Abstract
Merozoite surface protein 1 (MSP-1) is a high-molecular-weight protein expressed on the surface of the malaria merozoite in a noncovalent complex with other protein molecules. MSP-1 undergoes a series of proteolytic processing events, but no precise biological role for the various proteolytic fragments of MSP-1 or for the additional proteins present in the complex is known. Through the use of the yeast two-hybrid system, we have isolated genes encoding proteins that interact with a region of the amino-terminal proteolytic fragment of MSP-1 from the mouse parasite Plasmodium yoelii. This analysis has led to the isolation of two sequence-related molecules, one of which is the P. yoelii homologue of MSP-7 originally described in Plasmodium falciparum. BLAST analysis of the P. falciparum database has revealed that there are six related protein molecules present in this species encoded near each other on chromosome 13. In P. falciparum, we designated these molecules MSRP-1 to -5. Analysis of the P. yoelii database indicates a similar chromosomal organization for the two genes in the mouse parasite species. The three P. falciparum sequences with the highest degree of homology to the P. yoelii sequences isolated in the two-hybrid screen have been characterized at the molecular level (MSRP-1 to -3). Expression analysis indicated that the mRNAs are expressed at various levels in the different asexual stages. Immunofluorescence studies colocalized the expression of the MSRP molecules and the amino-terminal portion of MSP-1 to the surfaces of trophozoites. In vitro binding experiments confirmed the interaction between MSRP-1, MSRP-2, and the amino-terminal region of P. falciparum MSP-1.
Despite the immunological evidence of the importance of various merozoite surface proteins as targets for protective antibodies, the biological roles of many of these molecules remain unclear, especially with regard to their roles in parasite invasion. The emerging genomic and expressed sequence tag (EST) sequencing information will certainly lead to the identification of new antigenic and chemotherapeutic targets. To date, there is no effective subunit vaccine against blood stages of malaria, and this complex, dynamic parasite has acquired resistance to most readily available antimalarial drugs (1). Therefore, new strategies must be utilized to assess the functioning of these molecules and to aid in target identification and validation. The yeast two-hybrid system is well-suited for this role, as the system allows the detection of protein-protein interactions and facilitates the direct cloning of the interacting molecules (4, 11, 20). Such studies may aid in the dissection of various structure-function relationships of key parasite molecules.
A variety of different surface antigens within the preerythrocytic, erythrocytic, and sexual stages of the parasite are under investigation as potential vaccine candidates (12, 19). Substantial effort has been dedicated to the development of a vaccine directed against the asexual blood stages responsible for the clinical manifestation of the disease. One of the most extensively studied asexual blood stage antigens is merozoite surface protein 1 (MSP-1). MSP-1 is synthesized as a large precursor molecule of approximately 200 kDa. At the time of parasite egress from the erythrocyte, the molecule undergoes proteolytic processing into 83-, 30-, 38-, and 42-kDa regions that remain associated noncovalently on the surface of the merozoite (13, 21, 22). Coincident with invasion of the red blood cell, the 42-kDa region is processed again into 33- and 19-kDa fragments, with the remainder of the MSP-1 molecule being shed (2). Previously, by use of the two-hybrid system, an interaction between the 33- and 38-kDa regions of Plasmodium yoelii MSP-1 which spans the proteolytic cleavage site between these two regions has been shown (9). The 19-kDa region remains glycosylphosphatidylinositol (GPI) anchored on the surface of the merozoite throughout the invasion process (3). Protective immune responses produced in the mouse and primate models have been primarily directed at the carboxy-terminal 19-kDa portion of MSP-1 (5, 7, 8, 10, 14, 26). In other studies, immunization with the 83-kDa region has been shown to provide partial protection upon subsequent parasite challenge in mice (28).
The precise roles and functions of the different regions of MSP-1 have not been established. In addition, other proteins have been demonstrated biochemically to associate with MSP-1 (22, 24, 27, 30). Recently, using a biochemical approach, Holder and coworkers have identified several proteins (MSP-6 and MSP-7) that copurify with a shed MSP-1 complex (24, 30). In this study, we have used the yeast two-hybrid system to examine what proteins may interact with the different regions of MSP-1. We have used this system to identify proteins that interact with the amino-terminal portion of the 83-kDa region of P. yoelii MSP-1. In this study, we isolated members of a multigene family (PyMSP-7 and PyMSRP-2, for MSP-7-related protein) that interact with the amino-terminal portion of the 83-kDa region of P. yoelii MSP-1, and we also present the analysis and characterization of the Plasmodium falciparum homologues of these gene products.
