in the absence and presence of the phorbol ester PMA, the Ca2+ ionophore ionomycin, and the calcineurin inhibitor CsA. (A) Effects of treatment with PMA plus ionomycin and (B) effects of treatment with ionomycin plus CsA. Bars represent average ratio of luciferase/β-galactosidase activity in arbitrary units from three independent transfections ± SD, using β-galactosidase expression from an hCMV-β-gal plasmid for normalization. (C) Quantification of the expression level of Ets1 wild-type and the mutants in the transfected DG75 cells by Western blot using the C-275 anti-Ets1 antibody (Santa Cruz).
Ets1 Negatively Affects GM-CSF Transcription on Stimulation with Ionomycin in the Presence of CsA
To examine the role of calcium-dependent Ets1 phosphorylation on transcriptional activation of GM-CSF without interference of calcineurin activation, the transfected cells were treated with ionomycin in the presence of CsA. This Ca2+ increase, without calcineurin activation, led to a 3.8-fold inhibition of transactivation of the reporter in the presence of wild-type Ets1 (Figure 4B). In contrast, the negative effect was greatly reduced for all mutants, illustrated by a minimal inhibition of only 1.2-fold for the combined mutant, Ets1-m3 (Figure 4B). These results indicate that the activity of Ets1 is sensitive to activation of one or more calcium ion–dependent enzyme(s) other than calcineurin. Conversely, Ets1 with mutations in some or all of the CaMK II target serines progressively loses this sensitivity. We conclude that the serines in Ets1 identified as CaMK II phosphorylation sites in vitro play a role for negative calcium ion–dependent regulation of the GM-CSF promoter/enhancer in vivo.
CaMK II Target Serines in Ets1 Inhibit the Cooperative Transactivation of GM-CSF by Runx1/AML1
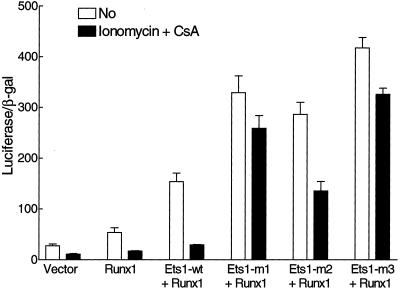
The GM-CSF promoter contains a binding site for proteins belonging to the Runx family of transcription factors. The site is only a few nucleotides from the Ets1 site. One Runx family member is Runx1, which is essential for development of hematopoietic stem cells and several functions in hematopoiesis and the immune system (Lutterbach and Hiebert, 2000; Tracey and Speck, 2000). Cooperation between Ets1 and Runx1 has been reported in the transcription of several promoters, and Runx1 has been shown to decrease autoinhibition of Ets1 DNA binding through interaction between the proteins (Kim et al., 1999; Goetz et al., 2000). To analyze whether the negative calcium ion–dependent regulation of GM-CSF was blocked by Runx1, the largest splice form of mouse Runx1 was overexpressed. As expected, a functional cooperation was found between Ets1 and Runx1 in regulation of transcription of GM-CSF. Although Ets1 overexpression had a slightly negative effect (Figure 4) and Runx1 a small positive effect (Figure 5), the effect of combined overexpression of both Ets1 and Runx1 was a profound 5.6-fold activation (Figure 5). Importantly, the negative effect of ionomycin plus CsA was not blocked when Ets1 was cooperating efficiently with Runx1, because a 5.1-fold inhibition was observed (Figure 5), compared with 3.8-fold inhibition without Runx1 coexpression (Figure 4). Furthermore, all three Ets1 mutants decreased the negative effect of ionomycin plus CsA to approximately the same extent in the presence of strong Runx1 cooperativity as in the absence of Runx1 overexpression. The high calcium ion concentration in the absence of calcineurin activity gave as much as 29.8-fold higher GM-CSF reporter transcription on overexpression of Ets1-m3 together with Runx1 compared with the vector control. These results show that the serines in Ets1 identified as CaMK II phosphorylation sites in vitro play a significant negative role in calcium ion regulation of the GM-CSF promoter/enhancer even when Ets1 is efficiently cooperating with Runx1.
Figure 5.
Effects of ionomycin plus CsA on activation of the GM-CSF promoter/enhancer by Ets1 wild type and mutants when the largest splice form of mouse Runx1 is overexpressed in DG75 cells. Assay conditions were as described in the legend to Figure 4.
