Abstract
Comitin is an F-actin binding and membrane-associated protein from Dictyostelium discoideum, which is present on Golgi and vesicle membranes and changes its localization in response to agents affecting the cytoskeleton. To investigate its in vivo functions we have generated knockout mutants by gene replacement. Based on comitin's in vitro functions we examined properties related to vesicular transport and microfilament function. Whereas cell growth, pinocytosis, secretion, chemotaxis, motility, and development were unaltered, comitin-lacking cells were impaired in the early steps of phagocytosis of Saccharomyces cerevisiae particles and of Escherichia coli, whereas uptake of latex beads was unaffected. Furthermore, the lack of comitin positively affected survival of pathogenic bacteria. Mutant cells also showed an altered response to hyperosmotic shock in comparison to the wild type. The redistribution of comitin during hyperosmotic shock in wild-type cells and its presence on early phagosomes suggest a direct involvement of comitin in these processes.
The actin cytoskeleton is important for cell architecture, cell motility, intracellular vesicle transport, phagocytosis, and endo- and exocytosis. This broad range of functions is supported by many actin-binding proteins, such as unconventional myosins (2, 23, 40), profilin, spectrin (7, 17, 53), and synapsin (56). These proteins are involved in actin polymerization and cross-linking the filaments into bundles or networks. Comitin, a 24-kDa protein, can also bundle actin filaments. It furthermore associates with membranes and, based on its properties, was proposed to provide a link between membrane vesicles and the actin-based microfilament system, and thus it belongs to the group of membrane anchors. Comitin was first identified in Dictyostelium discoideum as an F-actin binding protein (52, 42). It is a highly basic protein of 185 amino acids and consists of two domains. The C-terminal domain is composed of 41 residues possessing six repeats of the GYP(P)Q motif, which are also found in proteins of rather diverse functions such as Octopus rhodopsin, which is involved in light perception (44); annexins A7 and 11, Ca2+ and phospholipid binding proteins that function as a Ca2+ channel and mediate membrane fusion (12, 20) or are present in the nucleus (54); and synaptophysin, a protein present on synaptic vesicles (36). The N-terminal core domain of comitin is constructed of 144 residues and carries nearly the whole charge of the protein.
Comitin is a bifunctional protein. In addition to its actin binding activity it exhibits a mannose binding activity with which it might bind mannose residues in glycoproteins or glycolipids on the cytoplasmic surface of membrane vesicles, providing a mechanism for comitin's membrane association. The actin-binding site is primarily located between amino acids 90 and 135 of comitin; another binding site of lower affinity was mapped near the N terminus (30). Comitin's binding site on F-actin has been mapped near the actin N terminus in subdomain 1 (24). This site is different from the binding site on F-actin for other actin binding proteins, such as α-actinin, which contact two actin monomers in the filament (39). Immunoelectron microscopy, immunofluorescence studies, and biochemical data localized comitin to the Golgi region and to vesicles distributed throughout the cell and suggested a function for comitin as a mediator between the cytoskeleton and the membrane vesicle system (59).
In this work, we describe the generation of a comitin gene knockout in D. discoideum and the effects of the mutation on cellular processes requiring a functional actin cytoskeleton as well as membrane vesicle system. We present evidence that comitin is involved in phagocytosis and protection against osmotic stress and discuss the role of comitin-decorated vesicles during these processes in view of comitin's bifunctional role. We propose that the vesicles are guided by the cytoskeleton to their place of destiny where they fuse with the plasma membrane to allow formation of a phagocytic cup or protection during osmotic shock.
MATERIALS AND METHODS
D. discoideum strains and growth conditions.
D. discoideum strain AX2-214 (referred to as the wild type), an axenically growing derivative of wild strain NC4, and the comitin-negative mutants 1a1 and 3a1 used in this study were grown at 21°C in liquid medium with shaking at 160 rpm (15) or on SM agar plates with Klebsiella aerogenes (21).
Disruption of the comitin gene.
For construction of a comitin targeting vector, a 1.5-kb genomic DNA fragment carrying the comitin gene was obtained from HindIII- and EcoRV-cut DNA. This fragment was cloned into pIC19R (38). A 0.4-kb BglII/XbaI fragment, including the start codon ATG, was replaced by a blasticidin resistance cassette under the control of the actin 15 promoter and actin 8 terminator (1). The resulting vector was linearized and introduced into AX2 cells by electroporation. For selection, cells were grown in liquid nutrient medium containing blasticidin (3.5 μg/ml). Mutants lacking comitin were identified by colony blotting. For mutant characterization we used the previously described monoclonal antibodies (MAbs) (59) as well as a newly generated polyclonal antibody against recombinant comitin.
Growth and development of D. discoideum.
For the analysis of growth in shaking suspension under stress conditions, 5 × 104 cells/ml were inoculated and grown either in axenic medium at 15 or 27°C or in axenic medium at 21°C supplemented with 30 mM NaCl, 150 mM sorbitol, brefeldin A (25 or 50 μg/ml; Sigma, Deisenhofen, Germany), and 1 or 3 mM EGTA. For growth in shaken suspension on Escherichia coli B/r, E. coli cells were adjusted to a density of 1010 cells in Soerensen phosphate buffer (17 mM K-sodium phosphate, pH 6.0), and this suspension was inoculated with mutant and wild-type strains at various densities. Shaking was done at 160 rpm and 21°C. Cell numbers were determined by counting. For analysis of development, cells were grown to a density of 3 × 106 cells/ml, washed, and resuspended at a density of 108 cells/ml in Soerensen phosphate buffer. Cells (5 × 107) were deposited on phosphate agar plates. For development in shaking suspension, cells were washed in Soerensen phosphate buffer, resuspended at a density of 107 cells/ml, and shaken at 21°C and 160 rpm (49). Osmotic shock experiments were done as described in the work of Schuster et al. (50).
Fluorescence microscopy.