MATERIALS AND METHODS
Plasmid constructs.
A 1,296-bp fragment, corresponding to amino acids 82 to 514 of P. yoelii MSP-1 (18), was amplified from P. yoelii 17XL genomic DNA by using primers containing EcoRI sites. The resulting DNA fragment was cleaved with EcoRI and cloned directly into the EcoRI site of pBD-GAL-4-CAM (Stratagene), creating an in-frame fusion to the DNA binding domain of the yeast Gal4 protein. The resulting plasmid, termed Py83a, was sequenced and subsequently used to transform yeast strain PJ69-4a. Details of the yeast two-hybrid screen were essentially as described previously (9). Briefly, the colonies were initially selected for their ability to grow on SD-Leu− Trp− His− medium containing 3.0 mM 3-aminotriazole (3-AT). One hundred randomly chosen His+ colonies were then subjected to a second round of selection on SD-Leu− Trp− Ade− medium. Trp+ Leu+ His+ Ade+ colonies were also assayed for β-galactosidase expression. Activation domain library plasmids, rescued from positive interacting colonies, were subjected to DNA sequence analysis using a primer specific for the activation domain vector. To express the amino-terminal fragment of PfMSP-1 in Escherichia coli, a 1,203-bp fragment, corresponding to amino acids 55 to 456 of P. falciparum (strain 3D7) MSP-1, was amplified from genomic DNA by using primers containing EcoRI sites (25). The resulting EcoRI fragment was cloned into the EcoRI site of the pGEX4T-1 vector (Pharmacia Biotech), thus creating an in-frame fusion with glutathione S-transferase (GST). DNA fragments corresponding to P. falciparum MSRP-1 and MSRP-2 were amplified by PCR from P. falciparum 3D7 genomic DNA. PfMSRP-1, as a BamHI fragment, was ligated into pVR1020 (Vical) for a DNA-based vaccine. The plasmid for immunization was prepared by using the Endo-Mega kit (Qiagen). PfMSRP-1, as an EcoRI fragment, was ligated into the pGEX4T-1 vector for protein expression. PfMSRP-2 was amplified as a BamHI fragment and subsequently cloned into the poly(His) tag vector pET-28a (Novagen) for protein expression. All amplified DNA sequences and fusion junctions were confirmed by DNA sequencing.
Antisera.
Male BALB/cByJ mice 6 to 8 weeks old were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed in our AALAC-approved animal facility. The animals received three subcutaneous injections, 3 weeks apart. Each immunization consisted of 100 μg of recombinant protein or 200 μg of the DNA vaccine with Ribi adjuvant. Control animals (n = 1 per protein) were not immunized. Sera were obtained 2 weeks following the third immunization with recombinant PfMSRP-1 or PfMSRP-2. A guinea pig antiserum against a purified fragment of P. falciparum MSP-1 (amino acids 55 to 456) expressed in E. coli as a GST fusion protein was prepared commercially (Cocalico Biologicals, Inc., Reamstown, Pa.). The animals (n = 2) received three immunizations, 3 weeks apart. Each immunization consisted of 200 μg of recombinant protein with Ribi adjuvant administered subcutaneously. Sera were collected 2 weeks after the last boost. Preimmune sera were collected from the animals prior to immunization.
Sequence analysis.
A variety of different search algorithms were used to analyze the sequences obtained. TBLASTN searches were done at the National Center for Biotechnology Information (NCBI). All BLAST searches were done without the low-complexity filter and with all other settings kept at default. Searches for the P. yoelii sequences were performed using the 5× annotated sequences from the TIGR (The Institute for Genomic Research) P. yoelii database (http://www.ncbi.nih.nlm.gov/Malaria/plasmodiumblcus.html). Searches of the P. falciparum 3D7 database were done using GenBank blasts at NCBI (http://www.ncbi.nlm.nih.gov/Malaria/plasmodiumbl.html). The low-complexity filters were removed, and all NR, EST, GSS, STS, and HTGS databases were scanned.
