Abstract
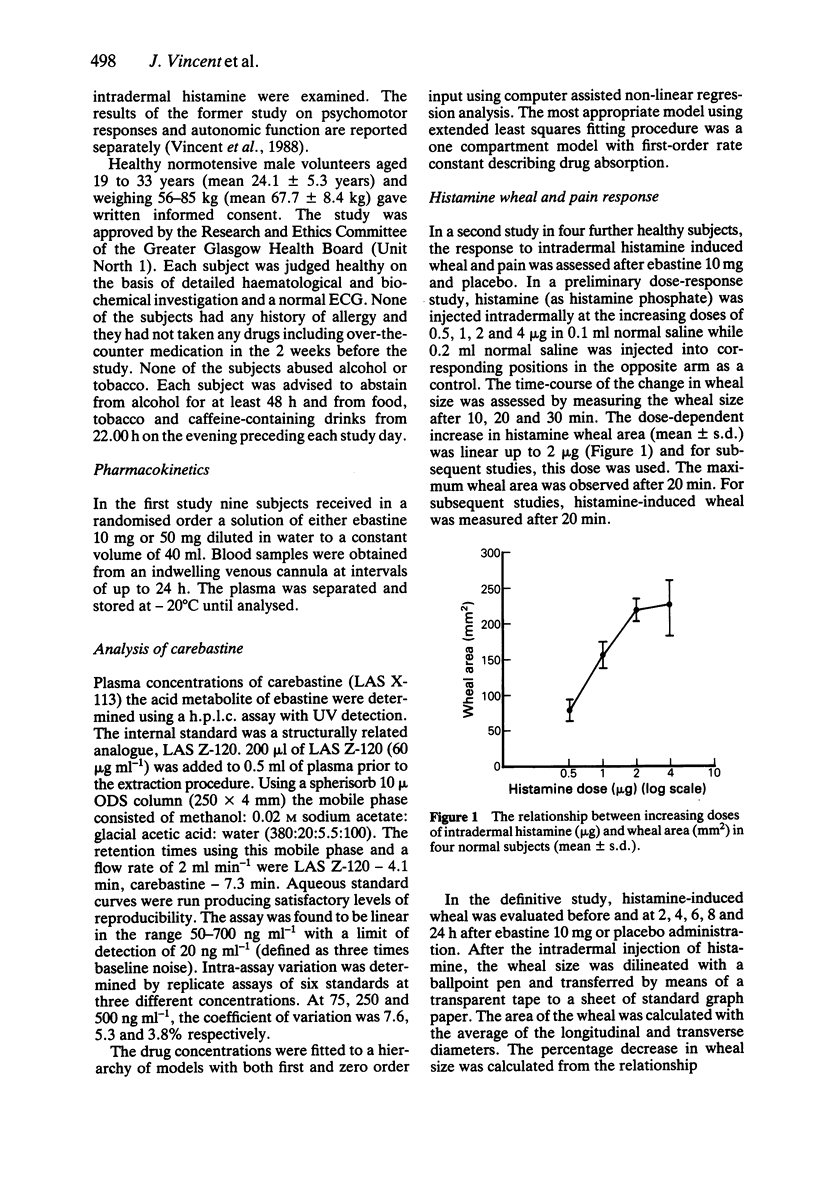
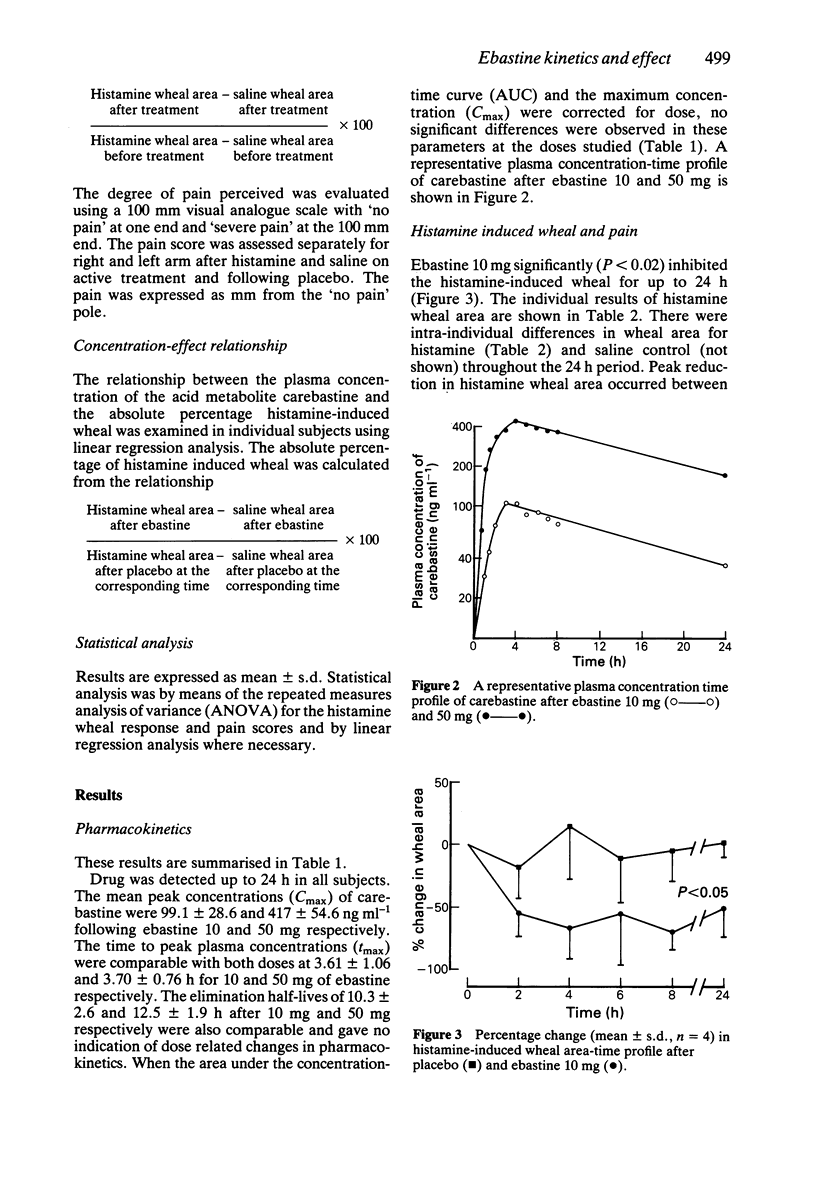
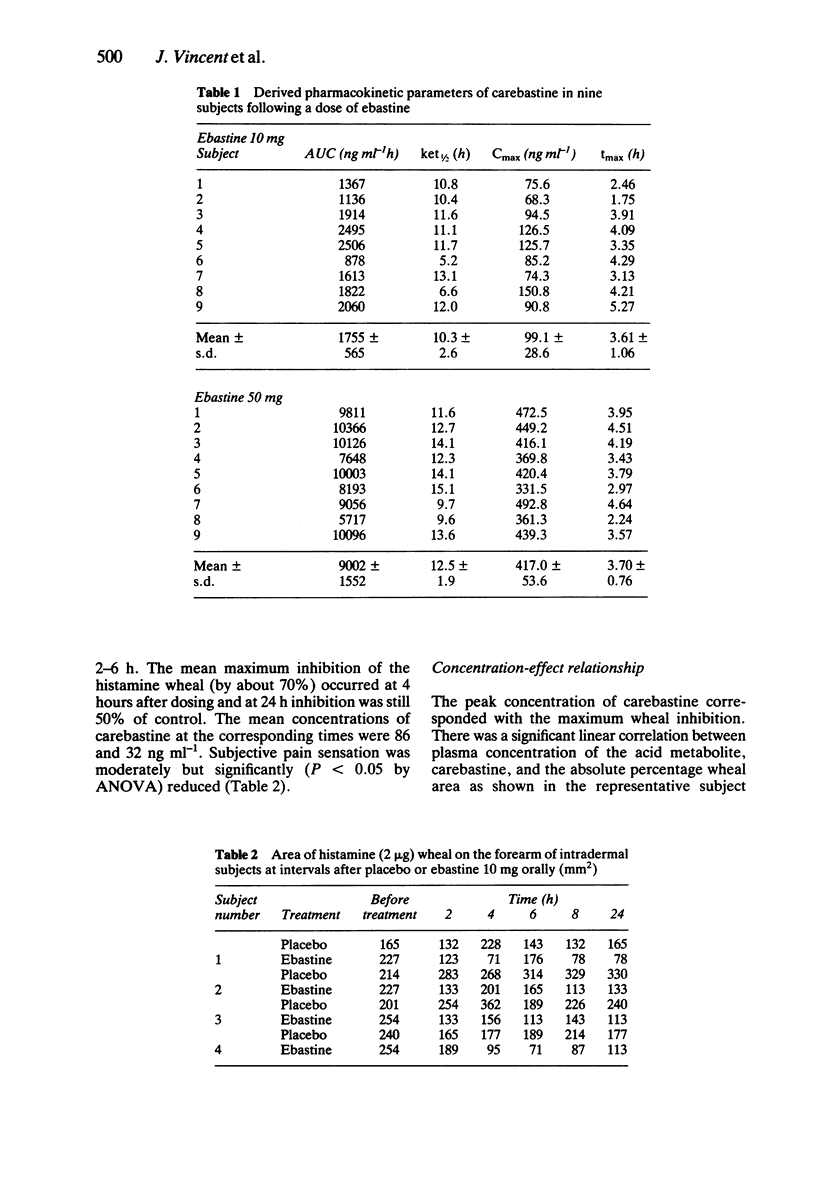
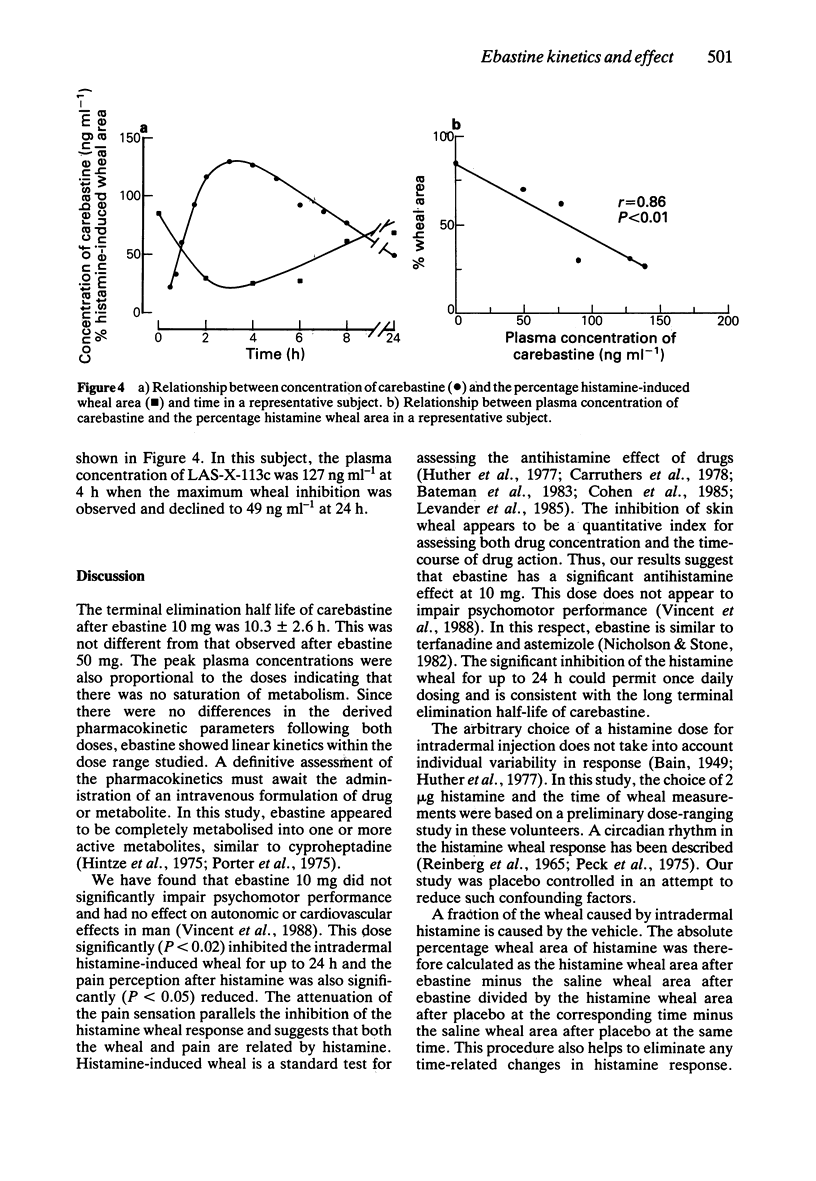
1. The kinetics and effects of ebastine 10 and 50 mg were studied after oral dosing in healthy subjects. 2. The parent drug was extensively metabolised during the first pass to its carboxylic acid derivative, carebastine. 3. The pharmacokinetics of carebastine were linear over the dose range studied and the terminal elimination half-life was 10.6 +/- 2.6 and 12.5 +/- 1.9 h respectively after 10 and 50 mg of ebastine. 4. Antihistamine (H1-receptor) activity was examined with intradermal histamine (2 micrograms). Oral ebastine reduced the histamine wheal area for up to 24 h and also reduced subjective local pain. 5. Antihistamine activity correlated well with plasma levels of carebastine in individual subjects. 6. Ebastine appears to have potential as an antihistamine for once a day dosing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIN W. A. The quantitative comparison of histamine antagonists in man. Proc R Soc Med. 1949 Aug;42(8):615–623. [PMC free article] [PubMed] [Google Scholar]