For fluorescence microscopy, cells were grown to a density of 3 × 106 cells/ml, harvested, and resuspended at a density of 5 × 105 cells/ml with Soerensen phosphate buffer. Cells were transferred onto glass coverslips and allowed to settle for 15 min. For fixation, cells were incubated in cold methanol (−20°C) for 10 min. Comitin distribution was determined by incubation with the comitin-specific MAbs 190-23-5, 190-68-1, and 190-340-8 followed by incubation with Cy3-labeled anti-mouse immunoglobulin G (59). Phagosomal cup formation was studied in mutant and wild-type cells expressing green fluorescent protein (GFP)-actin (60). Confocal images were taken with an inverted laser scanning microscope with a 63× PL Fluotar 1.32 oil immersion objective (Leica TCS SP; Leica Lasertechnik GmbH, Heidelberg, Germany). Conditions for image acquisition and processing were as previously described (37, 41).
Phagocytosis assay.
The phagocytosis assay to measure uptake of Saccharomyces cerevisiae particles was a modification of the method of Hed (29). D. discoideum cells of strain AX2 and the comitin-deficient mutant cell lines were grown to densities below 5 × 106 cells/ml, harvested, washed, and resuspended in Soerensen phosphate buffer to a density of 2 × 106 cells/ml in a 100-ml Erlenmeyer flask. Tetramethyl rhodamine isothiocyanate (TRITC)-labeled yeast cells (120 μl; 109 cells/ml) were added to 20 ml of the Dictyostelium cell suspension. Samples of 1 ml were withdrawn every 20 min and added to 100 μl of trypan blue solution (20 mg/ml dissolved in 20 mM sodium citrate containing 150 mM NaCl; Merck, Darmstadt, Germany), which quenches the fluorescence of noninternalized yeast cells. After 3 min of agitated incubation, cells were spun and the supernatant was removed carefully. After resuspension in Soerensen phosphate buffer, fluorescence was measured in a fluorimeter (PTI; Photo Med GmbH, Seefeld, Germany) using 544-nm light for excitation and recording emission at 574 nm. The measured fluorescence was calculated as relative fluorescence to AX2. Phagocytosis of E. coli B/r and latex beads (1 μm in diameter) was as described elsewhere (21). The phagocytosis was also assayed on a substratum. For this D. discoideum cells were allowed to settle on coverslips, and yeast cells were added. At different time points, the coverslips were briefly washed in Soerensen phosphate buffer, fixed in methanol, and embedded. More than 950 cells were analyzed for each time point with an Olympus microscope IX70.
Flow cytometry with GFP-tagged Legionella pneumophila is described by Hägele et al. (27). In brief, Dictyostelium cells (5 × 105 cells/ml) were inoculated with L. pneumophila Corby pBC(gfp)Pmip (multiplicity of infection, 100). After 3 h postinfection extracellular bacteria were removed by washing, infected Dictyostelium cells were transferred to fluorescence-activated cell sorter tubes, and GFP fluorescence was recorded in a flow cytometer (FACSalibur; Becton Dickinson) equipped with a 15-mW argon laser emitting at 488 nm and using the FL-1 channel of a FACS cytometer (Becton Dickinson).
Preparation of phagosomes.
Phagosomes from wild-type cells that had taken up paramagnetic iron beads (Polysciences Europe GmbH, Eppelheim, Germany) were prepared as described by Maniak et al. (37). Cells were opened by filtration through a cell cracker or alternatively by freeze-thawing, with both methods leading to identical results. The resulting homogenate was extracted three times with homogenization buffer using a magnet (wash steps). The final pellet represented phagosomes. The protein concentration was determined, and equal amounts of protein were loaded.
Miscellaneous methods.
Pinocytosis and secretion assays were done as described by Döring et al. (21), and chemotaxis assays were carried out as described by Rivero et al. (48). Southern and Northern blotting were performed as described previously (47). For expression of GFP-tagged comitin, vectors allowing N- as well as C-terminal fusions were used (60). DNA and RNA were transferred to nylon membranes (Pall; Filtron, Dreieich, Germany) and incubated with 32P-labeled probes generated by using a random prime labeling kit (Stratagene, La Jolla, Calif.). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was done as described by Laemmli (33). Western blotting was performed using MAbs, an anti-mouse immunoglobulin G peroxidase-conjugated antibody (Sigma) as secondary antibody, and the ECL detection system (Amersham, Braunschweig, Germany). Phosphodiesterase activity was determined according to the method of Gerisch et al. (26), and α-mannosidase and acid phosphatase activities were determined according to the method of Dimond et al. (19). Antibodies specific for the cell surface protein contact site A are described by Berthold et al. (8).
RESULTS
Generation and analysis of a comitin-lacking mutant.
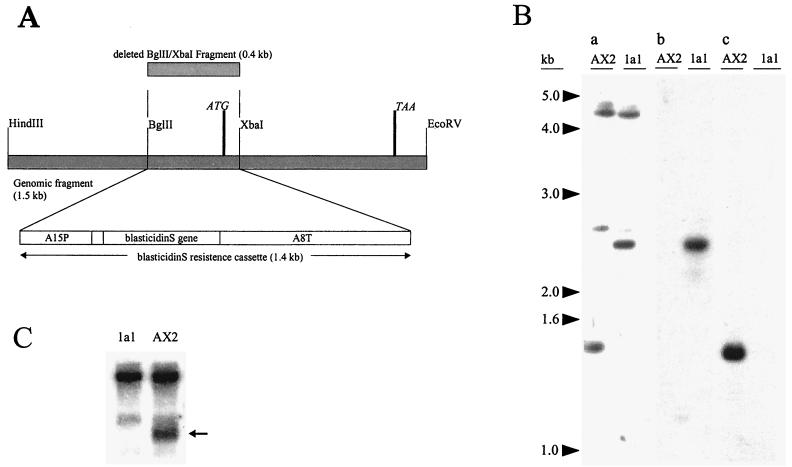
To investigate the comitin function in vivo we have generated D. discoideum knockout mutants by homologous recombination. We used a transformation vector that contained a 1.5-kb EcoRV/HindIII fragment carrying comitin gene sequences. Of this fragment a 0.4-kb BglII/XbaI fragment encompassing the ATG start codon was replaced by the 1.4-kb blasticidin resistance cassette (Fig. 1A). Several independent clones lacking comitin were isolated by colony blotting and confirmed by Western, Southern, and Northern blotting. Southern blot analysis revealed a replacement of the endogenous gene by the transformation vector, since a probe consisting of the 0.4-kb fragment that was deleted from the transformation vector no longer hybridized to the mutant DNA, whereas the endogenous gene in AX2 wild-type DNA was recognized (Fig. 1B). The mutant cells did not produce the comitin-specific mRNA anymore, nor was protein detectable when tested with polyclonal antibodies and MAbs (Fig. 1C and data not shown). In further studies we focused on two independently isolated mutants designated 1a1 and 3a1. The results obtained with both mutants were nearly identical; therefore, mainly data from mutant 1a1 are shown.
