Abstract
The acidity of mammalian secretory vesicles drives concentration and processing of their contents. Here, pH-sensitive green fluorescent protein (GFP) variants show that the ≥30-fold (H+) difference between secretory vesicles (pH ≤ 5.7) and the cytoplasm (pH = 7.2) in mammalian cells is not present in peptidergic and small synaptic vesicles of the Drosophila neuromuscular junction. First, we find that fluorescence from Topaz-tagged atrial natriuretic factor, a peptidergic vesicle pH indicator, is only modestly affected by collapsing the H+ gradient in type III synaptic boutons. Quantitation shows that peptidergic vesicles are nearly neutral (pH = 6.74 ± 0.05), even when temperature is elevated. Furthermore, small synaptic vesicles in glutamatergic synaptic boutons, studied with synaptophluorin, are as alkaline as peptidergic vesicles. Finally, yellow fluorescent protein measurements show that cytoplasmic pH is only slightly different than in mammals (pH = 7.4). Thus, the marked acidity of mammalian secretory vesicles is not conserved in evolution, and a modest vesicular H+ gradient is sufficient for supporting neurotransmission.
A major characteristic of mammalian secretory vesicles is that they are very acidic (i.e., luminal pH = 5.0–5.7) relative to the cytoplasm (pH = 7.2) (1). This large H+ concentration gradient is present in large dense core vesicles that contain hormones and neuropeptides and in small synaptic vesicles (SSVs) that contain small molecule neurotransmitters. Collapsing the secretory vesicle pH gradient disrupts aggregation, enzymatic processing, and packaging of peptides and eliminates the electrochemical driving force for vesicular uptake of small molecules (1). Therefore, the large vesicular pH gradient is essential for regulated secretion in mammalian neurons and endocrine cells. This has led to the assumption that a large vesicular H+ gradient is conserved in evolution. However, here we conduct in vivo imaging of pH-sensitive green fluorescent protein (GFP) variants transgenically targeted to peptidergic vesicles and SSVs to show that Drosophila neuromuscular junction secretory vesicles are only slightly acidic.
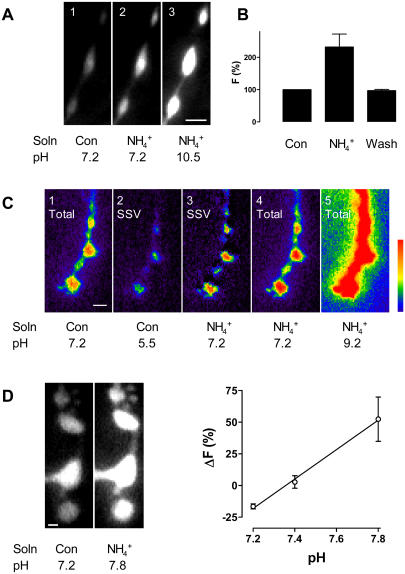
We began our studies by examining a neuropeptide/hormone called atrial natriuretic factor (ANF) tagged with the Topaz variant of green fluorescent protein (ANF-Tpz) in type III peptidergic neuromuscular junction boutons. Topaz fluorescence is quenched by acid with a neutral pK, and so collapsing the pH gradient in peptidergic vesicles of mammalian neuroendocrine cells produces a dramatic increase in ANF-Tpz fluorescence (2,3). To collapse the vesicular pH gradient, we applied an ammonium solution as described previously for Drosophila neuromuscular junctions (4). Ammonium and ammonia are in equilibrium (i.e., 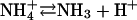 ). Since ammonia is uncharged, it crosses membranes and binds protons until there is no pH gradient between the vesicle lumen and the extracellular solution. Setting the pH inside type III bouton vesicles to 7.2 in Ca2+-free saline, which prevents activity-dependent release, only increased peptide fluorescence by 113 ± 7% (n = 5) (Fig. 1 A, panels 1 and 2), instead of the ≥10-fold effect expected.