Pattern profiles of both species of Plasmodium were performed using the InterPro database at the European Bioinformatics Institute (http://www.ebi.ac.uk/interpro/scan.html). ClustalW was used to produce the multiple alignments (http://www.ebi.ac.uk/cgi-bin/newclustalwpl), which were copied into Boxshade Hofmann, Barron (at http://bioweb.pasteur.fr/seqanal.interfaces/boxshade.html#letters) to produce the alignments. Prediction of the signal peptides was done using iPsort and Signal P (at http://hypothesiscreator.net/iPSORT/predict.cgi and http://www.cbs.dtu.dk/services/signalp/#submission, respectively) (23). The P. falciparum sequences were analyzed for the presence of introns by using the Glimmer M program at TIGR (http://www.tigr.org/softlab/glimmerm/). Preliminary sequence data were obtained from the TIGR website (http//www.tigr.org) and from the P. falciparum Sequencing Group at the Sanger Institute (ftp://ftp.sanger.ac.uk/pub).
Expression and purification of recombinant proteins.
The amino-terminal portions of PfMSP-1 (amino acids 55 to 456, termed Pf83a) and PfMSRP-1 were expressed as fusions with GST. The fusion proteins were expressed in E. coli BL-21 DE3 Codon Plus cells (Stratagene). Bacteria containing the GST-Pf83a plasmid were grown at 37°C to an optical density at 600 nm (OD600) of 1.0, then induced at 15°C overnight with 1.0 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Bacteria harboring the PfMSRP-1 plasmid were grown at 37°C to an OD600 of 1.2, then induced for 2.5 h at 37°C with 1.0 mM IPTG. Pellets of induced bacterial cultures were frozen overnight at −20°C. The cell pellets were then resuspended in 15 ml of a solution containing 1× TBS (25 mM Tris [pH 8.0], 138 mM NaCl, 27 mM KCl), 0.1% Triton X-100, and a bacterial protease inhibitor cocktail (Sigma P8465) and were lysed by sonication. GST-Pf83a and GST-PfMSRP-1 were recovered from the soluble fraction following centrifugation at 12,000 × g for 20 min. Proteins were purified using glutathione-agarose beads (Sigma) in a batchwise fashion. Elutions were preformed by rocking the matrix for 10 min at room temperature with 5 mM or 10 mM glutathione.
PfMSRP-2 was expressed in E. coli BL-21 DE3 Codon Plus cells by using the pET-28a plasmid vector (Novagen). The recombinant protein was expressed as an amino-terminal fusion with a six-histidine tag. Bacteria were grown at 37°C until the OD600 reached 0.8. Cultures were then induced overnight at 15°C with 1.0 mM IPTG. Pelleted cells were frozen overnight at −20°C. Cell pellets were then thawed and resuspended in 15 ml of 0.5 M sucrose-50 mM Tris-HCl (pH 7.4)-PIC-1 mg of lysozyme/ml. The mixture was incubated on ice for 30 min and then sonicated, and the protein was isolated from the soluble fraction prepared by centrifugation at 12,000 × g for 20 min. The protein was purified by batch elution from nitrilotriacetic acid (NTA)-agarose (Qiagen) with 50 mM sodium phosphate, 300 mM sodium chloride, and 200 mM imidazole. All recombinant proteins were concentrated using a Centricon-30 (Amicon). The purity of the protein was assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis followed by Coomassie blue staining (15). Protein concentrations were determined by a Bradford assay (protein reagent; Bio-Rad).
In vitro binding assays.
Binding assays were done as previously described (17). Briefly, 2.0 μg of recombinant His-Pf83a was mixed with 4.0 μg of recombinant GST-MSRP-1 or GST (alone) and NTA-agarose for 1.5 h at 4°C. The binding experiment was carried out in 0.1 ml of BB500 buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.1% Nonidet-P40, 10% glycerol, 1.0 mM dithiothreitol, PIC). Beads were washed three times in BB500 buffer. The interaction between Pf83a and MSRP-2 was tested by mixing 2.0 μg of recombinant GST-Pf83a or GST (alone) with 4.0 μg of His-MSRP-2 and glutathione agarose beads for 2 h at room temperature in the same buffer containing 100 mM NaCl and 0.2 mM EDTA (BB100 buffer). The glutathione agarose beads were washed three times with BB100 buffer; proteins were eluted by boiling the beads in 2× SDS sample buffer and then separated by SDS-polyacrylamide gel electrophoresis. The proteins were detected by immunoblotting and Coomassie blue staining.