- Bateman D. N., Chapman P. H., Rawlins M. D. The effects of astemizole on histamine-induced weal and flare. Eur J Clin Pharmacol. 1983;25(4):547–551. doi: 10.1007/BF00542126. [DOI] [PubMed] [Google Scholar]
- Bilzer W., Gundert-Remy U., Weber E. Relationship between antihistamic activity and plasma level of diphenhydramine. Eur J Clin Pharmacol. 1974 Aug 23;7(5):393–395. doi: 10.1007/BF00558213. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., Boobis A. R., Bulpitt C. J., Davies D. S. Influence of liver disease and environmental factors on hepatic monooxygenase activity in vitro. Eur J Clin Pharmacol. 1981;20(1):39–46. doi: 10.1007/BF00554665. [DOI] [PubMed] [Google Scholar]
- Carruthers S. G., Shoeman D. W., Hignite C. E., Azarnoff D. L. Correlation between plasma diphenhydramine level and sedative and antihistamine effects. Clin Pharmacol Ther. 1978 Apr;23(4):375–382. doi: 10.1002/cpt1978234375. [DOI] [PubMed] [Google Scholar]
- Cohen A. F., Hamilton M. J., Liao S. H., Findlay J. W., Peck A. W. Pharmacodynamic and pharmacokinetics of BW 825C: a new antihistamine. Eur J Clin Pharmacol. 1985;28(2):197–204. doi: 10.1007/BF00609692. [DOI] [PubMed] [Google Scholar]