Constitutively Active CaMK II Inhibits Ets1 Transactivation of GM-CSF
To further investigate the negative effect of Ca2+ on Ets1 transactivation of GM-CSF, we coexpressed the wild-type or mutant Ets1 proteins with inactive or constitutively active CaMK II in the DG 75 cells. As shown in Figure 6A, expression of constitutively active CaMK II dramatically decreased transactivation of GM-CSF by Ets1. The effect of CaMK II was dependent on the kinase activity of the enzyme, because no significant effect was obtained by expression of inactive CaMK II. The background GM-CSF reporter transcription in the absence of Ets1 overexpression was also inhibited by constitutively active CaMK II, suggesting that this transcription is dependent on endogenous Ets1 and/or an equivalent CaMK II–sensitive protein. Similar to the effect of ionomycin in the presence of the calcineurin inhibitor CsA, the inhibition by constitutively active CaMK II was greatly alleviated by the mutations of the CaMK II target sites in Ets1. Only a very small part of the negative effect of constitutively active CaMK II remained in the Ets1-m3 mutant with all four identified CaMK II sites in Ets1 mutated. Thus, the negative effect of CaMK II overexpression on GM-CSF transcription by Ets1 is dependent on the serines that are CaMK II phosphorylation sites.
Figure 6.
Decreased inhibition of the GM-CSF promoter/enhancer by constitutively active CaMK II on mutation of Ets1. (A) Cotransfection of constitutively active CaMK II T286D, inactive CaMK II T286A, or vector control with Ets1 wild type, mutant Ets1, or vector control in DG75 cells. (B) Same as A except that Runx1 was also cotransfected. Bars represent average ratio of luciferase/β-galactosidase activity in arbitrary units from three independent transfections ± SD, using β-galactosidase expression from an hCMV-β-gal plasmid for normalization.
To analyze whether CaMK II can affect cooperation between Ets1 and Runx1, we also analyzed the effect of constitutively active CaMK II when Runx1 was coexpressed. As shown in Figure 6B, constitutively active CaMK II could efficiently inhibit the synergism of Runx1 with wild-type Ets1. This inhibitory effect of constitutively active CaMK II was dramatically decreased by the Ets1 mutations even in the presence of Runx1. Compared with the wild-type, Ets1-m1, Ets1-m2, and Ets1-m3 gave 5.5-, 4.9-, and 8.7-fold higher transcriptional activity, respectively, in the presence of Runx1 and constitutively active CaMK II. These results show that inhibition of Ets1 by CaMK II also occurs when Ets1 cooperates with Runx1, which has the potential to relieve autoinhibition of Ets1.
We also analyzed the effect of overexpression of constitutively active calcineurin. As expected, calcineurin increased transcription of the GM-CSF reporter. None of the Ets1-m1, Ets1-m2, or Ets1-m3 mutations had any effect on the activation by calcineurin (data not shown). Thus, none of the analyzed CaMK II sites in Ets1 contribute significantly to the positive effect of calcineurin on the GM-CSF promoter/enhancer.
To analyze whether Ca2+ inhibition of Ets1 regulates GM-CSF transcription only in DG75 cells or in a broad range of cells, we analyzed the effects of the Ets1 mutations in the absence and presence of ionomycin plus CsA in other cell lines as well. The Ets1 mutations increased transcription of the GM-CSF reporter by Ets1 in all analyzed cell lines. The results with the B-lymphocyte cell line Raji and the myeloid cell line K562 are shown in Figure 7. In the Raji cell line, the mutations of Ets1 increased the activation of the GM-CSF reporter much more when the cells were treated with ionomycin plus CsA than without the treatment, showing that inhibition of wild-type Ets1 was Ca2+ dependent in this cell line as well (Figure 7A). However, in K562 cells, the increase in GM-CSF reporter activation was approximately equal in the presence and absence of ionomycin plus CsA treatment. The most likely explanation for the difference between the cell lines was a limiting CaMK II activity in K562 cells, which would lead to a lack of inhibitory effect of ionomycin plus CsA treatment. This was indeed the case, because expression of constitutively active CaMK II resulted in a strong inhibition of the GM-CSF reporter in Ets1-wt–transfected K562 cells but a much smaller inhibition of the reporter in cells transfected with mutant Ets1 (Figure 7B, bottom). The decrease in the inhibition by constitutively active CaMK II was from 46 ± 4% in Ets1-wt transfected K562 to only 25 ± 4% inhibition in the Ets1-m3–transfected cells. Transfection of Jurkat cells with Ets1-wt gave a low level of GM-CSF reporter transcription that also was more inhibited by cotransfection of CaMK II than the higher level of transcription in Ets1-m3 transfected Jurkat cells (data not shown).