Immunoblot analysis.
Purified recombinant proteins PfMSRP-1 and PfMSRP-2 were prepared, and 50 ng of each protein was analyzed by SDS-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel. Proteins were then transferred electrophoretically to a nitrocellulose membrane. Membranes were blocked for 1 h with TBS with Tween 20 and 6% nonfat dry milk. Membranes were then incubated with various sera at a 1:3,000 dilution in TBS with Tween 20, followed by goat polyclonal anti-mouse immunoglobulin G (IgG) conjugated with horseradish peroxidase. Membranes were developed using a stabilized TMB substrate (Promega).
Indirect immunofluorescence assay.
P. falciparum strain 3D7 parasites were grown using standard methods to 5% parasitemia (16, 29). Cultures were collected by centrifugation at 700 × g for 15 min. Parasitized cells were washed with an equal volume of phosphate-buffered saline (PBS) and then resuspended in an equal volume of RPMI medium. Thin blood smears were prepared, air dried, and fixed in acetone-methanol (1:1) for 20 min at −20°C. Fixed slides were air dried and stored at −20°C until use. Slides were warmed to room temperature and hydrated in PBS for 5 min. Fixed cells were incubated for 30 min at 37°C in a humidified chamber with preimmune mouse serum, immune mouse serum, preimmune guinea pig serum, or immune guinea pig serum (1:100) diluted in PBS. Slides were washed three times in PBS for 5 min with agitation. The slides were then incubated as above with a fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG (Zymed Laboratories) with 10% normal rabbit serum (Gibco) diluted 1:100 in PBS or a rhodamine-conjugated donkey anti-guinea pig IgG serum (Jackson ImmunoResearch Laboratories, Inc.) diluted 1:100 in PBS. Slides used for colocalization were incubated with the mouse sera, washed, and then incubated with the guinea pig serum. The slides were then incubated with their respective secondary antibodies. Slides were washed and mounted with Fluoromount G (Southern Biotechnology Associates, Inc., Birmingham, Ala.) and visualized by fluorescent microscopy.
RNA slot blots and Northern analysis.
P. falciparum strain 3D7 parasites were cultured by standard methods, and various stages were enriched by using sorbitol (16, 29). Parasites were treated with 5% d-sorbitol for 10 min at room temperature and then centrifuged at 500 × g. The top layer was removed, and the parasites were washed once with RPMI medium. Parasites were put back into culture and collected at 24, 36, and 48 h. The ring stages contained 20% rings, 0.5% trophozoites, 0.17% schizonts, and some merozoites. The trophozoite stages contained 7.4% trophozoites and 4.3% rings. The schizont stages contained 7.4% schizonts, 2.4% rings, and 2.9% trophozoites. For Northern analysis, parasites were grown in an asynchronous culture to 6% parasitemia. The parasites were then lysed with 0.01% saponin in PBS. RNA was isolated using the RNAgents total RNA isolation system (Promega). The RNA was precipitated with isopropanol and then again with ethanol. The sample was resuspended in diethyl pyrocarbonatae-treated water and quantitated by spectrophotometry. The integrity of the RNA was visualized on a 1.2% formaldehyde agarose gel. The RNA slot blot was prepared using a Schleicher and Schuell minifold. The RNA was mixed with 300 μl of 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.15 M formaldehyde and loaded in 15-, 10-, 5-, and 1-μg increments. The RNA for the Northern blots was transferred through capillary action. The blots were washed with 10× SSC, baked at 80°C for 2 h, and UV cross-linked for 30 s.
P. falciparum MSRP-1, MSRP-2, MSRP-3, and the MSP-1 probes were radiolabeled using the Prime It II random primer labeling kit (Stratagene). The blots were hybridized with the probe overnight at 42°C in 50% formamide, washed, and exposed to film at −80°C.
RESULTS
Isolation of the MSRPs.
