Abstract
Current therapies for breast cancer include treatments that are toxic and often result in drug resistance. Telomerase, a cellular reverse transcriptase that maintains the ends of chromosomes (telomeres), is activated in the vast majority of breast cancers (over 90% of breast carcinomas) but not in normal adjacent tissues. Telomerase is thus an attractive target for both diagnosis and therapy because of its distinct pattern of expression. We address the use of telomerase in the diagnostics of breast pathology, as well as the use of telomerase inhibitors in the treatment and prevention of breast cancer.
Keywords: breast cancer, inhibitors, telomerase, telomeres, therapy
Overview of telomeres and the use of telomerase to compensate for telomere loss
Human chromosomes contain repeated TTAGGG DNA sequences at their ends (telomeres) that provide genomic stability (protect the ends from being recognized as DNA breaks needing repair) and a source of expendable DNA (a solution to the inability of the replication machinery to copy the extreme ends of chromosomes: the end replication problem) (see [1] for a review). A significant amount of truncation can occur during DNA replication without causing deleterious effects. Human telomeres progressively shorten with ongoing cell division until they reach a critical length that induces replicative senescence [2].
Recent research has led to increased knowledge of the structure and function of telomeres. Telomeres end in G-rich single-stranded 3' overhangs, and can form a lariat structure called a t-loop. Telomere binding proteins such as TRF1 and TRF2 are thought to protect against nucleases and prevent DNA recombination or end joining [1]. Recent research has shown an increasing connection between DNA damage repair proteins (eg Mre11/Rad50/Nbs1, Ku, etc) and telomere biology in mammals. Double-strand break recognition and repair factors associated with the BRCA1-associated genome surveillance complex have, for instance, been linked to telomeres and telomere binding proteins [3]. BRCA1 or BRCA2 might also play a role in telomere structure and function.
Although other mechanisms to maintain telomere stability are possible, the mechanism for lengthening telomeres in humans is almost always by the reactivation or upregulation of telomerase [4]. Human telomerase is a protein complex consisting of a human telomerase reverse transcriptase catalytic subunit (hTERT) that uses the human telomerase RNA component (hTR) of the complex as a template for adding TTAGGG repeats to the end of the chromosome [5,6,7]. Telomerase is only expressed in a small number of proliferating cell types, such as germ line and somatic stem cells. Most normal human cells lack telomerase activity and their telomeres shorten with each cell division, until they enter replicative senescence. Cells that lose critical cell-cycle checkpoint functions escape this initial growth arrest and divide until they enter crisis when chromosome end fusions and apoptosis occur. Cells remain in this crisis period unless a rare cell acquires a mechanism, such as telomerase expression, that can lengthen telomeres. As cells continue to proliferate, the maintenance of telomeres involves a collection of factors including telomere binding and associated proteins, the telomerase ribonucleoprotein complex and, potentially, DNA damage repair proteins. A rare cell that can maintain telomeres is then able to grow continuously (ie becomes immortal) and this is generally believed to be a critical step towards cancer progression [8].
Telomerase as a diagnostic target: incidence of telomerase in breast cancer
The development of the telomeric repeat amplification protocol (TRAP) assay led to an expansion in the ability to detect telomerase activity in human cancer cells [9]. This sensitive polymerase chain reaction (PCR)-based assay can detect as few as 1 to 10 positive cells or 0.01% in a mixed population. Although preliminary data showed 88% of all stages of breast carcinoma having positive TRAP [9], closer investigation and careful handling of initially negative samples revealed the value may be closer to 95% [10]. As reviewed by Shay and Bacchetti, 75% of breast carcinoma in situ lesions, 88% of ductal and lobular carcinomas, 5% of adjacent tissues, and none of the normal tissues were TRAP-positive [9]. Yashima et al detected a progressive increase in the mean telomerase levels with the severity of histopathological change: 14% in benign breast diseases, 92% in carcinoma in situ lesions, and 94% in invasive breast cancers [11]. Expression of the hTERT mRNA can also be detected using real-time quantitative reverse transcriptase-PCR, and this assay revealed a statistical link between hTERT mRNA levels and the aggressiveness of breast tumors [12]. Both this semi-automated assay and the TRAP assay provide suitable methods for breast cancer diagnosis, but should be used in conjunction with other diagnostic tools to rule out false results.