Figure 7.
Effects of Ets1 wild-type and mutants on the GM-CSF promoter/enhancer in (A) Raji cells and (B) K562 cells in the absence and presence of ionomycin plus CsA. Bars represent average ratio of luciferase/β-galactosidase activity in arbitrary units from three independent transfections ± SD, using β-galactosidase expression from an hCMV-β-gal plasmid for normalization. The results from cotransfections of Ets1 wild type, mutant Ets1, or vector control with constitutively active CaMK II T286D compared with vector control are shown below the bars in B. These numbers are the average inhibitions in per cent from three independent transfections ± SD.
To analyze whether the Ets domain containing part of Ets1 was needed for the decrease in GM-CSF reporter activation by wild-type Ets1 on ionomycin plus CsA treatment, we constructed a C-terminal deletion derivative of the Ets1 expression plasmid, ΔEts1(1–315). This deletion derivative was included in the analysis in Raji cells, in which, as in DG75 cells, ionomycin plus CsA treatment had a negative effect on GM-CSF activation on expression of Ets1-wt. The deletion was found to block the negative effect of ionomycin plus CsA, and this treatment even led to a small increase in transcription (Figure 7A). Furthermore, this deletion of Ets1 resulted in loss of most of the inhibitory effect of ionomycin plus CsA treatment or of CaMK II overexpression in DG75 cells (data not shown). This shows that the Ets domain or a sequence C-terminal to it is needed for the inhibition of GM-CSF transcription by Ca2+ activation of CaMK II inhibition of Ets1.
CaMK II Inhibits Ets1 Binding to the GM-CSF Promoter In Vivo
The GM-CSF promoter contains several Ets1 binding sites (Figure 8A), and transactivation of the GM-CSF promoter by Ets1 requires interaction of Ets1 with at least one intact Ets1 binding site, GM5 (Thomas et al., 1995; Thomas et al., 1997; McKinlay et al., 1998). To examine whether Ca2+ signaling and phosphorylation by CaMK II affects Ets1 binding to the promoter in vivo, ChIP PCR analysis was performed. A pair of primers flanking the strongest Ets1 binding sites in the GM-CSF promoter was used (Figure 8A). The results in Figure 8B show that treatment with either ionomycin plus CsA or overexpression of constitutively active CaMK II led to a strong reduction of in vivo binding of transfected Ets1 to the DNA segment containing the strongest Ets1 binding sites in the GM-CSF promoter of the reporter plasmid. No significant effect by either ionomycin plus CsA or coexpression of CaMK II was observed when the mutant Ets1-m3 was expressed, showing that the CaMK II phosphorylation sites were important for the reduction in the binding of Ets1 to the promoter in vivo.
Figure 8.
Binding of wild-type and mutant Ets1 to the GM-CSF promoter in vivo: decreased binding of wild-type Ets1 but not the Ets1-m3 mutant on ionomycin plus CsA treatment or expression of constitutively active CaMK II. (A) Schematic drawing of the GM-CSF reporter plasmid. The 716-bp human GM-CSF enhancer and the 647-bp human GM-CSF promoter segments (Cockerill et al., 1993; Jenkins et al., 1995; Shannon et al., 1997) are indicated. The positions of the primers used in ChIP, ChIP1 and ChIP2, relative to the weak (gray) and strong (black) Ets1 binding sites (Thomas et al., 1995) are indicated. (B) The amounts of the PCR product after ChIP with wild-type and mutant Ets1. Where indicated, either the cells were treated with ionomycin plus CsA, or constitutively active CaMK II was expressed (MATERIALS AND METHODS).