FIG. 1.
Generation and analysis of comitin knockout mutants. (A) Construction of the replacement vector. (B) Southern blot analysis of the comitin-lacking clone 1a1. Genomic DNA of AX2 wild-type and 1a1 mutant cells was digested with HindIII and EcoRV, separated in a 0.7% agarose gel, and blotted onto nylon membrane. (a) Hybridization with the comitin-specific 1.5-kb HindIII/EcoRV genomic fragment shows the 1.5-kb fragment in AX2. This is shifted to 2.5 kb in 1a1. Additional signals correspond to the GYP(P)Q motif coding sequences of other genes. (b) Blasticidin resistance cassette recognized the 2.5-kb band in 1a1. No signal was seen in AX2 cells. (c) Hybridization with the 0.4-kb BglII/XbaI fragment deleted from the replacement vector marked the 1.5-kb band in the wild type. No signal was detected in 1a1 cells. (C) Northern blot analysis of the comitin-lacking mutant. Total RNA from vegetative cells, mutant and control, was isolated; separated in a 1.2% agarose gel under denaturing conditions; and transferred onto a nylon membrane. The blot was probed with the comitin full-length cDNA, which recognizes all RNA species harboring a GYPPQ repeat (Noegel et al. [42]). The 0.7-kb message for comitin was lacking in the mutant (arrow).
Growth, pinocytosis, secretion, chemotaxis, and development are not impaired in comitin-lacking cells.
As comitin is both a vesicle- and F-actin-associated protein, we addressed aspects of the actin cytoskeleton as well as of intracellular transport processes when assaying for defects in the mutant. We analyzed growth behavior under different temperatures as detailed in Materials and Methods and did not detect any differences compared to the wild type. Furthermore, addition of EGTA, a Ca2+ chelator that should block Ca2+-dependent processes, and brefeldin A, a drug known to inhibit transport processes, affected growth similarly in wild-type and mutant cells, leading to lower cell densities at saturation (data not shown).
Fluid-phase endocytosis depends on membrane flow and on the rearrangement of the actin cytoskeleton. In general, growth in liquid medium is already a measure of pinocytotic activity, and the results mentioned above are an indication that this property is not substantially altered in comitin-lacking mutants. In addition we performed a quantitative assay and measured the pinocytotic activity by monitoring the uptake of 3H-labeled dextran, since the pinocytosed radioactivity corresponds to the amount of liquid volume taken up. These experiments also did not reveal differences between wild-type and comitin-lacking cells (data not shown).
Exocytotic processes were studied by following the secretion of the lysosomal enzymes α-mannosidase and acid phosphatase and were found to be unaltered. Similarly, the development-specific secretion of the enzyme phosphodiesterase was unaffected. Correct posttranslational modification was analyzed for the contact site A, a developmentally regulated cell surface glycoprotein, which was properly O- and N-glycosylated as assessed using specific antibodies, and was transported to the cell surface (8, 28) (data not shown). Likewise, PsA, a lipid-linked cell surface glycoprotein of prespore cells, was properly expressed and posttranslationally modified (22, 31). This implies that modifications occurring in the endoplasmic reticulum (ER) and Golgi as well as transport of proteins to the cell surface are not impaired.
Mutant cells underwent normal development, expressed the developmental markers at the same time as wild-type cells, and formed fruiting bodies showing normal morphology. Furthermore, the expression of stage-specific genes followed the pattern observed in AX2 wild type. In chemotaxis assays the cells reacted towards cyclic AMP and migrated with similar speed and orientation as wild-type cells (data not shown).
Comitin-lacking cells show a defect in phagocytosis.
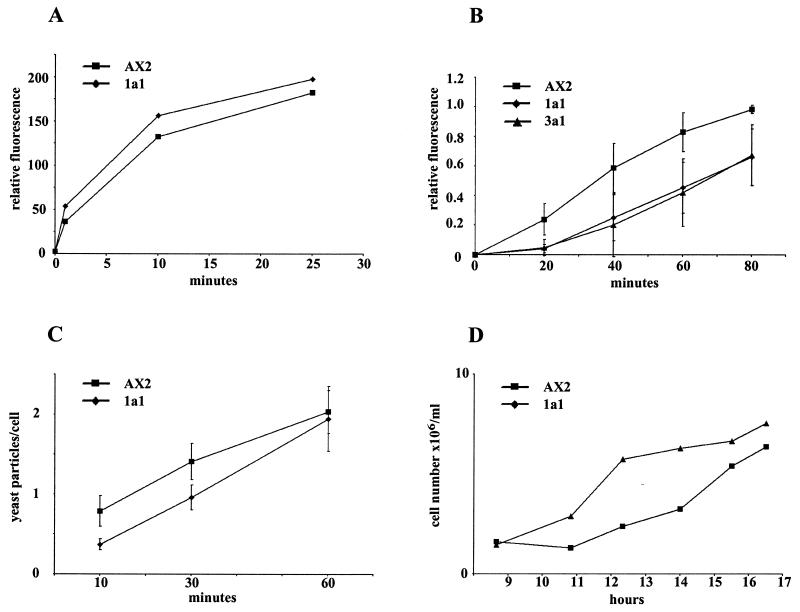
Several assays are available for the analysis of phagocytosis in Dictyostelium. We have tested uptake of bacteria, latex beads, and yeast particles in suspension in phosphate buffer. The particles differ in size and, more importantly, in the nature of their surface. E. coli B/r and yeast particles have a hydrophilic surface, whereas the latex beads have a very hydrophobic surface and the receptors responsible for the uptake will differ (16, 57). We found that the uptake of latex beads was comparable between mutant and wild-type strains (Fig. 2A), whereas phagocytosis of E. coli and yeast particles was impaired in the mutant strains. For yeast uptake, cells were fed with heat-killed TRITC-labeled yeast cells. The lack of comitin resulted in a marked defect in uptake of yeast cells. This defect was mainly due to a lag in initiating internalization. At 40 min comitin-null cells showed an uptake of 39% compared to wild-type cells. After 80 min the uptake of mutant cells reached 67.5% of the wild-type level (Fig. 2B). To investigate this in more detail we determined the time-dependent uptake of yeast particles by allowing cells to take up TRITC-labeled yeast particles in suspension and fixing them at the indicated time points. The numbers of yeast cells taken up were determined by microscopic analysis. At early points (10 min) D. discoideum wild-type cells had ingested 0.79 yeast particle/cell, whereas mutant cells took up only 0.37 yeast particle/cell as determined by immunofluorescence analysis. After 60 min both cell types reached a steady-state level of internalized yeast which was then maintained (Fig. 2C). When comparing the actual numbers, this behavior is even more obvious. After 10 min, 71.3% of all mutant cells had not taken up a yeast cell, whereas 52.3% of the AX2 wild type had ingested at least one particle. The difference between mutant and wild type decreases over time, and at 60 min there is no longer a prominent difference between wild type and mutant. The observed impairment in phagocytosis therefore appears to reside mainly in initial uptake and not in the downstream processing of the phagosomes.