Detection of telomerase activity in preoperative specimens, such as in fine-needle aspirates (FNAs), may improve diagnostic accuracy [13,14]. FNA cytology is known to be accurate, cost effective and have minimal risk [14]; however, difficulties still occasionally occur using cytology alone. Two groups separately compared the diagnostic utility of telomerase assays of FNAs with cytology preparations [13,14]. Poremba et al showed that 92% of FNAs from breast cancer patients were telomerase-positive, 94% of FNAs from patients with benign breast lesions were telomerase-negative (the positive cases were all fibroadenomas), and there was a strong correlation between TRAP and histologic diagnosis of atypia [13]. Hiyama et al observed that all atypical or intermediate cases with detectable telomerase activity in the FNAs were found to be carcinomas after surgery [14]. Furthermore, six out of seven tumors without telomerase activity were diagnosed as benign, while one half of the cases with detectable telomerase activity, initially designated by cytology as benign, were subsequently diagnosed as cancer. Detecting telomerase activity in FNAs is thus equivalent, if not better, than detection by cytology [14], and can be used in conjunction with other diagnostic tests. Finally, tumor-derived telomerase RNA found in the serum of breast cancer patients may have implications in diagnosis and in follow-up monitoring studies [15].
Telomerase activity and prognosis in breast cancer
With the increasing number of breast cancers detected by screening procedures, a marker is needed to stratify the risk of subsequent invasive cancer. Hoos et al found a significant correlation between telomerase activity and tumor size, lymph node status, and stage [16]. A significant association between telomerase-positive infiltrating breast carcinomas and lymphovascular invasion, a fundamental step in breast cancer metastasis and a predictor of survival, has also been observed, making telomerase a useful prognostic marker [17]. Clark et al reported, in a prognostic study involving 398 patients with lymph node-positive breast cancer, that increased telomerase activity was associated with decreased disease-free survival [18]. High telomerase activity in breast cancer is moreover associated with genetic aberrations in 3q (gain), 8q (gain), and 17p (deletion) [19]. These aberrations are common in breast cancers and involve the hTR (on 3q), c-myc (on 8q), and p53 (on 17p) genes, all of which have been associated with telomerase regulation [19]. Understanding the link between telomerase activity and genetic changes associated with breast cancer remain an important area of research today.
Telomerase inhibition as an anticancer approach
The average telomere length in breast cancer cells is usually well below that of normal cells. This difference in telomere length coupled with the more rapid rate of cell division in cancer cells makes the inhibition of telomerase an attractive potential breast cancer therapeutic target. Treatment with telomerase inhibitors may not have the toxicity found with other chemotherapeutic agents since telomerase is absent in most somatic cells (Fig. 1). While normal, proliferating telomerase-positive stem cells may also initially be affected, their telomeres are well above the critically short length that induces a DNA damage/growth arrest mechanism. Furthermore, most stem cells are quiescent, and telomere shortening normally only occurs with cell division. Since most breast cancer cells have very short telomeres, treatment with telomerase inhibitors should lead to growth arrest and cell death.
Figure 1.
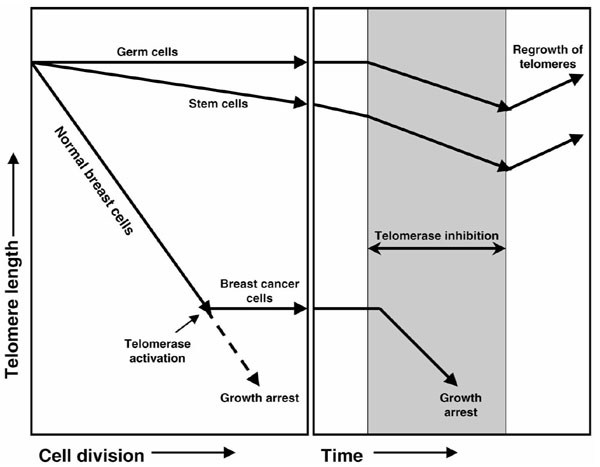
Effects of telomerase inhibitors in breast cancer therapy based on reviews by Krupp et al [1] and White et al [20]. Normal breast tissues do not have telomerase activity and their telomeres progressively shorten with each cell division. When telomeres become short, cells undergo growth arrest. In rare circumstances, telomerase may be activated and a cell can become immortal, leading to accumulations of mutations and cancer. Inhibition of telomerase would lead to progressive shortening of telomeres. While normal, telomerase-competent proliferating cells, such as germ and stem cells, would be affected, their telomeres are well above the critically short length to induce a DNA damage/growth arrest mechanism. Since most breast cancer cells exhibit telomere lengths close to the critically short limit, treatment with telomerase inhibitors would lead to growth arrest and cell death. With the removal of inhibitors, telomerase would be active and telomere lengths might return to their original size.