DISCUSSION
A broad range of cells, including T lymphocytes and mantle zone B lymphocytes, produce GM-CSF in response to different immune-activating and inflammatory stimuli (Gasson, 1991; Pistoia and Corcione, 1995). One major signal in T cells responding to TCR activation is an increase in intracellular Ca2+. Activation of GM-CSF expression by Ca2+ occurs through the phosphatase calcineurin (Shannon et al., 1997). Other immune and inflammatory stimuli, such as BCR activation, also bring about signaling by the second messenger Ca2+ (Healy et al., 1997; Benschop et al., 2001). BCR activation of B lymphocytes and TCR activation of T lymphocytes lead to Ca2+-dependent phosphorylation of Ets1 (Pognonec et al., 1990; Fisher et al., 1991; Rabault and Ghysdael, 1994). In the present study, we have shown that Ca2+ signaling not only positively regulates transcription from the GM-CSF promoter/enhancer through calcineurin activation of the transcription factors NF-AT, NF-κB, and AP-1 but also negatively regulates it through CaMK II phosphorylation of serines in the auto-inhibitory domain for DNA binding of the transcription factor Ets1 (Figure 9).
Figure 9.
Schematic illustrating the discussed models for regulation of GM-CSF transcription. The binding of NF-AT, NF-κB, Ets1, AP-1, and Runx1 transcription factors to the promoter is shown. For simplicity, only one NF-AT site and one Ets1 site are drawn, although the GM-CSF promoter/enhancer contains multiple binding sites for these factors (Thomas et al., 1995; Shannon et al., 1997; Thomas et al., 1997; McKinlay et al., 1998; Shang et al., 1999). The autoinhibitory domain of Ets1 (gray) and the Ets domain are also shown. Nonphosphorylated serines at positions 251, 257, 282, and 285 in the autoinhibitory domain are indicated with S and phosphorylation of these serines with P. Ca2+ signaling, which can be induced by TCR or BCR activation or by ionomycin treatment, positively regulates transcription from the GM-CSF promoter/enhancer through calcineurin activation of the transcription factors NF-AT, NF-κB, and AP-1 (Shannon et al., 1997). In the present study, we show that Ca2+ signaling can also negatively regulate the GM-CSF promoter/enhancer through CaMK II phosphorylation of serines in the autoinhibitory domain for DNA binding of Ets1. Serine phosphorylation in the autoinhibitory domain stabilizes the conformation that inhibits the DNA binding of the Ets domain (Cowley and Graves, 2000). The negative effect of wild-type Ets1 suggests that it functions as a dominant inhibitory protein that decreases transactivation by a functionally related protein(s), such as the p42 splice form of Ets1, that lacks the autoinhibitory domain or another Ets family member(s) that is less inhibited by CaMK II. The finding that the mutations also increased the Ets1 activity in the absence of Ca2+/CaMK II–activating treatment may, as illustrated, indicate that another kinase can mediate a partial phosphorylation in the absence of Ca2+ signaling. The circular arrow indicates a possible dynamic balance between Ca2+-dependent CaMK II and autophosphorylation that makes CaMK II independent of Ca2+ (Lukas et al., 1998). The intermediate nucleotides between the Runx1 and Ets1 sites in the GM-CSF promoter constitute a binding site for AP-1. The results suggest that when Ets1 functions in cooperation with nearby AP-1, at least a large part of the autoinhibition through phosphorylation of the autoinhibitory domain remains and is not relieved by interaction between Ets1 and Runx1.