The amino-terminal portion of the 83-kDa region (amino acids 82 to 514) of P. yoelii MSP-1 was fused to the yeast GAL4p DNA binding domain and used to screen a Py17XL cDNA blood stage library using the two-hybrid system (9). The transformation yielded approximately 5.0 × 106 transformants. Fifteen of the His+ Ade+,β-Gal+ clones were randomly selected, and individual activation domain plasmids were isolated. PCR and restriction analysis revealed that the clones possessed single plasmids with inserts ranging from 1,075 to 1,438 bp. Through sequence alignments with ClustalW, 12 of the 15 clones were found to be unique overlapping sequences that could be divided into two sets, with set A having 7 members and set B containing 5 members.
Analysis of the P. yoelii genome database allowed the retrieval of the full-length sequence of each of the clones. Interestingly, the two genes were located adjacent to each other on the same contig (TIGR c5m591) (see Fig. 1A). The first gene was 972 bp in length, encoding a protein of 323 amino acids, and was identified by Holder and coworkers as being homologous to P. falciparum MSP-7 (24). Hence we designated this gene PyMSP-7. The second gene was 1,227 bp in length, encoding a protein of 408 amino acids, and is structurally related to PyMSP-7; it was designated PyMSRP-2 (for MSP-7-related protein). Both of the P. yoelii genes were predicted to have an amino-terminal signal peptide by analysis with both the iPSORT and Signal P programs. No other recognizable motifs or domains were predicted by various motif search programs except the presence of a carboxy-terminal proline- and serine-rich extension in PyMSRP-2. This type of sequence extension was not seen in any of the additional related sequences seen in other plasmodium species (see below). As seen in Fig. 1B, amino acid sequence alignment revealed 25% sequence identity and 43% similarity between the proteins. In each case, several independent clones were isolated in the two-hybrid screen. The minimal interacting region deduced from the sequences of these independent clones encompassed amino acids 127 to 408 for PyMSRP-2, and amino acids 113 to 324 for PyMSP-7. We have identified additional clones of both PyMSRP-2 and PyMSP-7 from the recently sequenced P. yoelii activation domain library and have shown that removal of an additional 26 amino acids from PyMSRP-2 (presence of amino acids 143 to 408) or an additional 44 amino acids from PyMSP-7 (presence of amino acids 157 to 323) results in molecules that fail to interact with the amino-terminal end of MSP-1 in the two-hybrid system (data not shown).
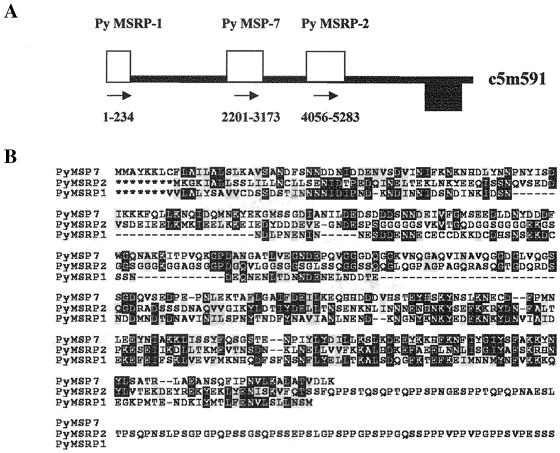
FIG. 1.
(A) Schematic representation of P. yoelii contig TIGR c5m591 indicating the relative positions of PyMSP-7, PyMSRP-2, and PyMSRP-1 (partial sequence). The contig is 10,334 bp in length. The contig is represented in this orientation to show the potential syntenic relationship to P. falciparum by using an evolutionarily conserved hypothetical protein (indicated by the black box; e value, 100−132). (B) ClustalW alignment and Boxshade representation of the amino acid sequences of PyMSP-7, PyMSRP-2, and PyMSRP-1 (partial sequence). Identical residues are indicated by white letters on a black background, whereas similar residues are indicated by black letters on a grey background. Dashes represent gaps to facilitate alignment, and asterisks were included to allow proper alignment.
Analysis of the P. yoelii EST database has revealed the partial cDNA sequence of a third related molecule, which we have termed PyMSRP-1. These clones have been rescued from the cDNA library, and the DNA sequence has been confirmed. The partial sequence (250 amino acids) showed significant homology to the two sequences described above, and a portion of the identified sequence mapped to the extreme end of the same contig (see Fig. 1A). ClustalW alignment of PyMSRP-1 with the other two molecules (Fig. 1B) revealed that the partial sequence of PyMSRP-1 is 20% identical and 39% similar to PyMSP-7 and 31% identical and 48% similar to PyMSRP-2. Efforts are under way to obtain additional sequences of this gene and determine if it also interacts with MSP-1.