). Since ammonia is uncharged, it crosses membranes and binds protons until there is no pH gradient between the vesicle lumen and the extracellular solution. Setting the pH inside type III bouton vesicles to 7.2 in Ca2+-free saline, which prevents activity-dependent release, only increased peptide fluorescence by 113 ± 7% (n = 5) (Fig. 1 A, panels 1 and 2), instead of the ≥10-fold effect expected.
FIGURE 1 .
(A) ANF-Tpz responses in type III boutons. (1) Peptide fluorescence under control conditions in Ca2+-free saline; (2) approximate doubling of fluorescence after collapsing the vesicular pH gradient at pH 7.2; (3) increase in signal after setting the vesicular pH to 10.5. Bar is 2 μm. (B) The ammonium effect is reversible at pH 7.2 (n = 4). (C) Synaptophluorin responses in type I boutons. (1) Pseudo-color representation of synaptophluorin fluorescence under control conditions, which includes the vesicular and surface signals; (2) vesicular signal revealed after quenching surface fluorescence with pH 5.5 medium; (3) vesicular signal at pH 7.2. Obtained by subtracting surface signal (1 − 2) from total signal after setting the pH to 7.2 in all compartments with ammonium (4). (5) Total synapto-phluorin signal after setting the pH to 9.2, which was used with 4 to calculate the pK of the indicator. Bar is 2 μm. (D) Cytoplasmic YFP responses in type I boutons. (Left) Fluorescence increase after setting the pH to 7.8. Bar is 1 μm. (Right) Change in YFP fluorescence versus cytoplasmic pH; n = 4 for pH 7.2, 4 for pH 7.4, and 3 for pH 7.8.
We considered explanations for this small response that do not involve an alkaline vesicular lumen. First, we showed that this is not due to ANF-Tpz on the extracellular surface of terminals because application of pH 5.5 medium did not reduce the peptide signal: fluorescence changed by 1.9 ± 5.1% (n = 4). Thus, ANF-Tpz was only present inside the nerve terminals. Second, we tested whether sufficient ammonium was applied to collapse the vesicular pH gradient. Ammonium dose-response results showed that 50 mM ammonium, which is effective in larval neuromuscular junction (4), gave a maximal response (data not shown). Hence, ammonium was not limiting. Third, we investigated whether the pK of the fluorescent protein might be perturbed dramatically by some difference in the Drosophila milieu (e.g., lower ionic strength). Intravesicular pH was varied by applying ammonium solutions set at different pH values (e.g., Fig. 1 A, panels 2 and 3). Using the Henderson-Hasselbach equation, we calculated the pK of ANF-Tpz in Drosophila peptidergic vesicles to be 7.29 ± 0.04 (n = 5), a value only slightly different than in mammalian cells (3). Thus, the modest response to collapsing the pH gradient could not be attributed to insensitivity of the pH indicator. Fourth, we showed that the absence of extracellular Ca2+ in Fig. 1 A to prevent muscle contraction was not relevant: the fluorescence increase induced by collapsing the vesicular pH gradient was statistically identical in Ca2+-containing saline (119 ± 15%, n = 4). Fifth, we verified that ANF-Tpz, like ANF-GFP (5–8), is targeted to secretory vesicles in Drosophila. ANF-Tpz fluorescence paralleled the known distribution of peptidergic vesicles: it was high in type III boutons and low in type I boutons, even though the UAS driver used here induces expression in both of these nerve terminals. Also, depolarizing terminals for 5 min after collapsing the pH gradient induced release of 65% ± 5% of the ANF-Tpz (n = 3), a robust response for neuronal peptide secretion. Hence, secretory vesicles must have contained ANF-Tpz, as had been found in mammalian cells (3). Sixth, the ammonium effect was reversible, showing that release and photobleaching did not affect our pH assay (Fig. 1 B). Thus, we were left to conclude that the small response to collapsing the vesicular pH gradient must reflect a limited vesicular H+ gradient. The effect of collapsing the pH gradient combined with the calibrated indicator pK showed that the pH in peptidergic vesicles is 6.74 ± 0.05 (n = 5) (Table 1).