It is interesting that the Ca2+ second messenger can elicit both positive and negative effects on transcription of the same gene. Even though Ca2+ is a ubiquitous second messenger, Ca2+ signals lead to GM-CSF expression in only a small fraction of all cell types, including T cells and certain B cells. Furthermore, a positive Ca2+ signaling pathway dependent on calcineurin combined with a negative pathway dependent on CaMK II (Figure 9) would lead to a Ca2+ activation of GM-CSF transcription only when the activity or quantity of components of the former pathway dominate over those of the latter pathway. Moreover, Ca2+ signaling after BCR or TCR activation displays both amplitude and frequency modulation. Foreign antigen triggers a large biphasic Ca2+ response in naive B cells, whereas tolerant B cells display an increased basal Ca2+ level and the self-antigen stimulates low Ca2+ oscillations (Healy et al., 1997). Similarly, T cells induced to anergy display an elevated basal Ca2+ concentration, and comparatively low-amplitude Ca2+ responses are found when T-cell anergy is induced by altered peptide ligands (Gajewski et al., 1990, 1994a,b; Sloan-Lancaster et al., 1996). T cells stimulated through the TCR display Ca2+ oscillations with a period of ∼100 s (Gajewski et al., 1990, 1994a,b; Sloan-Lancaster et al., 1996). It is also notable in this context that immature B cells have a higher-amplitude Ca2+ response to antigen than mature B cells and that α-hemolysin of uropathogenic E. coli induces Ca2+ oscillations with a period of ∼700 s in renal epithelial cells (Uhlen et al., 2000; Benschop et al., 2001). Therefore, because differential Ca2+ signaling plays a key role in the distinct responses to self and nonself, it is not surprising that transcription of a cytokine can be both positively and negatively regulated by Ca2+. The importance of plasticity in the Ca2+ signaling system is in line with the participation in GM-CSF regulation of at least three calcineurin-activated transcription factors, NF-AT, AP-1, and NF-κB (Shannon et al., 1997; Shang et al., 1999), that each display a different pattern of amplitude and frequency modulation in response to Ca2+ signaling (Dolmetsch et al., 1997, 1998). Moreover, the frequency of intracellular Ca2+ oscillations can be decoded through CaMK II, because its activity is highly sensitive to the temporal pattern of Ca2+ oscillations, and CaMK II is known to be a key mediator of many Ca2+ effects (Dupont and Goldbeter, 1998; Lukas et al., 1998).
Wild-type Ets1 negatively affected GM-CSF transcription on Ca2+ stimulation of DG75 and Raji cells in the presence of a calcineurin inhibitor, whereas Ets1 with mutated CaMK II target serines resulted in increased transactivation of GM-CSF. Likewise, wild-type Ets1 but not mutant Ets1 had a negative effect in DG75 cells when constitutively active CaMK II was expressed. The negative effect of wild-type Ets1 suggests that it functions as a dominant inhibitory protein that decreases transactivation by a functionally related protein(s) (Figure 9). Such a related protein could be the p42 splice form of Ets1 (Figure 9) that lacks the autoinhibitory exon VII domain (Koizumi et al., 1990; Wasylyk et al., 1992; Fisher et al., 1994). There is also a large family of Ets1-related Ets transcription factors that can function as either transcriptional activators or repressors (Mimeault, 2000; Yordy and Muise-Helmericks, 2000; Lelievre et al., 2001). Significantly, the DG75 cell line contains large amounts of proteins binding to DNA sites for Ets family members in EMSA (Nilsson et al., 1995). Therefore, an alternative possibility is that another Ets family member, less inhibited by CaMK II, contributes to GM-CSF transcription, at least when Ets1 is Ca2+ inhibited (Figure 9). Such a contribution could also explain why phosphorylation of the four inhibitory CaMK II sites decreases DNA binding of Ets1 50-fold in vitro (Cowley and Graves, 2000), whereas expression of this mutant gave a 6- to 9-fold higher increase in transcription than the wild type on Ca2+ stimulation in the presence of CsA or expression of constitutively active CaMK II (Figures 4B and 6A). We also found that the Ca2+-dependent phosphorylation of Ets1 reduced the DNA binding of Ets1 to the GM-CSF promoter in vivo to a level that resembles the decrease in GM-CSF transcription in vivo (compare Figures 4B, 6A, and 8).
Transfection analysis of Ets1 with deletion of the C-terminal part, containing the DNA binding Ets domain, showed that the Ets domain or a sequence C-terminal to it is needed for the inhibition by Ca2+ activation in the presence of calcineurin inhibitor (Figure 7). However, the Ets domain is not only interacting with DNA but is also one of the domains participating in the interaction enabling the synergy with Runx1 (Kim et al., 1999), which is important for GM-CSF expression (Figures 5 and 6B). Perhaps the Ets domain also participates in another relevant protein–protein interaction(s), because Ets1 binds DNA cooperatively with several binding partners, including NF-κB and the AP-1 proteins Jun/Fos (references in Cowley and Graves, 2000). Thus, either the DNA binding of Ets1 or a protein interaction site in the Ets domain or on its C-terminal side is needed for the dominant interference.