Advances in development of telomerase inhibitors
The telomerase protein complex allows for multiple sites for inhibition. Recent research includes targeting the RNA component of the telomerase (antisense oligonucleotides, hammerhead ribozymes), inhibition of the catalytic subunit (dominant negative mutant hTERT, reverse transcriptase inhibitors), immunotherapy, and small molecule inhibitors (reviewed in [20]). It is important in most approaches to confirm that telomerase inhibitors are acting specifically in a telomere-dependent mechanism. First, telomerase inhibitors should almost always lack immediate toxicity to the cell; they should reduce telomerase activity without initially compromising cellular proliferation. Second, without telomerase activity, telomeres should progressively shorten with each cell division. Cells should ultimately die or undergo growth arrest and the time required should be related to initial telomere length. Over 100 manuscripts have been published on telomerase inhibitors but only a small number meet the presented criteria [20], and fewer still have been tested in breast cancer cells.
Antisense oligonucleotides, dominant negative mutant hTERT and reverse transcriptase inhibitors have been studied in breast cancer [20,21,22]. The most widely used reverse transcriptase inhibitor is 3'-azido-3'-deoxythymidine (AZT) (reviewed in [20]). Melana et al observed that AZT inhibited the growth of breast cancer cells and telomerase activity at lower doses than in normal breast cells [21]. Multani et al, however, showed that AZT reduced the telomeric signals, as detected by fluorescence in situ hybridization, in human MCF-7 breast carcinoma cells within 72 h [22], a time too short to be explained by the inhibition of telomerase and progressive telomere shortening. The effects of AZT, while effective in inhibiting breast cancer cell growth in these studies, may not be due to a specific telomere-dependent mechanism, and telomerase inhibition may just be a side effect [20]. Whereas further research is needed to prove the specificity of AZT, studies using both antisense oligonucleotides, such as 2'-O-methyl RNA directed against the hTR template region, and dominant negative mutant hTERT have been shown to be effective specific telomerase inhibitors in immortalized breast epithelial and breast carcinoma cells in vitro (reviewed in [20]).
Standard chemopreventive agents have also been investigated for a possible role in telomerase inhibition and control of breast cancer. Retinoic acid and tamoxifen have already been shown to inhibit telomerase activity in breast cancer cells [23,24], probably as a secondary consequence of inhibition of proliferation. While these agents are known for their antiproliferative effects, not much is known on their effects on immortalization, a critical step towards breast cancer progression [8]. We have recently investigated the possibility of using these agents or specific telomerase inhibitors to prevent cellular immortalization as a breast cancer chemoprevention strategy. We found that long-term treatment of precrisis breast epithelial cells with nontoxic doses of either chemopreventive or antitelomerase agents significantly lowered the frequency of spontaneous immortalization [25]. Our studies provide a new model for the screening of novel chemopreventive agents that target cellular immortalization.
Conclusion
Telomerase is an attractive target for diagnosis and therapy since it is expressed in over 90% of breast cancer cells, while it is not expressed in most normal cells. Whereas most of the progress on the role of telomerase in breast cancer has been in diagnostics, research into telomerase inhibitors is increasing. Inhibition of telomerase in vitro leads to progressive telomere shortening, eventually resulting in growth arrest or cell death due to the critically short telomeres inducing a DNA damage response. Breast cancer cells with already short telomeres would be most affected by telomerase inhibitors, whereas normal stem cells with longer telomeres would be relatively resistant. The effects of telomerase inhibitors would depend on initial telomere length and rate of cell division, and it may take weeks to months to see changes in tumor size. Combining telomerase inhibitors with current therapies to reduce tumor burden may thus provide a better regimen to target breast cancer and prevent recurrence.
Abbreviations
AZT = 3'-azido-3'-deoxythymidine; FNA = fine-needle aspirate; hTERT = human telomerase reverse transcriptase catalytic subunit; hTR = human telomerase RNA component; PCR = polymerase chain reaction; TRAP = telomeric repeat amplification protocol.