FIG. 2.
Phagocytosis of latex beads, yeast particles, and E. coli B/r and internalization of yeast particles over time. (A) Phagocytosis of fluorescent latex beads (1 μm in diameter) is comparable in AX2 wild type and mutant 1a1. A representative experiment is shown. (B) Reduced phagocytic uptake of yeast cells in comitin-lacking strains 1a1 and 3a1. D. discoideum cells were incubated with TRITC-labeled yeast cells under shaking conditions for the times indicated. Samples were withdrawn and incubated with trypan blue solution to quench fluorescence of noningested yeast. After resuspension in Soerensen phosphate buffer, fluorescence was measured at 544 nm for excitation and 574 nm for emission. The differences were found to be significant (10−2 for the 20- and 40-min results and 10−3 for the 60- and 80-min results). (C) Quantitative analysis of yeast particles taken up at individual time points. Internalization of yeast particles over time was determined by counting internalized particles in fixed cells. The 10-, 30-, and 60-min analyses of AX2 control and 1a1 mutant are shown. The data represent the means of three independent experiments. For each time point 950 to 1,300 cells were analyzed. Average deviations are indicated in B and C (error bars). (D) Growth of AX2 and 1a1 mutant strain on E. coli B/r. A suspension of E. coli B/r (1010 cells/ml) was inoculated with equal numbers of logarithmically growing wild-type and mutant cells. Cell numbers were determined by counting. A representative experiment is shown. It was performed six times.
Impairment in the initial phases of uptake was also observed when the growth of mutant and wild-type strains in shaking suspension with E. coli B/r as the food source was determined. Although inoculation was carried out with equal cell numbers, the mutant cells exhibited an extended lag phase before resuming growth at rates similar to those of wild-type cells (Fig. 2D).
Infection experiments with L. pneumophila Corby demonstrated that the lack of comitin had a positive effect on intracellular bacterial numbers compared to the wild type (51). In a subsequent analysis, by using flow cytometry and GFP-tagged Legionella we found that 39% of comitin-lacking cells harbored the bacteria, compared to 26% of AX2 control cells, indicating that processing of the phagosome is less efficient in the mutant.
Comitin is present on phagosomes.
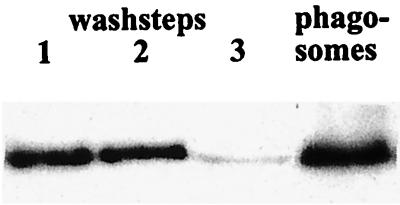
A broad range of F-actin proteins are involved in phagocytosis, but not all of them contribute to the formation of the phagosome layer. Comitin shows a vesicular distribution; its presence on phagosomes has not been analyzed so far. To demonstrate comitin incorporation into the protein layer associated with the membrane of early phagosomes, we loaded D. discoideum wild-type cells with magnetic iron beads. After 10 min, cells were lysed by freeze-thaw or by passage through a cell cracker, vesicles containing the magnetic beads were isolated by magnetic fractionation and carefully washed, and the protein content was analyzed by Western blotting. Comitin was enriched in the phagosome fraction relative to other proteins (Fig. 3 and data not shown) as is actin (45). GFP-tagged comitin behaved similarly and was also found in the phagosomal fraction, whereas α-actinin was enriched in the cytosol and only a weak signal was observed in the phagosome fractions (data not shown). α-Actinin had been shown previously to accumulate on the phagosome only during later stages of phagocytosis (25).
FIG. 3.
Comitin is present in isolated phagosomal membranes. Lanes 1 through 3 show successive wash fractions of phagosomal preparations; the last lane represents protein of the phagosome fraction. Protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% acrylamide), blotted onto nitrocellulose, and probed with the comitin-specific MAb 190-68-1. The 24-kDa signal of comitin decreased in the wash fractions and was present in the phagosome fraction.
We also analyzed the comitin distribution during phagocytosis by immunofluorescence studies. It was, however, difficult to prove an accumulation of comitin around the phagosome since comitin is present throughout the cells making it difficult to detect a specific enrichment in areas of phagosome formation.
Phagosomal cup formation is normal in mutant cells.
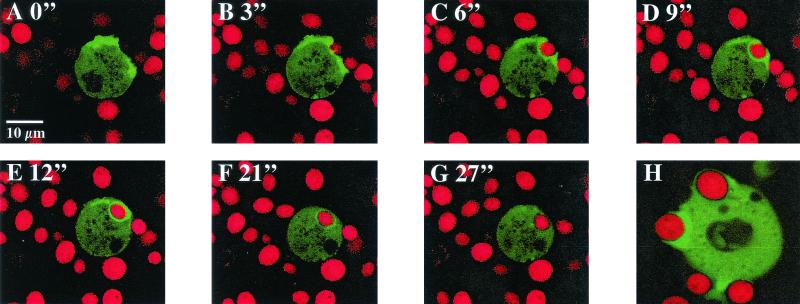
Actin accumulation on phagosomes is essential for phagocytosis, and accumulation of actin on phagosomes can conveniently be taken as a measure for formation of phagosomes (3, 4, 45). To study phagosomal cup formation we monitored actin assembly during the uptake of yeast particles in vivo in comitin-lacking cells expressing a GFP-actin fusion protein and compared it with the process in wild-type cells that also expressed GFP-actin. Wild-type and mutant cells were incubated with heat-killed yeast cells labeled with TRITC and analyzed by confocal laser scanning microscopy. We found that the F-actin accumulation on phagocytic cups was unaltered in mutant cells in comparison to wild-type cells. At the beginning of the sequence shown, actin became enriched beneath the cell surface at contact sites with the particle. Then GFP-actin enriched extensions that formed on leading edges off the cell surface began to engulf the yeast cell until it was surrounded by a continuous ring of GFP-actin. Once the yeast particle was completely taken up, GFP-actin disappeared from the phagosome (Fig. 4). The process and time course of actin accumulation and disappearance of actin from the phagosome were nearly indistinguishable in AX2 cells (data not shown).