We conclude that interaction of another protein(s) with Ets1 and/or with the promoter in vivo decreases the negative effect of the Ets1 phosphorylations, or alternatively, the Ca2+/CaMK II–activating treatments in vivo caused a quantitative but not a qualitative change in the phosphorylation of these sites. The latter alternative is supported by the finding that the mutations also increased the Ets1 activity, although to a lower extent, in the absence of Ca2+/CaMK II–activating treatment. This may indicate that another kinase can mediate a partial phosphorylation in the absence of Ca2+ signaling. Yet another alternative could be that there is a dynamic balance between Ca2+-dependent CaMK II and autophosphorylation that makes CaMK II independent of Ca2+ (Lukas et al., 1998). The discussed models for regulation of the effect of Ets1 on GM-CSF transcription are schematically illustrated in Figure 9.
Binding of the Runx1 transcription factor has been reported to facilitate Ets1 DNA binding at the Tβ3 and Tβ4 elements in the TCRβ enhancer and at the transcription control region of murine leukemia virus by counteracting autoinhibition of Ets1 (Kim et al., 1999; Goetz et al., 2000). Nevertheless, mutation of CaMK II target serines in Ets1 relieved inhibition of the cooperative transactivation of GM-CSF by Ets1 and Runx1 (Figures 5 and 6B). Presumably, the reason for the apparent difference between our results and those of the previous studies is not the slightly higher number of nucleotides between the Runx1 and Ets1 binding sites at the GM-CSF promoter, because the relief of autoinhibition was unaffected even if the distance between the two sites was expanded so that it became greater than that present in the GM-CSF promoter (Goetz et al., 2000). The main difference between the Runx1 and Ets1 sites of the GM-CSF promoter and the other promoters/enhancers is that the intermediate nucleotides in the GM-CSF promoter constitute a binding site for an AP-1 transcription factor (Figure 9), and together, the AP-1 and Ets binding sites constitute the important CLE0 element of the promoter (Shannon et al., 1997; Thomas et al., 1997). Our results suggest that when Ets1 functions in cooperation with nearby AP-1, then at least a large part of the autoinhibition through phosphorylation of the exon VII domain remains and is not relieved by overexpression of Runx1. It is notable in this context that the interaction of Ets1 with Runx1 is to a large extent through the autoinhibitory exon VII domain (Kim et al., 1999), and it is therefore possible that exon VII phosphorylation has an effect on this interaction.
In summary, we have shown that Ca2+ has a dual role in positively regulating GM-CSF through calcineurin and negatively through Ets1 phosphorylation by CaMK II. The role of this regulatory mechanism for the plasticity in the amplitude- and frequency-modulated immune and inflammatory Ca2+ responses is an important issue for future studies.
ACKNOWLEDGMENTS
This work was supported by a grant from the Swedish Cancer Society.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–03–0149. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–03–0149.
REFERENCES
- Benschop RJ, Brandl E, Chan AC, Cambier JC. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol. 2001;167:4172–4179. doi: 10.4049/jimmunol.167.8.4172. [DOI] [PubMed] [Google Scholar]
- Bories JC, Willerford DM, Grevin D, Davidson L, Camus A, Martin P, Stehelin D, Alt FW. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature. 1995;377:635–638. doi: 10.1038/377635a0. [DOI] [PubMed] [Google Scholar]
- Cockerill PN, Shannon MF, Bert AG, Ryan GR, Vadas MA. The granulocyte-macrophage colony-stimulating factor/interleukin 3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci USA. 1993;90:2466–2470. doi: 10.1073/pnas.90.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley DO, Graves BJ. Phosphorylation represses Ets-1 DNA binding by reinforcing autoinhibition. Genes Dev. 2000;14:366–376. [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Dupont G, Goldbeter A. CaM kinase II as frequency decoder of Ca2+ oscillations. Bioessays. 1998;20:607–610. doi: 10.1002/(SICI)1521-1878(199808)20:8<607::AID-BIES2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Estrov Z, Talpaz M, Ku S, Harris D, LaPushin R, Koller CA, Hirsh-Ginsberg C, Huh Y, Yee G, Kurzrock R. Molecular and biologic characterization of a newly established Philadelphia-positive acute lymphoblastic leukemia cell line (Z-33) with an autocrine response to GM-CSF. Leukemia. 1996a;10:1534–1543. [PubMed] [Google Scholar]