TABLE 1.
pH of Drosophila peptidergic vesicles, SSVs, and nerve terminal cytoplasm
| Indicator | °C | pH | |
|---|---|---|---|
| Peptidergic vesicle | ANF-Tpz | 22 | 6.74 ± 0.05 (n = 5) |
| 37 | 6.64 ± 0.04 (n = 4) | ||
| SSV | Synaptophluorin | 22 | 6.63 ± 0.13 (n = 5) |
| Cytoplasm | YFP | 22 | 7.4 (n = 4)* |
The method used for cytoplasmic pH does not yield a standard error.
One potential basis for the difference between warm-blooded mammals and cold-blooded invertebrates such as Drosophila is physiological temperature. Since the vesicular pH gradient is generated by an enzyme, the vacuolar ATPase, it was conceivable that luminal acidity could increase with temperature. Therefore, to test whether temperature is a significant factor, neuromuscular junction preparations were maintained at 37°C for 30 min. Under these conditions, we first determined the pK of ANF-Tpz (pK (37°C) = 6.81 ± 0.08 (n = 4)). We then used this value along with the effect of collapsing the vesicular H+ gradient at 37°C to determine the temperature dependence of luminal pH. As shown in Table 1, this change in temperature did not significantly alter vesicle pH. Results acquired at 30°C also supported this conclusion (data not shown). Therefore, the nearly neutral pH of Drosophila peptidergic vesicles cannot be attributed to temperature.
Previous studies of the Drosophila neuromuscular junction have utilized synaptophluorin to study SSV exocytosis and endocytosis (4,9). At glutamatergic type I boutons, much of the synaptophluorin signal originates from the cell surface, but the fluorescence from SSVs can be measured by quenching surface fluorescence with a pH 5.5 solution. Our finding that a pH 5.5 extracellular solution did not quench luminal protein fluorescence in Drosophila peptidergic vesicles validates this approach (see above). Synaptophluorin fluorescence is increased by collapsing the pH gradient in fly SSVs (4), showing that these organelles are more acidic than the extracellular medium. However, acid quenching of surface signal and collapsing the vesicular H+ gradient were not combined on the same preparation, and the pK of synaptophluorin was not measured in Drosophila. Therefore, it is not possible to determine the luminal pH of Drosophila SSVs from prior studies.
Quenching the surface synaptophluorin signal (Fig. 1 C, panel 2) and applying ammonium at pH 7.2 (Fig. 1 C, panel 4) revealed the change in SSV synaptophluorin signal upon collapsing pH gradients (Fig. 1 C, panel 3). Then we varied pH after collapsing the H+ gradient with ammonium (Fig. 1 C, panels 4 and 5) to determine that the pK of synaptophluorin in type I boutons is 7.55 ± 0.06 (n = 5). Finally, combining these measurements allowed us to calculate that the pH in SSVs is 6.63 ± 0.13 (n = 5) (Table 1). This is statistically identical to the results found with ANF-Tpz in type III boutons. Thus, as expected from mammalian studies, peptidergic vesicles and SSVs maintain the same luminal pH. However, in contrast to mammalian secretory vesicles, these organelles are nearly neutral in Drosophila.
The driving force for vesicular uptake of neurotransmitters is generated by the H+ electrochemical gradient, which depends on the difference in pH between vesicles and the cytoplasm. However, cytoplasmic pH has not been measured in Drosophila nerve terminals. We reasoned that a cytoplasmic fluorescent protein with a neutral pK would be an appropriate indicator. Therefore, we used flies expressing cameleon, a construct that contains cyan and yellow fluorescent proteins (CFP and YFP) (10). Although CFP in insensitive to physiological pH, YFP's fluorescence spectra and pH sensitivity are similar to Topaz fluorescent protein (2). Typically, cameleon is used by exciting CFP (e.g., with 440 nm light), which then undergoes fluorescence resonance energy transfer with YFP that depends on Ca2+. However, this fluorescence resonance energy transfer (and its Ca2+ dependence) can be precluded by directly exciting YFP with longer wavelengths of excitation (e.g., 500 nm). Therefore, we directly excited YFP and used this fluorescence as a cytoplasmic pH indicator.