- Hintze K. L., Wold J. S., Fischer L. J. Disposition of cyproheptadine in rats, mice, and humans and identification of a stable epoxide metabolite. Drug Metab Dispos. 1975 Jan-Feb;3(1):1–9. [PubMed] [Google Scholar]
- Hüther K. J., Renftle G., Barraud N., Burke J. T., Koch-Weser J. Inhibitory activity of terfenadine on histamine-induced skin wheals in man. Eur J Clin Pharmacol. 1977 Nov 14;12(3):195–199. doi: 10.1007/BF00609860. [DOI] [PubMed] [Google Scholar]
- Levander S., Hägermark O., Ståhle M. Peripheral antihistamine and central sedative effects of three H1-receptor antagonists. Eur J Clin Pharmacol. 1985;28(5):523–529. doi: 10.1007/BF00544062. [DOI] [PubMed] [Google Scholar]
- Nicholson A. N., Stone B. M. Performance studies with the H1-histamine receptor antagonists, astemizole and terfenadine. Br J Clin Pharmacol. 1982 Feb;13(2):199–202. doi: 10.1111/j.1365-2125.1982.tb01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton D. M., Webster D. R. Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines). Clin Pharmacokinet. 1985 Nov-Dec;10(6):477–497. doi: 10.2165/00003088-198510060-00002. [DOI] [PubMed] [Google Scholar]