- Estrov Z, Talpaz M, Ku S, Harris D, Van Q, Beran M, Hirsch-Ginsberg C, Huh Y, Yee G, Kurzrock R. Z-138: a new mature B-cell acute lymphoblastic leukemia cell line from a patient with transformed chronic lymphocytic leukemia. Leuk Res. 1998;22:341–353. doi: 10.1016/s0145-2126(97)00191-4. [DOI] [PubMed] [Google Scholar]
- Estrov Z, Talpaz M, Zipf TF, Kantarjian HM, Ku S, Ouspenskaia MV, Hirsch-Ginsberg C, Huh Y, Yee G, Kurzrock R. Role of granulocyte-macrophage colony-stimulating factor in Philadelphia (Ph1)-positive acute lymphoblastic leukemia: studies on two newly established Ph1-positive acute lymphoblastic leukemia cell lines (Z-119 and Z-181) J Cell Physiol. 1996b;166:618–630. doi: 10.1002/(SICI)1097-4652(199603)166:3<618::AID-JCP17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- Fisher CL, Ghysdael J, Cambier JC. Ligation of membrane Ig leads to calcium-mediated phosphorylation of the proto-oncogene product, Ets-1. J Immunol. 1991;146:1743–1749. [PubMed] [Google Scholar]
- Fisher RJ, Fivash M, Casas-Finet J, Erickson JW, Kondoh A, Bladen SV, Fisher C, Watson DK, Papas T. Real-time DNA binding measurements of the ETS1 recombinant oncoproteins reveal significant kinetic differences between the p42 and p51 isoforms. Protein Sci. 1994;3:257–266. doi: 10.1002/pro.5560030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Lancki DW, Stack R, Fitch FW. “Anergy” of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994a;179:481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Qian D, Fields P, Fitch FW. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci USA. 1994b;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Schell SR, Fitch FW. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990;144:4110–4120. [PubMed] [Google Scholar]
- Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- Goetz TL, Gu TL, Speck NA, Graves BJ. Auto-inhibition of Ets-1 is counteracted by DNA binding cooperativity with core-binding factor alpha2. Mol Cell Biol. 2000;20:81–90. doi: 10.1128/mcb.20.1.81-90.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- Hughes K, Antonsson Å, Grundström T. Calmodulin dependence of NFkappaB activation. FEBS Lett. 1998;441:132–136. doi: 10.1016/s0014-5793(98)01537-3. [DOI] [PubMed] [Google Scholar]
- Jenkins F, Cockerill PN, Bohmann D, Shannon MF. Multiple signals are required for function of the human granulocyte-macrophage colony-stimulating factor gene promoter in T cells. J Immunol. 1995;155:1240–1251. [PubMed] [Google Scholar]
- Kim WY, Sieweke M, Ogawa E, Wee HJ, Englmeier U, Graf T, Ito Y. Mutual activation of Ets-1 and AML1 DNA binding by direct interaction of their autoinhibitory domains. EMBO J. 1999;18:1609–1620. doi: 10.1093/emboj/18.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Fisher RJ, Fujiwara S, Jorcyk C, Bhat NK, Seth A, Papas TS. Isoforms of the human ets-1 protein: generation by alternative splicing and differential phosphorylation. Oncogene. 1990;5:675–681. [PubMed] [Google Scholar]
- Lars N, Paschalis S. The human I alpha 1 and I alpha 2 germline promoter elements: proximal positive and distal negative elements may regulate the tissue specific expression of C alpha 1 and C alpha 2 germline transcripts. Int Immunol. 1993;5:271–282. doi: 10.1093/intimm/5.3.271. [DOI] [PubMed] [Google Scholar]
- Lelievre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- Liles WC. Immunomodulatory approaches to augment phagocyte-mediated host defense for treatment of infectious diseases. Semin Respir Infect. 2001;16:11–17. doi: 10.1053/srin.2001.22724. [DOI] [PubMed] [Google Scholar]
- Lukas TJ, Mirzoeva S, Watterson DM. Calmodulin-regulated protein kinases. In: Van Eldik L, Watterson DM, editors. Calmodulin and Signal Transduction. New York: Academic Press; 1998. pp. 65–168. [Google Scholar]