FIG. 4.
Distribution of a GFP-actin fusion protein during phagocytosis. AX2 wild-type cells and 1a1 mutant cells expressing a GFP-actin fusion protein were fed with TRITC-labeled yeast cells. Phagocytosis was observed by confocal laser scanning microscopy at the indicated times (in minutes). GFP-actin distribution did not differ between the two Dictyostelium strains. Thus, we only show the series of confocal images for the mutant 1a1. (A and B) Enrichment of actin beneath the cell surface at sites of contact with the yeast particle. (C and D) GFP-actin-stained leading edges begin to engulf the yeast cell. (E) The engulfed yeast cell is surrounded by a ring of GFP-actin. (F and G) GFP-actin disassembled from the mature phagosome. (H) AX2 cell forming a phagocytic cup which shows an enrichment of F-actin.
Comitin-lacking cells show an increased sensitivity towards osmotic shock.
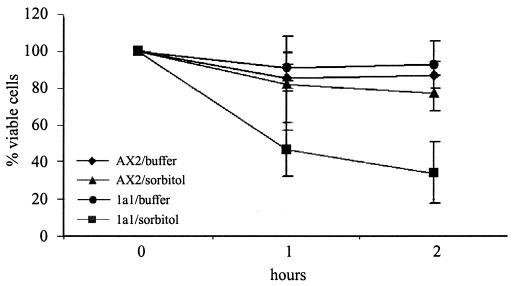
Several Dictyostelium mutants lacking actin-associated proteins exhibit a reduced resistance against osmotic shock (48). This has often been taken as evidence that an intact cytoskeleton is required in stress responses. To determine the survival rate in response to osmotic stress, wild-type and mutant cells were treated up to 2 h with 0.4 M sorbitol and afterwards were diluted into Soerensen phosphate buffer. We found a markedly reduced survival rate of the comitin-lacking mutant in comparison to AX2 cells. The osmotic shock reduced the viability of 1a1 cells to 47% after 1 h and to 34% after 2 h in hypertonic medium, whereas more than 77% of AX2 cells survived this treatment (Fig. 5). Lack of comitin thus resulted in a markedly reduced survival rate of D. discoideum cells under these conditions of acute osmotic shock, whereas the ability of the mutant strains to grow in the presence of increased osmolarity by supplementing the axenic medium with either 30 mM NaCl or 115 mM sorbitol was comparable to that of the wild type.
FIG. 5.
Resistance to hyperosmotic shock of strains AX2 and 1a1. Cells were shaken in phosphate buffer in the presence and absence of 0.4 M sorbitol for the times indicated, diluted into phosphate buffer, and plated on SM agar plates with K. aerogenes. Cell viability after 1 and 2 h of treatment was determined as the percentage of colonies in relation to the number of colonies observed at 0 h, which was assigned 100%. Each point is the average of colony counts from five plates. Data represent the average of four independent experiments (error bars, standard deviations).
Comitin redistribution during osmotic shock.
Exposure to high osmolarities evokes cell volume changes which affect a broad range of metabolic pathways (34, 58). In yeast, osmotic stress evokes changes in fusion pathways and in vacuolar morphologies (9); in mammalian cells, hyperosmotic shock induces inhibition of the ER export, the ER-to-Golgi-transport and the ER-Golgi intermediate compartment transport steps whereas the retrograde transport is not impaired by cell swelling. This cell response leads to the collapse of the ER-Golgi intermediate compartment and Golgi apparatus into the ER (35).
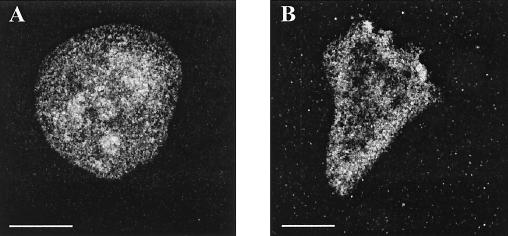
We studied the comitin distribution in osmotically shocked cells. In general, exposure to high osmolarities causes a rounding up of the cells. In immunofluorescence studies, MAb 190-68-1 stains vesicles throughout the cells. After treatment of AX2 cells for 90 min with 0.4 M sorbitol, the vesicles appeared larger in size and were more enriched in the vicinity of the plasma membrane (Fig. 6).
FIG. 6.
Altered distribution of comitin in response to hyperosmotic shock. AX2 cells were incubated in 0.4 M sorbitol for the times indicated. The cells were allowed to settle on coverslips and fixed. Shown are cells after 90 min of incubation without (A) and with (B) sorbitol. The MAb 190-68-1 stained vesicles in the control cell, which were distributed throughout the cell. After treatment with sorbitol, the vesicles were enriched in the vicinity of the cell boundaries. Bar, 10 μm.
DISCUSSION
Up to now the function of comitin was elucidated mainly by biochemical methods. Here we study mutant cells lacking the protein and demonstrate a role for comitin in phagocytic processes and in protection against osmotic shock. Our previous analysis showed that a lack of comitin does not cause phenotypic defects during development when cells are grown under conditions that resemble those in the natural environment of Dictyostelium (46). We now focused our experiments primarily on actin- and membrane-mediated cellular processes.