- Peck A. W., Fowle A. S., Bye C. A comparison of triprolidine and clemastine on histamine antagonism and performance tests in man: implications for the mechanism of drug induced drowsiness. Eur J Clin Pharmacol. 1975 Aug 14;8(6):455–463. doi: 10.1007/BF00562321. [DOI] [PubMed] [Google Scholar]
- Porter C. C., Arison B. H., Gruber V. F., Titus D. C., Vandenheuvel W. J. Human metabolism of cyproheptadine. Drug Metab Dispos. 1975 May-Jun;3(3):189–197. [PubMed] [Google Scholar]
- REINBERG A., GHATA J., SIDI E. CIRCADIAN REACTIVITY RHYTHMS OF HUMAN SKIN TO HISTAMINE OR ALLERGEN AND THE ADRENAL CYCLE. J Allergy. 1965 May-Jun;36:273–283. doi: 10.1016/0021-8707(65)90086-9. [DOI] [PubMed] [Google Scholar]
- Richards D. M., Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Astemizole. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1984 Jul;28(1):38–61. doi: 10.2165/00003495-198428010-00003. [DOI] [PubMed] [Google Scholar]
- Vincent J., Sumner D. J., Reid J. L. Ebastine: the effect of a new antihistamine on psychomotor performance and autonomic responses in healthy subjects. Br J Clin Pharmacol. 1988 Nov;26(5):503–508. doi: 10.1111/j.1365-2125.1988.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. Sedation and antihistaminics. Arzneimittelforschung. 1982;32(9A):1193–1195. [PubMed] [Google Scholar]