For these experiments, we collapsed the pH gradient with ammonium solutions set at varying pH values. When pH was clamped to 7.8, cytoplasmic YFP fluorescence increased (Fig. 1 D, left panels). Thus, the cytoplasm was more acidic than 7.8. Furthermore, setting the pH to 7.2 decreased the YFP signal, showing that cytoplasmic pH was more basic than 7.2. Finally, the change evoked by setting the pH to 7.4 was not significant (n = 4) (Fig. 1 D, right graph). A cytoplasmic pH of 7.4 implies that there is only a fivefold H+ concentration gradient in Drosophila neuromuscular junction secretory vesicles, rather than the ≥30-fold gradient found in mammals.
Our studies of Drosophila peptidergic vesicles and SSVs demonstrate that the extremely acidic pH that is characteristic of mammalian secretory vesicles is not evolutionarily conserved and so must not be essential for neurotransmission. Fully collapsing the vesicular pH gradient in mammalian cells disrupts condensation, packaging, and processing of secreted peptides and uptake of small molecule neurotransmitters. However, the effect of maintaining a smaller H+ gradient has never been tested. Here modest luminal acidification was found at the Drosophila neuromuscular junction, a synapse that is essential for survival of the animal. In fact, our results clarify why synaptophluorin responses are so small in this preparation (4,9) and luminal processing enzymes have less acidic pH optima in fruit flies than in mammals (11). More generally, we have shown that a very acidic pH is not a defining characteristic for neurosecretory vesicles. Rather, previously reported large vesicular H+ gradients may represent a feature associated with the evolution of vertebrates in general or mammals specifically.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
We thank Chaoming Zhou and Chandra Ziegler for technical assistance, and G. W. Davis (University of California, San Francisco) for UAS-n-Syb-pH flies.
This research was supported by National Institutes of Health grant NS32385 to E.S.L.
References
- 1.Schoonderwoert, V., and G. J. M. Martens. 2001. Proton pumping in the secretory pathway. J. Membr. Biol. 182:159–169. [DOI] [PubMed] [Google Scholar]
- 2.Tsien, R. Y. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509–544. [DOI] [PubMed] [Google Scholar]
- 3.Han, W., D. Li, and E. S. Levitan. 2002. A new green fluorescent protein construct for localizing and quantifying peptide release. Ann. N. Y. Acad. Sci. 971:627–633. [DOI] [PubMed] [Google Scholar]
- 4.Poskanzer, K. E., and G. W. Davis. 2004. Mobilization and fusion of a non-recycling pool of synaptic vesicles under conditions of endocytic blockade. Neuropharmacology. 47:714–723. [DOI] [PubMed] [Google Scholar]
- 5.Rao, S., C. Lang, E. S. Levitan, and D. L. Deitcher. 2001. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49:159–172. [DOI] [PubMed] [Google Scholar]
- 6.Shakiryanova, D., A. Tully, R. S. Hewes, D. L. Deitcher, and E. S. Levitan. 2005. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat. Neurosci. 8:173–178. [DOI] [PubMed] [Google Scholar]
- 7.Heifetz, Y., and W. F. Wolfner. 2004. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc. Natl. Acad. Sci. USA. 101:6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain, Q. M., and J. Ewer. 2004. Use of targetable GFP-tagged neuropeptide for visualizing neuropeptide release following execution of a behavior. J. Neurobiol. 59:181–191. [DOI] [PubMed] [Google Scholar]
- 9.Poskanzer, K. E., K. W. Marek, S. T. Sweeney, and G. W. Davis. 2003. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 426:559–563. [DOI] [PubMed] [Google Scholar]
- 10.Diegelmann, S., A. Fiala, C. Leibold, T. Spall, and E. Buchner. 2002. Transgenic flies expressing cameleon 2.1 under UAS control. Genesis. 34:95–98. [DOI] [PubMed] [Google Scholar]
- 11.Han, M., D. Park, P. J. Vanderzalm, R. E. Mains, B. A. Eipper, and P. H. Taghert. 2004. Drosophila uses two distinct neuropeptide amidating enzymes, dPAL1 and dPAL2. J. Neurochem. 90:129–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.