The actin cytoskeleton has been implicated in responses to osmotic shock in previous studies. Lack of the F-actin cross-linking proteins α-actinin and Dictyostelium filamin (previously called gelation factor or ABP120) resulted in a reduced resistance toward cell volume changes (48). For myosin mutants a similar observation has been made (32). In both instances the increased sensitivity towards osmotic stress was discussed as being the result of a reduced strength of the cortical cytoskeleton. A role of the actin cytoskeleton in the adaptation to situations of changed osmotic conditions was also described for melanoma cells deficient in mammalian filamin. These mutant cells were not capable of activating potassium channels and of regulating their cell volume when swollen in diluted solutions. Reexpression of filamin corrected these defects (13). Experiments with yeast cells point out a rapid and reversible disassembly and redistribution of the actin cytoskeleton in response to osmotic stress, and additional genetic and morphological analyses performed with an osmosensitive mutant hint at an actin-binding protein being involved in actin redistribution during osmotic shock (14).
Lack of comitin resulted in an increased sensitivity towards osmotic shock, observed by a markedly reduced survival rate of mutants compared to wild-type cells. As comitin was characterized as an F-actin binding protein which bundles filaments in vitro, it might act like α-actinin or myosin and physically strengthen the cytoskeleton. In cells it was always found in association with membranes, and it could well be that a redistribution of comitin-containing vesicles is involved in the generation of resistance to osmotic shock.
Comitin also has a role in phagocytosis. Phagocytosis is generally considered an actin-driven process, and it was suggested that actin polymerization is responsible for formation of pseudopods that surround a particle. This view is supported by the assembly of actin and the accumulation of actin binding proteins on the phagosome and by mutant analysis in Dictyostelium, which demonstrated the roles of actin binding proteins in phagocytosis (43). F-actin is also important for the subsequent processes, since intracellular transport and fusion of the phagosome with endocytic organelles can be inhibited by cytochalasin D (55). Phagocytosis also involves an increase in membrane area when pseudopods form and a loss of membrane when the particle is engulfed. These changes in membrane area are quite significant, especially in cells such as phagocytes, and Dictyostelium is considered a natural phagocyte. A polarized insertion of new membrane at sites of particle uptake could ensure pseudopod extension and would counteract a loss of membrane during phagocytic uptake. A similar mechanism was proposed for the membrane area increase that occurs when pseudopods form during cell movement, and in both cases endocytic vesicles have been proposed as the source of membranes (10, 11; I. Mellman, Letter, J. Cell Biol. 149:529-530, 2000). Motility is, however, not impaired in the comitin-lacking mutants. Fusion of endosomes with phagosomes is also important for phagosome maturation; Desjardins et al. (18) showed that early phagosomes fuse preferentially with early endocytic vesicles, and Bajno et al. (5) provided evidence that endosomal vesicles accumulate in the vicinity of phagosomes and fuse before phagosome sealing.
Our results indicate that comitin is present on early phagosomes. The deficiency in comitin resulted in a reduction of phagocytic efficiency. Notably, only the early steps of phagocytosis were affected, whereas the processing of the phagosome-containing yeast particles or E. coli appeared to be normal and actin accumulation around the phagosome occurred as in the wild type. These results and the presence of comitin on early and late endosomes (59) support the notion of a general mechanism of membrane insertion during phagocytosis as discussed above and comitin's role as a vesicle-associated protein in this process.
The phagocytosis defect is not a general defect, as the uptake of latex beads was comparable in wild-type and mutant cells. We have documented a specific defect in the phagocytosis of E. coli and of yeast particles., whereas uptake of latex beads and L. pneumophila (data not shown) was normal or slightly increased, respectively. During phagocytosis a particle is first recognized by the cell surface and bound via specific or nonspecific receptors. Work by Vogel et al. (57) identified different recognition sites for phagocytosis in mutants with altered phagocytic properties. One class of receptors mediated binding of particles containing terminal glucose as it is present on E. coli B/r, and the other one allows binding of hydrophobic particles (i.e., latex beads). The defect in the comitin mutant appears to be associated with the specific receptor and might reflect a defect in adhesion to the particles or in early processes of uptake and processing. Independent of this defect, in experiments studying the uptake and survival of pathogenic bacteria in Dictyostelium host strains, we observed that comitin-lacking mutants were more permissive to infections with L. pneumophila and showed a delayed degradation of Salmonella enterica serovar Typhimurium (51). This defect is clearly separable from the altered uptake characteristics and alludes to processes further downstream.
The findings presented here link comitin to intracellular trafficking and suggest that comitin participates in the early processes of uptake as well as the maturation of the phagosomes. The data support our working model for comitin (30) and are compatible with data from the structural modeling of the molecular domains (6). Comitin is a dimer with a lectin-binding activity and an actin-binding activity. The lectin domains bind to mannose residues at the cytoplasmic side of vesicles, which allows a close contact between vesicles and might be a first step towards fusion. Moreover, comitin is also able to bind a vesicle via its lectin activity and anchor it at actin filaments due to its actin binding activity, thus helping to direct vesicles to the sites where they are required.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft, Köln Fortune, and the Fonds der Chemischen Industrie.
We thank Barbara Peracino for her help with phagocytosis assays, G. Gerisch for kindly providing antibodies, Rolf Müller for help with phagocytosis analysis, and Berthold Gassen for production of MAbs.