Acknowledgments
Acknowledgements
Supported in part by the Susan G Komen Breast Cancer Foundation Fellowship (99-3061), DAMD (17-00-1-0438), Division of Cancer Prevention, National Cancer Institute (CN85139 and CN85143), and Geron Corporation (Menlo Park, CA)
References
- Krupp G, Klapper W, Parwaresch R. Cell proliferation, carcinogenesis and diverse mechanisms of telomerase regulation. Cell Mol Life Sci. 2000;57:464–486. doi: 10.1007/PL00000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383–389. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- Zhou BS, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang S, Weinrich SL, Avilion AA, Chiu C, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrews WH, Greider CW, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergern RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Bacchetti S. Telomere maintenance in tumour cells. Cancer Surveys. 1996;28:197–216. [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Carey LA, Hedican CA, Henderson GS, Umbricht CB, Dome JS, Varon D, Sukumar S. Careful histological confirmation and microdissection reveal telomerase activity in otherwise telomerase-negative breast cancers. Clin Cancer Res. 1998;4:435–440. [PubMed] [Google Scholar]
- Yashima K, Milchgrub S, Gollahon LS, Maitra A, Saboorian MH, Shay JW, Gazdar AF. Telomerase enzyme activity and RNA expression during the multistage pathogenesis of breast carcinoma. Clin Cancer Res. 1998;4:229–234. [PubMed] [Google Scholar]
- Bieche I, Nogues C, Paradis V, Olivi R, Bedossa P, Lidereau R, Vidaud M. Quantitation of hTERT gene expression in sporadic breast tumors with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6:452–459. [PubMed] [Google Scholar]
- Poremba C, Shroyer KR, Frost M, Diallo R, Fogt F, Schafer KL, Burger H, Shroyer AL, Dockhorn-Dworniczak B, Boecker W. Telomerase is a highly sensitive and specific molecular marker in fine-needle aspirates of breast lesions. J Clin Oncol. 1999;17:2020–2026. doi: 10.1200/JCO.1999.17.7.2020. [DOI] [PubMed] [Google Scholar]
- Hiyama E, Saeki T, Hiyama K, Takashima S, Shay JW, Matsuura Y, Yokoyama T. Telomerase activity as a marker of breast carcinoma in fine-needle aspirated samples. Cancer Cytopathol. 2000;90:235–238. [PubMed] [Google Scholar]
- Chen X, Bonnefoi H, Pelte M-F, Lyautey J, Lederrey C, Movarekhi S, Schaeffer P, Mulachy HE, Meyer P, Stroun M, Anker P. Telomerase RNA as a detection marker in the serum of breast cancer patients. Clin Cancer Res. 2000;6:3827–3831. [PubMed] [Google Scholar]
- Hoos A, Hepp HH, Kaul S, Ahlert T, Bastert G, Wallweiner D. Telomerase activity correlates with tumor aggressiveness and reflects therapy effect in breast cancer. Int J Cancer. 1998;79:8–12. doi: 10.1002/(SICI)1097-0215(19980220)79:1<8::AID-IJC2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mokbel KM, Parris CN, Ghilchik M, Amerasinghe CN, Newbold RF. Telomerase activity and lymphovascular invasion in breast cancer. Eur J Surg Oncol. 2000;26:30–33. doi: 10.1053/ejso.1999.0736. [DOI] [PubMed] [Google Scholar]
- Clark GM, Osborne CK, Levitt D, Wu F, Kim NW. Telomerase activity and survival of patients with node-positive breast cancer. J Natl Cancer Inst. 1997;89:1874–1881. doi: 10.1093/jnci/89.24.1874. [DOI] [PubMed] [Google Scholar]
- Loveday RL, Greenman J, Drew PJ, Monson JRT, Kerin MJ. Genetic changes associated with telomerase activity in breast cancer. Int J Cancer. 1999;84:516–520. doi: 10.1002/(SICI)1097-0215(19991022)84:5<516::AID-IJC12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- White LK, Wright WE, Shay JW. Telomerase inhibitors. Trends Biotechnol. 2001;19:114–120. doi: 10.1016/s0167-7799(00)01541-9. [DOI] [PubMed] [Google Scholar]
- Melana SM, Holland JF, Pogo BGT. Inhibition of cell growth and telomerase activity of breast cancer cells in vitro by 3'-azido-3'-deoxythymidine. Clin Cancer Res. 1998;4:693–696. [PubMed] [Google Scholar]
- Multani AS, Furlong C, Pathak S. Reduction of telomeric signals in murine melanoma and human breast cancer cell lines treated with 3'-azido-3'-deoxythymidine. Int J Oncol. 1998;13:923–925. doi: 10.3892/ijo.13.5.923. [DOI] [PubMed] [Google Scholar]
- Choi SH, Kang HK, Im EO, Kim YJ, Bae YT, Choi YH, Lee KH, Chung HY, Chang HK, Kim ND. Inhibition of cell growth and telomerase activity of breast cancer cells in vitro by retinoic acids. Int J Oncol. 2000;17:971–976. doi: 10.3892/ijo.17.5.971. [DOI] [PubMed] [Google Scholar]
- Aldous WK, Marean AJ, DeHart MJ, Matej LA, Moore KH. Effects of tamoxifen on telomerase activity in breast carcinoma cell lines. Cancer. 1999;85:1523–1529. doi: 10.1002/(SICI)1097-0142(19990401)85:7<1523::AID-CNCR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Herbert B-S, Wright AC, Passons CM, Kopelovich L, Ali I, Wright WE, Shay JW. Effects of chemopreventive and anti-telomerase agents on the spontaneous immortalization of breast epithelial cells. J Natl Cancer Inst. 2001;93:39–45. doi: 10.1093/jnci/93.1.39. [DOI] [PubMed] [Google Scholar]


