Abstract
1 Fosinopril sodium is the first phosphorus-containing angiotensin-converting enzyme (ACE) inhibitor to be studied clinically as an antihypertensive agent. It is an ester prodrug that is hydrolysed in vivo to the active diacid ACE inhibitor, SQ 27, 519. 2 In a three-way crossover study, nine healthy male subjects (age range 20-34 years) each received an intravenous 7.5 mg dose of SQ 27, 519-[14C] and two oral 10 mg doses of [14C]-fosinopril sodium, administered as a capsule and in solution. 3 After the intravenous dose of SQ 27, 519, the 0 to 96 h recovery of radioactivity averaged 44 and 46% of the dose in urine and faeces, respectively, indicating substantial biliary secretion. Only intact SQ 27, 519 was detected in the plasma, urine, and faeces following the intravenous dose of SQ 27, 519. 4 After oral doses of fosinopril sodium, about 75% of the radioactivity in plasma and urine was present as SQ 27, 519; the remainder corresponded mainly to a beta-glucuronide conjugate of SQ 27, 519 (15-20%), and a monohydroxylated analogue of SQ 27, 519 (about 5%). Negligible amounts of fosinopril sodium were present, indicating complete hydrolysis of the prodrug. 5 For the solution and capsule doses, respectively, the oral absorption of fosinopril sodium averaged 32% and 36% and the oral bioavailability of SQ 27, 519 averaged 25% and 29%. 6 The average values for clearance (39 ml min-1), renal clearance (17 ml min-1), Vss (10 1), and plasma protein binding (approximately 95%), indicated that SQ 27, 519 was slowly cleared from the body and not distributed extensively into extravascular sites.
Full text
PDF
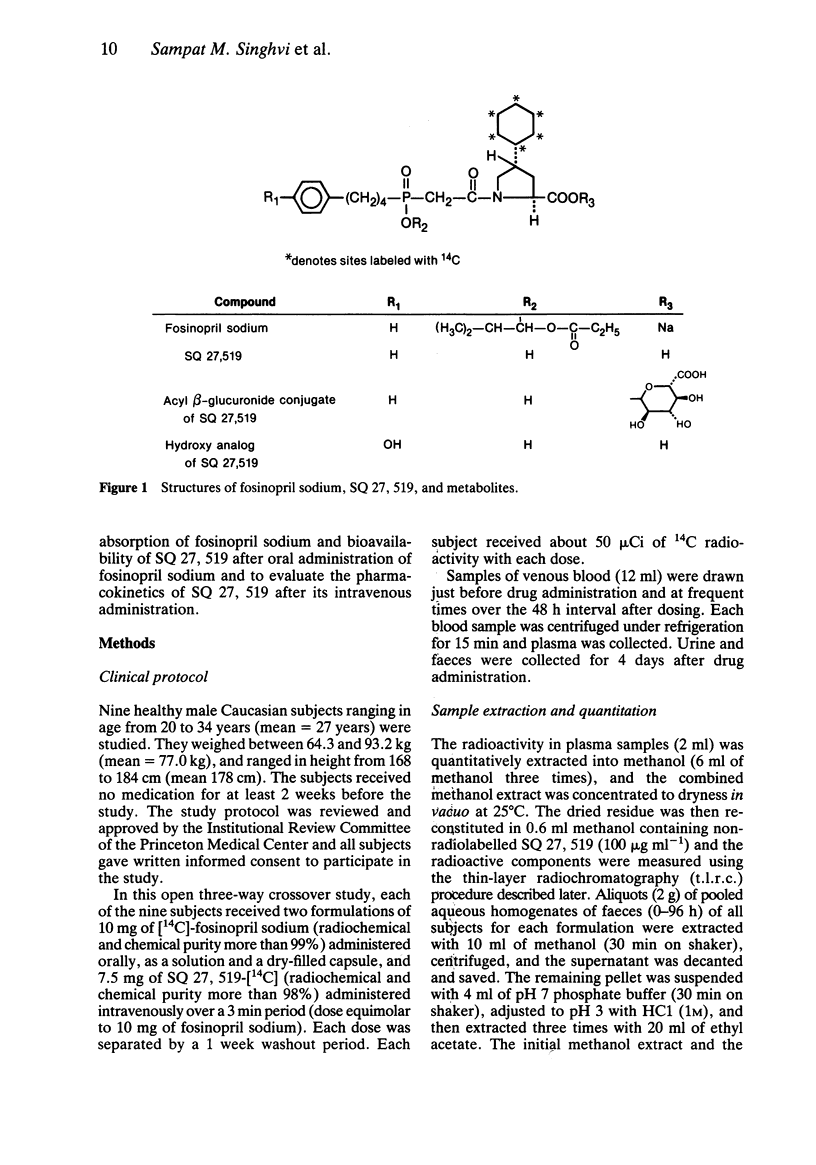



Selected References
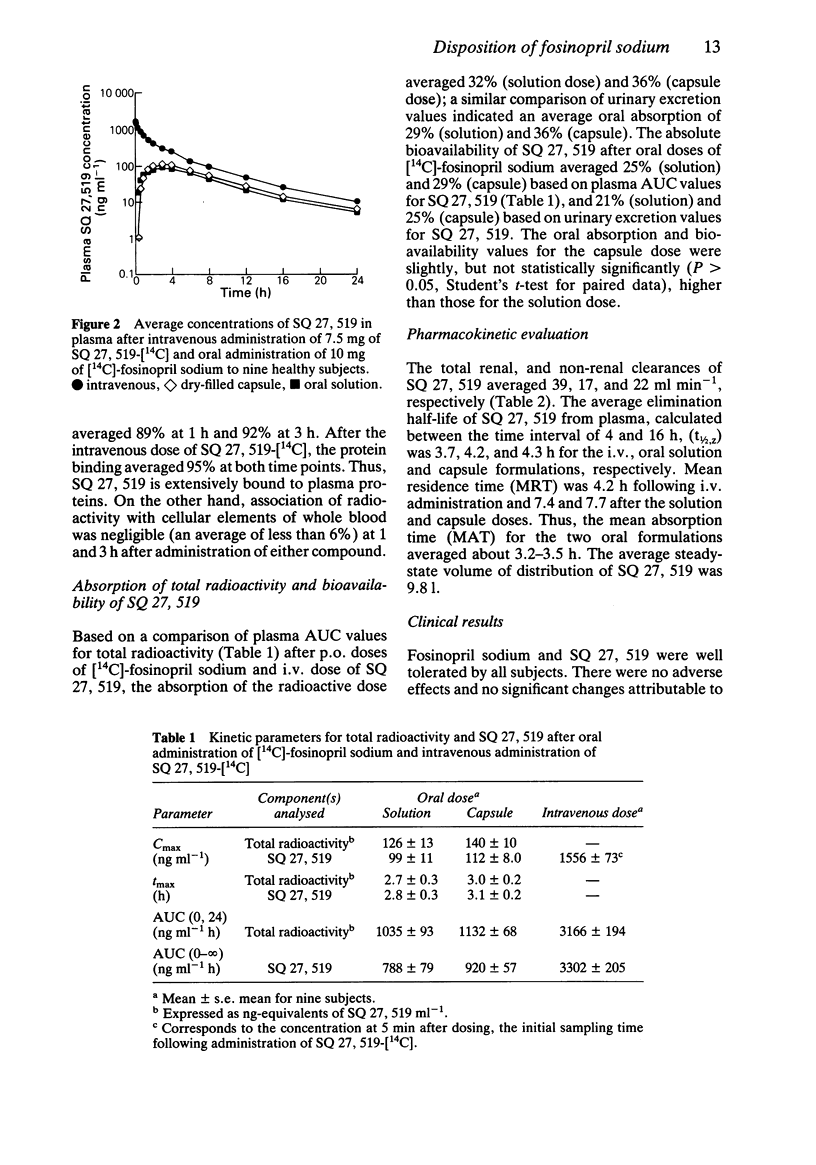
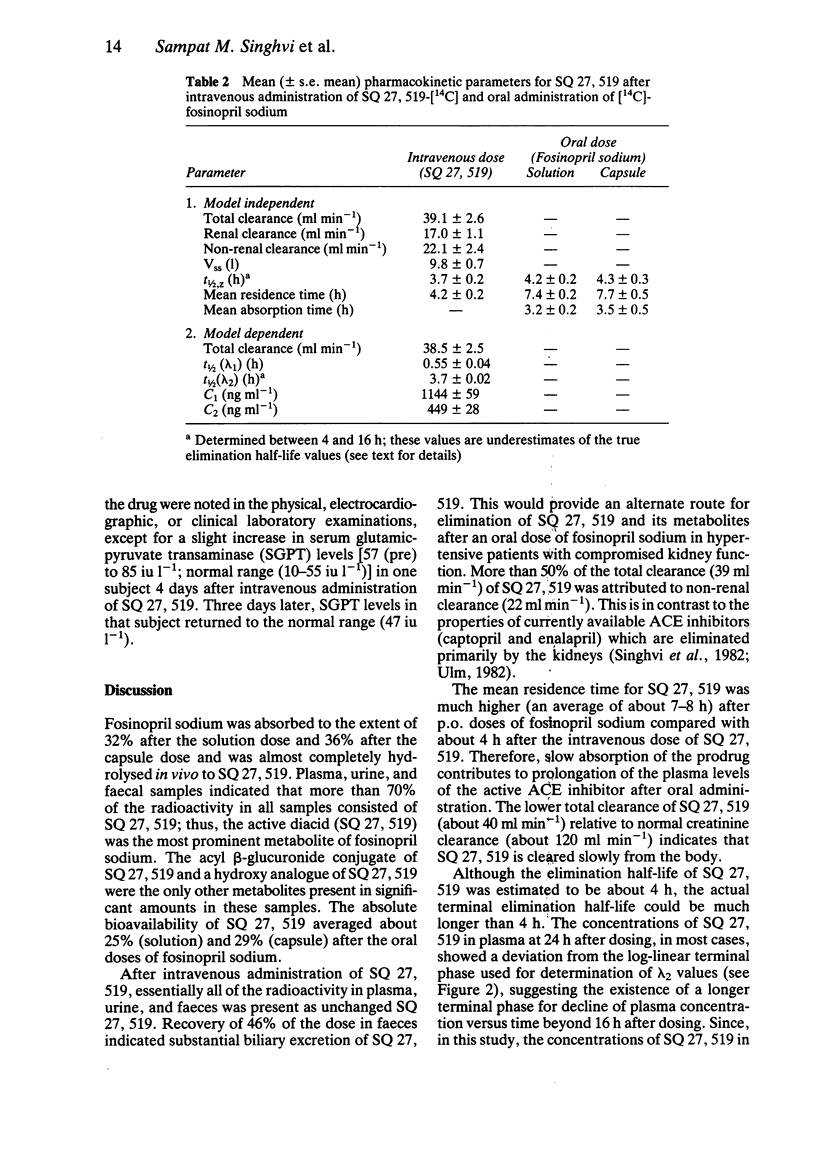
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Faed E. M. Properties of acyl glucuronides: implications for studies of the pharmacokinetics and metabolism of acidic drugs. Drug Metab Rev. 1984;15(5-6):1213–1249. doi: 10.3109/03602538409033562. [DOI] [PubMed] [Google Scholar]
- Singhvi S. M., Heald A. F., Gadebusch H. H., Resnick M. E., Difazio L. T., Leitz M. A. Human serum protein binding of cephalosporin antibiotics in vitro. J Lab Clin Med. 1977 Feb;89(2):414–420. [PubMed] [Google Scholar]
- Sinhvi S. M., Duchin K. L., Willard D. A., McKinstry D. N., Migdalof B. H. Renal handling of captopril: effect of probenecid. Clin Pharmacol Ther. 1982 Aug;32(2):182–189. doi: 10.1038/clpt.1982.145. [DOI] [PubMed] [Google Scholar]
- Yeh K. C., Kwan K. C. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978 Feb;6(1):79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]