REFERENCES
- 1.Adachi, H., T. Hasebe, K. Yoshinaga, T. Ohta, and K. Sutoh. 1994. Isolation of Dictyostelium discoideum cytokinesis mutants by restriction enzyme-mediated integration of the blasticidin S resistance marker. Biochem. Biophys. Res. Commun. 205:1808-1814. [DOI] [PubMed] [Google Scholar]
- 2.Adams, R. J., and T. D. Pollard. 1986. Propulsion of organelles isolated from Acanthamoeba along actin-filaments by myosin-I. Nature 322:754-756. [DOI] [PubMed] [Google Scholar]
- 3.Allen, L. H., and A. Aderem. 1995. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J. Exp. Med. 182:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison, A. C., P. Davies, and S. De Petris. 1971. Role of contractile microfilaments in macrophage movement and endocytosis. Nat. New Biol. 232:153-155. [DOI] [PubMed] [Google Scholar]
- 5.Bajno, L., X. R. Peng, A. D. Schreiber, H. P. Moore, W. S. Trimble, and S. Grinstein. 2000. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barre, A., E. J. M. Van Damme, W. J. Peumans, and P. Rougé. 1999. Homology modelling of the core domain of the endogenous lectin comitin: structural basis for its mannose-binding activity. Plant Mol. Biol. 39:969-978. [DOI] [PubMed] [Google Scholar]
- 7.Beck, K. A., J. A. Buchanan, V. Malhorta, and W. J. Nelson. 1994. Golgi spectrin: identification of an erythroid β-spectrin homolog associated with the Golgi complex. J. Cell Biol. 127:707-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertholdt, G., J. Stadler, S. Bozzaro, B. Fichtner, and G. Gerisch. 1985. Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 16:187-202. [DOI] [PubMed] [Google Scholar]
- 9.Bone, N., J. B. Millar, T. Toda, and J. Armstrong. 1998. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 8:135-144. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher, M. S. 1988. Fibroblasts on the move. J. Cell Biol. 106:235-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bretscher, M. S. 1996. Moving membrane up to the front of migrating cells. Cell 85:465-467. [DOI] [PubMed] [Google Scholar]
- 12.Burns, A. L., K. Magendzo, A. Shirva, M. Srivastava, E. Rojas, M. R. Alijani, and H. P. Pollard. 1989. Calcium channel activity of purified human synexin and structure of the human synexin gene. Proc. Natl. Acad. Sci. USA 86:3798-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantiello, H. F., A. G. Prat, J. V. Bonventre, C. C. Cunningham, J. H. Hartwig, and D. A. Ausiello. 1993. Actin-binding protein contributes to cell volume regulatory ion channel activation in melanoma cells. J. Biol. Chem. 268:4596-4599. [PubMed] [Google Scholar]
- 14.Chowdhury, S., K. W. Smith, and M. C. Gustin. 1992. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol. 11:8561-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claviez, M., K. Pagh, H. Maruta, W. Baltes, P. Fisher, and G. Gerisch. 1982. Electron microscopic mapping of monoclonal antibodies on the tail region of D. discoideum myosin. EMBO J. 1:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornillon, S., E. Pech, M. Benghezal, K. Ravanel, E. Gaynor, F. Letourneur, F. Bruckert, and P. Cosson. 2000. Phg1p is a nine-transmembrane protein superfamily member involved in dictyostelium adhesion and phagocytosis. J. Biol. Chem. 275:34287-34292. [DOI] [PubMed] [Google Scholar]
- 17.De Matteis, M. A., and J. S. Morrow. 2000. Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 113:2331-2343. [DOI] [PubMed] [Google Scholar]
- 18.Desjardins, M., N. N. Nzala, R. Corsini, and C. Rondeau. 1997. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 110:2303-2314. [DOI] [PubMed] [Google Scholar]
- 19.Dimond, R. L., D. A. Knecht, K. B. Jordan, R. A. Burns, and G. P. Livi. 1983. Secretory mutants in the cellular slime mold Dictyostelium discoideum. Methods Enzymol. 96:815-828. [DOI] [PubMed] [Google Scholar]
- 20.Döring, V., M. Schleicher, and A. A. Noegel. 1991. D. discoideum annexin VII (synexin). J. Biol. Chem. 266:17509-17515. [PubMed] [Google Scholar]
- 21.Döring, V., F. Veretout, R. Albrecht, B. Mühlbauer, C. Schlatterer, M. Schleicher, and A. A. Noegel. 1995. The in vivo role of annexin VII (synexin): characterization of an annexin VII-deficient Dictyostelium mutant indicates an involvement in Ca2+-regulated processes. J. Cell Sci. 108:2065-2076. [DOI] [PubMed] [Google Scholar]
- 22.Early, A. E., J. G. Williams, H. E. Meyer, S. B. Por, K. L. Williams, and A. A. Gooley. 1988. Structural characterization of Dictyostelium discoideum prespore-specific gene D19 and of its product, cell surface glycoprotein PsA. Mol. Cell. Biol. 8:3458-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fath, K. R., and D. R. Burgess. 1993. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. Biochemistry 31:4779-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulgenzi, G., L. Graciotti, A. L. Granata, A. Corsi, P. Fucini, A. A. Noegel, H. M. Kent, and M. Stewart. 1998. Location of the binding site of the mannose-specific lectin comitin on F-actin. J. Mol. Biol. 284:1255-1263. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa, R., and M. Fechheimer. 1994. Differential localization of α-actinin and the 30 kD actin-bundling protein in the cleavage furrow, phagocytic cup, and contractile vacuole of Dictyostelium discoideum. Cell Motil. Cytoskel. 29:46-56. [DOI] [PubMed] [Google Scholar]
- 26.Gerisch, G., D. Malchow, V. Riedel, E. Müller, and M. Every. 1972. Cyclic AMP phosphodiesterase and its inhibitor in slime mould development. Nat. New Biol. 235:90-92. [DOI] [PubMed] [Google Scholar]
- 27.Hägele, S., R. Köhler, H. Merkert, M. Schleicher, J. Hacker, and M. Steinert. 2000. Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella. Cell. Microbiol. 2:165-171. [DOI] [PubMed] [Google Scholar]
- 28.Harloff, C., Gerisch, G., and A. A. Noegel. 1989. Selective elimination of the contact site A protein of Dictyostelium discoideum by gene disruption. Genes Dev. 3:2011-2019. [DOI] [PubMed] [Google Scholar]
- 29.Hed, J. 1986. Methods for distinguishing ingested from adhering particles. Methods Enzymol. 132:198-204. [DOI] [PubMed] [Google Scholar]
- 30.Jung, E., P. Fucini, M. Stewart, A. A. Noegel, and M. Schleicher. 1996. Linking microfilaments to intracellular membranes: the actin-binding and vesicle-associated protein comitin exhibits a mannose-specific lectin activity. EMBO J. 15:1238-1246. [PMC free article] [PubMed] [Google Scholar]
