Abstract
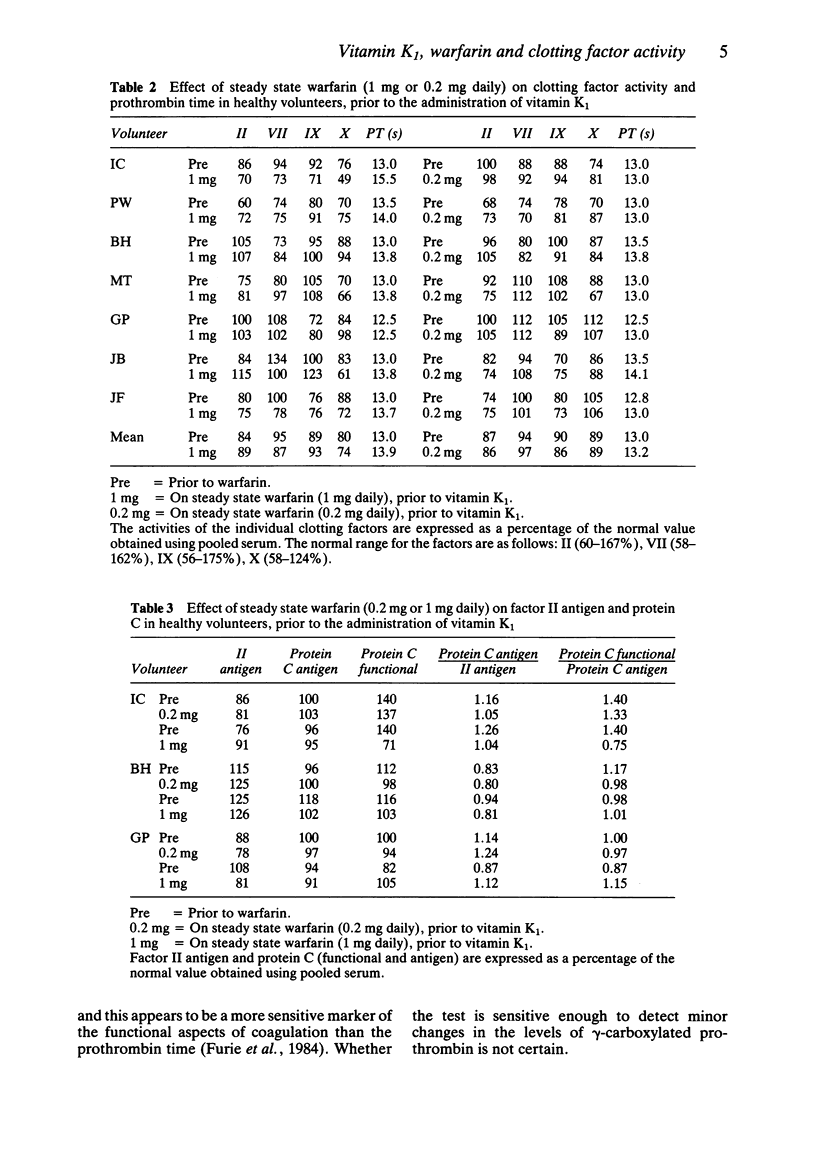
1 The effect of low dose steady state warfarin (0.2 mg and 1 mg daily) on clotting factor activity and vitamin K1 metabolism was studied in seven healthy volunteers. 2 Steady state plasma warfarin concentrations were 41-99 ng ml-1 for the 0.2 mg dose and 157-292 ng ml-1 for the 1 mg dose. 3 There was a significant prolongation of the mean prothrombin time (0.9 s) after 1 mg warfarin daily, but no significant change in prothrombin time after 0.2 mg warfarin daily. There was no significant change in individual clotting factor activity (II, VII, IX or X) with either dose of warfarin. 4 Following the administration of a pharmacological dose of vitamin K1 (10 mg), all seven volunteers had detectable levels of vitamin K1 2,3-epoxide with both doses of warfarin (Cpmax 31-409 ng ml-1). 5 Both the Cpmax and the AUC for vitamin K1 2,3-epoxide were significantly greater on 1 mg of warfarin daily than 0.2 mg daily (P less than 0.01). 6 The apparent dissociation between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity, produced by warfarin, may reflect the insensitivity of functional clotting factor assays to a small reduction in clotting factor concentration.