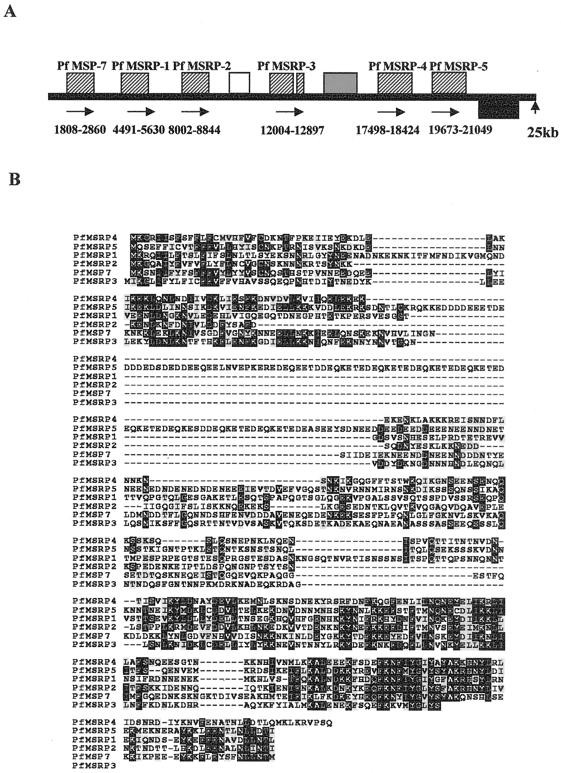
BLAST analysis of the P. falciparum 3D7 database using the isolated P. yoelii sequences revealed the presence of a related multigene family including PfMSP-7, described by Holder and coworkers (24). As depicted in Fig. 2A, there are six genes encoding molecules related to the P. yoelii sequences. We have designated the additional genes PfMSRP-1 through PfMSRP-5. The contigs were aligned in the manner shown due to the presence of a conserved sequence (e value, 10−132 for comparison of the sequences from the two species) that was transcribed from the opposite strand and was unrelated to the MSRPs. Again, the iPSORT and Signal P programs predicted that all six sequences contained a presumptive signal sequence. By using sequences derived from the database, primers were designed to amplify four of the six P. falciparum genes (PfMSP-7 and PfMSRP-1, -2 and -3) and the sequences in the database confirmed by DNA sequence analysis. Figure 2B depicts an alignment of the P. falciparum sequences. Three different gene prediction programs (Phat, Genefinder, and Glimmer M) were in agreement for the identification of all the genes except one (PfMSRP-3), the difference being the presence or absence of an intron. Of particular interest, PfMSRP-1 appears to contain two small inserts of approximately 20 to 25 amino acids relative to the other molecules, whereas PfMSRP-5 appears to contain a single large amino acid insert relative to the other molecules. As depicted in Fig. 2A, the region of highest sequence conservation is near the carboxy terminus of each molecule, but the significance of this region is not known. Further analysis of the pattern of expression of several of these genes is discussed below. Sequences related to the P. yoelii and P. falciparum gene products are also present in the P. berghii, P. vivax, and P. knowlesi databases. Table 1 shows individual pairwise comparisons of the six P. falciparum gene products and the three P. yoelii gene products (PyMSRP-1 is a partial sequence). Only PfMSRP-4 and PfMSRP-5 represent preliminary sequence information, and thus the comparative values obtained may not be absolutely correct due to the possibility of DNA sequence errors. In general, in comparing any protein with any other family member, the values range from approximately 20 to 37% identity with 39 to 60% similarity. Interestingly, the two sequences initially isolated in the P. yoelii two-hybrid screen, PyMSP-7 and PyMSRP-2, are most similar to PfMSRP-1 and PfMSRP-2.
FIG. 2.
(A) Schematic representation of P. falciparum contig Sangar MAL13P3-.0.000007 indicating the relative positions of PfMSP-7 and the PfMSRPs. The contig is 25,780 bp in length. The contig is represented in this orientation to show the potential syntenic relationship to P. yoelii by using an evolutionarily conserved hypothetical protein (indicated by a black box). A second sequence related to this molecule (28% identical) is also present on the contig (indicated by a grey box). (B). ClustalW alignment and Boxshade representation of the amino acid sequences of PfMSP-7 and the five PfMSRPs. Identical residues are indicated by white letters on a black background, whereas similar residues are indicated by black letters on a grey background. Dashes represent gaps to facilitate alignment.