- 31.Krefft, M., L. Voet, J. H. Gregg, and K. L. Williams. 1985. Use of a monoclonal antibody recognizing a cell surface determinant to distinguish prestalk and prespore cells of Dictyostelium discoideum slugs. J. Embryol. Exp. Morphol. 88:15-24. [PubMed] [Google Scholar]
- 32.Kuwayama, H., M. Ecke, G. Gerisch, and P. J. Van Haastert. 1996. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science 271:207-209. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 34.Lang, F., G. L. Busch, M. Ritter, H. Völkl, S. Waldegger, E. Gulbins, and D. Häussinger. 1998. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78:247-306. [DOI] [PubMed] [Google Scholar]
- 35.Lee, T. H., and A. D. Linstedt. 1999. Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol. Biol. Cell 10:1445-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leube, R. E., P. Kaiser, A. Seiter, R. Zimbelmann, W. W. Franke, H. Rehm, P. Knaus, P. Prior, H. Betz, and H. Reinke. 1987. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO J. 6:414-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniak, M., R. Rauchenberger, R. Albrecht, J. Murphy, and G. Gerisch. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by green fluorescent protein tag. Cell 83:915-924. [DOI] [PubMed] [Google Scholar]
- 38.Marsh, J. L., M. Erfle, and E. J. Wykes. 1984. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32:481-485. [DOI] [PubMed] [Google Scholar]
- 39.McGough, A., M. Way, and D. DeRosier. 1994. Determination of the alpha-actinin-binding site on actin filaments by cryoelectron microscopy and image analysis. J. Cell Biol. 126:433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mermall, V., J. G. McNally, and K. G. Miller. 1994. Transport of cytoplasmic particles catalysed by an unconventional myosin in living Drosophila embryos. Nature 369:560-562. [DOI] [PubMed] [Google Scholar]
- 41.Mohrs, M. R., K.-P. Janssen, T. Kreis, A. A. Noegel, and M. Schleicher. 2000. Cloning and characterization of β-COP from Dictyostelium discoideum. Eur. J. Cell Biol. 79:350-357. [DOI] [PubMed] [Google Scholar]
- 42.Noegel, A. A., G. Gerisch, F. Lottspeich, and M. Schleicher. 1990. A protein with homology to the C-terminal repeat of octopus rhodopsin and synaptophysin is a member of a multigene family in D. discoideum. FEBS Lett. 266:18-22. [DOI] [PubMed] [Google Scholar]
- 43.Noegel, A. A., and M. Schleicher. 2000. The actin cytoskeleton of Dictyostelium: a story told by mutants. J. Cell Sci. 113:759-766. [DOI] [PubMed] [Google Scholar]
- 44.Ovchinnikov, Y. A., N. G. Abdulaev, A. S. Zolotarev, I. D. Aratamonov, I. A. Bespalov, A. E. Dergachev, and M. Tsuda. 1988. Octopus rhodopsin amino acid sequence deduced from cDNA. FEBS Lett. 232:69-72. [DOI] [PubMed] [Google Scholar]
- 45.Peracino, B., J. Borleis, T. Jin, M. Westphal, J. M. Schwartz, L. Wu, E. Bracco, G. Gerisch, P. Devreotes, and S. Bozzaro. 1998. G protein β-subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J. Cell Biol. 141:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponte, E., F. Rivero, M. Fechheimer, A. Noegel, and S. Bozzaro. 2000. Severe developmental defects in Dictyostelium null mutants for actin binding proteins. Mech. Dev. 91:153-161. [DOI] [PubMed] [Google Scholar]
- 47.Rivero, F., R. Furukawa, A. A. Noegel, and M. Fechheimer. 1996. Dictyostelium discoideum cells lacking the 34,000-dalton actin-binding protein can grow, locomote, and develop, but exhibit defects in regulation of cell structure and movement: a case of partial redundancy. J. Cell Biol. 135:965-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivero, F., B. Köppel, B. Peracino, S. Bozzaro, F. Siegert, C. J. Weijer, M. Schleicher, R. Albrecht, and A. A. Noegel. 1996. The role of the cortical cytoskeleton: F-actin crosslinking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J. Cell Sci. 109:2679-2691. [DOI] [PubMed] [Google Scholar]
- 49.Schleicher, M., G. Gerisch, and G. Isenberg. 1984. New actin binding proteins from Dictyostelium discoideum. EMBO J. 3:2095-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuster, S. S., A. A. Noegel, F. Oehme, G. Gerisch, and M. I. Simon. 1996. The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 15:3880-3889. [PMC free article] [PubMed] [Google Scholar]
- 51.Skriwan, C., M. Fajardo, S. Hägele, M. Horn, M. Wagner, R. Michel, G. Krohne, M. Schleicher, J. Hacker, and M. Steinert. 2002. Various bacterial pathogens and symbionts infect the amoeba Dictyostelium discoideum. Int. J. Med. Microbiol. 291:615-624. [DOI] [PubMed] [Google Scholar]
- 52.Stratford, C. A., and S. S. Brown. 1985. Isolation of an actin binding protein from membranes of D. discoideum. J. Cell Biol. 100:727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Temesvari, L., L. Zhang, B. Fodera, K. P. Janssen, M. Schleicher, and J. A. Cardelli. 2000. Inactivation of lmpA, encoding a LIMPII-related endosomal protein, suppresses the internalization and endosomal trafficking defects in profilin-null mutants. Mol. Biol. Cell 11:2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towle, C. A., and B. V. Treadwell. 1992. Identification of a novel mammalian annexin. J. Biol. Chem. 267:5416-5423. [PubMed] [Google Scholar]
- 55.Toyohara, A., and K. Inaba. 1989. Transport of phagosomes in mouse peritoneal macrophages. J. Cell Sci. 94:143-153. [DOI] [PubMed] [Google Scholar]
- 56.Valtorta, F., F. Benfenati, and P. Greengard. 1992. Structure and function of the synapsins. J. Biol. Chem. 267:7195-7198. [PubMed] [Google Scholar]
- 57.Vogel, G., L. Thilo, H. Schwarz, and R. Steinhart. 1980. Mechanism of phagocytosis in Dictyostelium discoideum: phagocytosis is mediated by different recognition sites as disclosed by mutants with altered phagocytotic properties. J. Cell Biol. 86:456-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson, P. A. 1989. Accumulation of cAMP and calcium in S49 mouse lymphoma cells following hypoosmotic swelling. J. Biol. Chem. 264:14735-14740. [PubMed] [Google Scholar]
- 59.Weiner, O. H., J. Murphy, G. Griffiths, M. Schleicher, A. A. Noegel. 1993. The actin-binding protein comitin (p24) is a component of the Golgi apparatus. J. Cell Biol. 123:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westphal, M., A. Jungbluth, M. Heidecker, B. Mühlbauer, C. Heizer, J. M. Schwartz, G. Marriott, and G. Gerisch. 1997. Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion protein. Curr. Biol. 7:176-183. [DOI] [PubMed] [Google Scholar]