Full text
PDF

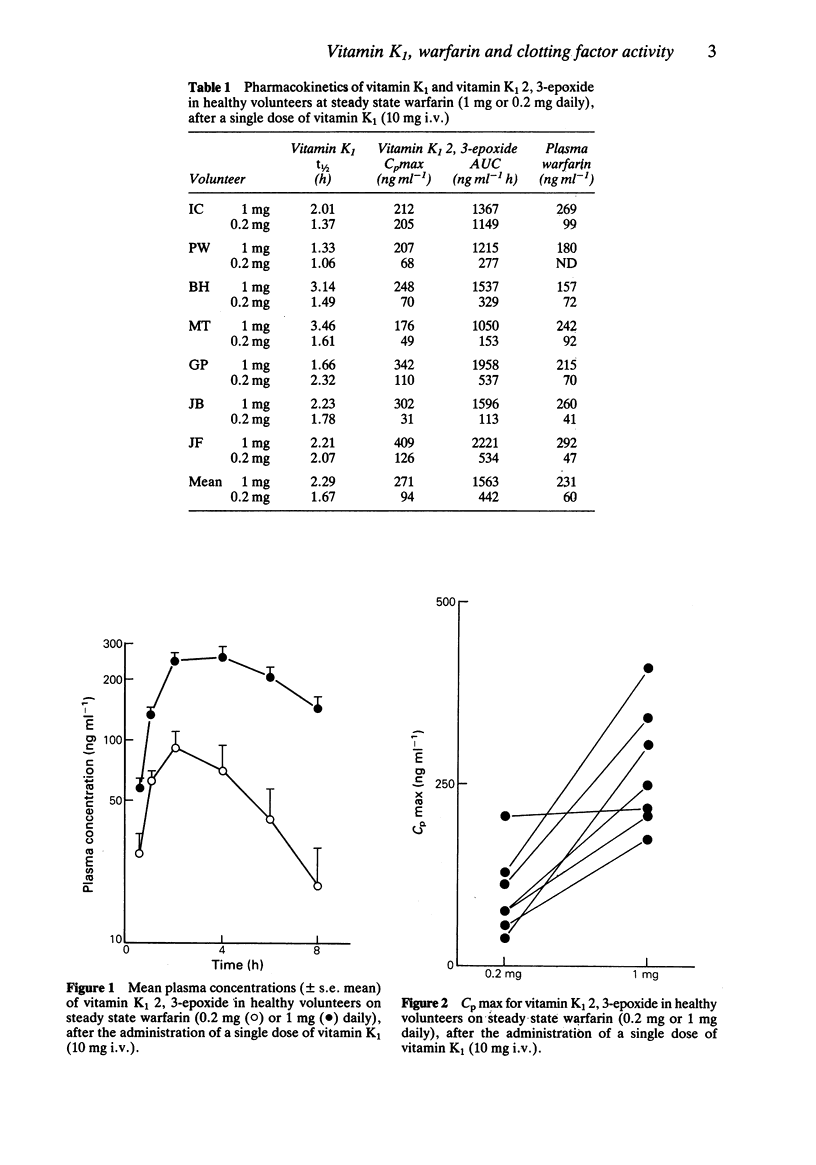
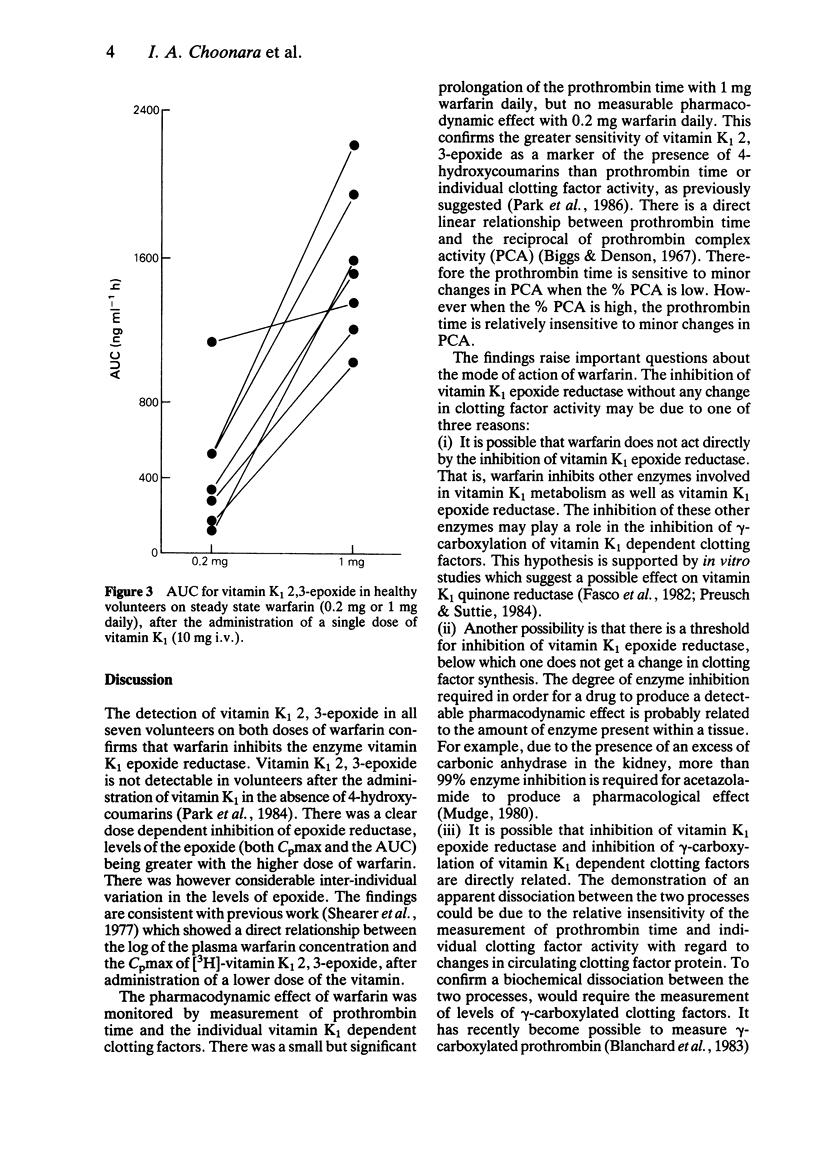


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. G., Matschiner J. T. Warfarin and the inhibition of vitamin K activity by an oxide metabolite. Nature. 1972 May 5;237(5349):32–33. doi: 10.1038/237032a0. [DOI] [PubMed] [Google Scholar]
- Bell R. G. Metabolism of vitamin K and prothrombin synthesis: anticoagulants and the vitamin K--epoxide cycle. Fed Proc. 1978 Oct;37(12):2599–2604. [PubMed] [Google Scholar]
- Bern M. M., Suzuki K., Mann K., Tracy P., Hoyer L., Jensen W., Gallivan M., Arkin C., Davis G. Response of protein C and protein C inhibitor to warfarin therapy in patient with combined deficiency of Factors V and VIII. Thromb Res. 1984 Dec 15;36(6):485–495. doi: 10.1016/0049-3848(84)90188-9. [DOI] [PubMed] [Google Scholar]
- Biggs R., Denson K. W. Standardization of the one-stage prothrombin time for the control of anticoagulant therapy. Br Med J. 1967 Jan 14;1(5532):84–88. doi: 10.1136/bmj.1.5532.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R. A., Furie B. C., Kruger S. F., Waneck G., Jorgensen M. J., Furie B. Immunoassays of human prothrombin species which correlate with functional coagulant activities. J Lab Clin Med. 1983 Feb;101(2):242–255. [PubMed] [Google Scholar]
- Breckenridge A. M., Cholerton S., Hart J. A., Park B. K., Scott A. K. A study of the relationship between the pharmacokinetics and the pharmacodynamics of the 4-hydroxycoumarin anticoagulants warfarin, difenacoum and brodifacoum in the rabbit. Br J Pharmacol. 1985 Jan;84(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- Choonara I. A., Scott A. K., Haynes B. P., Cholerton S., Breckenridge A. M., Park B. K. Vitamin K1 metabolism in relation to pharmacodynamic response in anticoagulated patients. Br J Clin Pharmacol. 1985 Dec;20(6):643–648. doi: 10.1111/j.1365-2125.1985.tb05123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein D. J., Bergum P. W., Bajaj S. P., Rapaport S. I. Radioimmunoassays for protein C and factor X. Plasma antigen levels in abnormal hemostatic states. Am J Clin Pathol. 1984 Nov;82(5):573–581. doi: 10.1093/ajcp/82.5.573. [DOI] [PubMed] [Google Scholar]