- Lutterbach B, Hiebert SW. Role of the transcription factor AML-1 in acute leukemia and hematopoietic differentiation. Gene. 2000;245:223–235. doi: 10.1016/s0378-1119(00)00014-7. [DOI] [PubMed] [Google Scholar]
- McKinlay LH, Tymms MJ, Thomas RS, Seth A, Hasthorpe S, Hertzog PJ, Kola I. The role of Ets-1 in mast cell granulocyte-macrophage colony-stimulating factor expression and activation. J Immunol. 1998;161:4098–4105. [PubMed] [Google Scholar]
- Mimeault M. Structure-function studies of ETS transcription factors. Crit Rev Oncog. 2000;11:227–253. [PubMed] [Google Scholar]
- Muthusamy N, Barton K, Leiden JM. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature. 1995;377:639–642. doi: 10.1038/377639a0. [DOI] [PubMed] [Google Scholar]
- Nghiem P, Saati SM, Martens CL, Gardner P, Schulman H. Cloning and analysis of two new isoforms of multifunctional Ca2+/calmodulin-dependent protein kinase: expression in multiple human tissues. J Biol Chem. 1993;268:5471–5479. [PubMed] [Google Scholar]
- Nilsson L, Grant P, Larsson I, Pettersson S, Paschalis S. The human Iα1 region contains a TGF-β1 responsive enhancer and a putative recombination hotspot. Int Immunol. 1995;7:1191–1204. doi: 10.1093/intimm/7.8.1191. [DOI] [PubMed] [Google Scholar]
- Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- Pistoia V, Corcione A. Relationships between B cell cytokine production in secondary lymphoid follicles and apoptosis of germinal center B lymphocytes. Stem Cells. 1995;13:487–500. doi: 10.1002/stem.5530130506. [DOI] [PubMed] [Google Scholar]
- Pognonec P, Boulukos KE, Bosselut R, Boyer C, Schmitt-Verhulst AM, Ghysdael J. Identification of a Ets1 variant protein unaffected in its chromatin and in vitro DNA binding capacities by T cell antigen receptor triggering and intracellular calcium rises. Oncogene. 1990;5:603–610. [PubMed] [Google Scholar]
- Rabault B, Ghysdael J. Calcium-induced phosphorylation of ETS1 inhibits its specific DNA binding activity. J Biol Chem. 1994;269:28143–28151. [PubMed] [Google Scholar]
- Shang C, Attema J, Cakouros D, Cockerill PN, Shannon MF. Nuclear factor of activated T cells contributes to the function of the CD28 response region of the granulocyte macrophage-colony stimulating factor promoter. Int Immunol. 1999;11:1945–1956. doi: 10.1093/intimm/11.12.1945. [DOI] [PubMed] [Google Scholar]
- Shannon MF, Coles LS, Vadas MA, Cockerill PN. Signals for activation of the GM-CSF promoter and enhancer in T cells. Crit Rev Immunol. 1997;17:301–323. doi: 10.1615/critrevimmunol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Steinberg TH, Allen PM. Selective activation of the calcium signaling pathway by altered peptide ligands. J Exp Med. 1996;184:1525–1530. doi: 10.1084/jem.184.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RS, Tymms MJ, McKinlay LH, Shannon MF, Seth A, Kola I. ETS1, NFkappaB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene. 1997;14:2845–2855. doi: 10.1038/sj.onc.1201125. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Tymms MJ, Seth A, Shannon MF, Kola I. ETS1 transactivates the human GM-CSF promoter in Jurkat T cells stimulated with PMA and ionomycin. Oncogene. 1995;11:2135–2143. [PubMed] [Google Scholar]
- Tracey WD, Speck NA. Potential roles for RUNX1 and its orthologs in determining hematopoietic cell fate. Semin Cell Dev Biol. 2000;11:337–342. doi: 10.1006/scdb.2000.0186. [DOI] [PubMed] [Google Scholar]
- Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. Alpha-hemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature. 2000;405:694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- Wasylyk C, Kerckaert JP, Wasylyk B. A novel modulator domain of Ets transcription factors. Genes Dev. 1992;6:965–974. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- Xie XQ, Pardali E, Holm M, Sideras P, Grundström T. AML and Ets proteins regulate the I alpha1 germ-line promoter. Eur J Immunol. 1999;29:488–498. doi: 10.1002/(SICI)1521-4141(199902)29:02<488::AID-IMMU488>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]