- Fasco M. J., Hildebrandt E. F., Suttie J. W. Evidence that warfarin anticoagulant action involves two distinct reductase activities. J Biol Chem. 1982 Oct 10;257(19):11210–11212. [PubMed] [Google Scholar]
- Furie B., Liebman H. A., Blanchard R. A., Coleman M. S., Kruger S. F., Furie B. C. Comparison of the native prothrombin antigen and the prothrombin time for monitoring oral anticoagulant therapy. Blood. 1984 Aug;64(2):445–451. [PubMed] [Google Scholar]
- Griffin J. H., Evatt B., Zimmerman T. S., Kleiss A. J., Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981 Nov;68(5):1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Malia R. G., Preston F. E., Mitchell V. E. Evidence against vitamin K deficiency in normal neonates. Thromb Haemost. 1980 Dec 19;44(3):159–160. [PubMed] [Google Scholar]
- Mikami S., Tuddenham E. G. Studies on immunological assay of vitamin K dependent factors. II. Comparison of four immunoassay methods with functional activity of protein C in human plasma. Br J Haematol. 1986 Jan;62(1):183–193. doi: 10.1111/j.1365-2141.1986.tb02915.x. [DOI] [PubMed] [Google Scholar]
- Nielsen-Kudsk F. A pharmacokinetic program for TI-59. J Pharmacol Methods. 1980 Jun;3(4):345–355. doi: 10.1016/0160-5402(80)90076-5. [DOI] [PubMed] [Google Scholar]
- Park B. K., Choonara I. A., Haynes B. P., Breckenridge A. M., Malia R. G., Preston F. E. Abnormal vitamin K metabolism in the presence of normal clotting factor activity in factory workers exposed to 4-hydroxycoumarins. Br J Clin Pharmacol. 1986 Mar;21(3):289–293. doi: 10.1111/j.1365-2125.1986.tb05192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. K., Leck J. B., Wilson A. C., Serlin M. J., Breckenridge A. M. A study of the effect of anticoagulants on [3H]vitamin K1 metabolism and prothrombin complex activity in the rabbit. Biochem Pharmacol. 1979 Apr 15;28(8):1323–1329. doi: 10.1016/0006-2952(79)90433-7. [DOI] [PubMed] [Google Scholar]
- Park B. K., Scott A. K., Wilson A. C., Haynes B. P., Breckenridge A. M. Plasma disposition of vitamin K1 in relation to anticoagulant poisoning. Br J Clin Pharmacol. 1984 Nov;18(5):655–662. doi: 10.1111/j.1365-2125.1984.tb02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusch P. C., Suttie J. W. Relationship of dithiothreitol-dependent microsomal vitamin K quinone and vitamin K epoxide reductases inhibition of epoxide reduction by vitamin K quinone. Biochim Biophys Acta. 1984 Mar 22;798(1):141–143. doi: 10.1016/0304-4165(84)90022-9. [DOI] [PubMed] [Google Scholar]
- Shearer M. J., McBurney A., Breckenridge A. M., Barkhan P. Effect of warfarin on the metabolism of phylloquinone (vitamin K1):dose-response relationships in man. Clin Sci Mol Med. 1977 Jun;52(6):621–630. doi: 10.1042/cs0520621. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Suttie J. W. Vitamin K-dependent formation of gamma-carboxyglutamic acid. Annu Rev Biochem. 1977;46:157–172. doi: 10.1146/annurev.bi.46.070177.001105. [DOI] [PubMed] [Google Scholar]
- Viganò S., Mannucci P. M., Solinas S., Bottasso B., Mariani G. Decrease in protein C antigen and formation of an abnormal protein soon after starting oral anticoagulant therapy. Br J Haematol. 1984 Jun;57(2):213–220. [PubMed] [Google Scholar]
- Willingham A. K., Matschiner J. T. Changes in phylloquinone epoxidase activity related to prothrombin synthesis and microsomal clotting activity in the rat. Biochem J. 1974 Jun;140(3):435–441. doi: 10.1042/bj1400435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Park B. K. Quantitative analysis of pharmacological levels of vitamin K1 and vitamin K1 2,3-epoxide in rabbit plasma by high-performance liquid chromatography. J Chromatogr. 1983 Oct 14;277:292–299. doi: 10.1016/s0378-4347(00)84847-1. [DOI] [PubMed] [Google Scholar]