TABLE 1.
TBLASTN comparison of the six P. falciparum and three P. yoelii gene productsa
| Gene product | E value (% identity, % similarity)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PfMSP-7 | PfMSRP-1 | PfMSRP-2 | PfMSRP-3 | PfMSRP-4 | PfMSRP-5 | PyMSRP-1 | PyMSRP-2 | PyMSP-7 | |
| PfMSP-7 | 0.0 | 5 × 10−29 (25, 43) | 2 × 10−28 (25, 43) | 1 × 10−26 (27, 45) | 6 × 10−25 (23, 45) | 8 × 10−34 (30, 48) | 3 × 10−8 (23, 43) | 4 × 10−17 (26, 45) | 7 × 10−18 (25, 45) |
| PfMSRP-1 | 2 × 10−26 (31, 58) | 0.0 | 2 × 10−28 (26, 42) | 1 × 10−17 (29, 54) | 2 × 10−28 (37, 58) | 6 × 10−35 (27, 49) | 9 × 10−9 (32, 53) | 1 × 10−31 (29, 45) | 7 × 10−16 (30, 48) |
| PfMSRP-2 | 8 × 10−27 (28, 44) | 1 × 10−28 (26, 42) | 0.0 | 1 × 10−20 (27, 50) | 2 × 10−31 (28, 50) | 2 × 10−23 (34, 56) | 2 × 10−7 (29, 52) | 2 × 10−19 (32, 54) | 2 × 10−19 (29, 46) |
| PfMSRP-3 | 6 × 10−25 (25, 48) | 3 × 10−15 (28, 53) | 3 × 10−18 (26, 49) | 0.0 | 9 × 10−21 (27, 41) | 2 × 10−22 (25, 48) | 6 × 10−4 (35, 57) | 8 × 10−12 (25, 41) | 2 × 10−13 (22, 43) |
| PfMSRP-4 | 5 × 10−25 (23, 45) | 1 × 10−28 (37, 58) | 3 × 10−31 (28, 50) | 2 × 10−23 (27, 42) | 0.0 | 4 × 10−33 (33, 58) | 5 × 10−10 (28, 56) | 3 × 10−18 (25, 42) | 5 × 10−14 (33, 60) |
| PfMSRP-5 | 8 × 10−21 (22, 44) | 8 × 10−35 (27, 49) | 3 × 10−33 (30, 48) | 1 × 10−24 (26, 49) | 7 × 10−33 (33, 58) | 0.0 | 3 × 10−27 (27, 47) | 4 × 10−20 (27, 49) | |
| PyMSRP-1 | 4 × 10−12 (22, 42) | 3 × 10−13 (22, 44) | 7 × 10−12 (23, 44) | 5 × 10−14 (25, 47) | 1 × 10−16 (26, 49) | 1 × 10−6 (23, 41) | 1 × 10−52 | 6 × 10−8 (31, 49) | 4 × 10−4 (20, 39) |
| PyMSRP-2 | 4 × 10−17 (26, 45) | 1 × 10−31 (29, 45) | 4 × 10−19 (32, 54) | 3 × 10−15 (24, 41) | 4 × 10−18 (25, 42) | 3 × 10−27 (27, 47) | 3 × 10−4 (30, 46) | 0.0 | 2 × 10−16 (25, 43) |
| PyMSP-7 | 6 × 10−18 (25, 45) | 6 × 10−16 (30, 48) | 2 × 10−19 (29, 46) | 3 × 10−16 (23, 44) | 6 × 10−14 (33, 60) | 2 × 10−20 (27, 49) | 2 × 10−4 (31, 57) | 2 × 10−16 (25, 43) | 0.0 |
A partial amino acid sequence was used for PyMSRP-1. Genes in the stub were used as queries in a custom blast at the Malaria Genetics/Genomic database at NCBI.
Values in italics were obtained by selective TBLASTN comparison at the P. yoelii genome database at TIGR. Boldfaced values highlight the homology to PfMSP-7 and PyMSRP-2, originally isolated in the two-hybrid screen.
Pattern of P. falciparum MSRP expression.
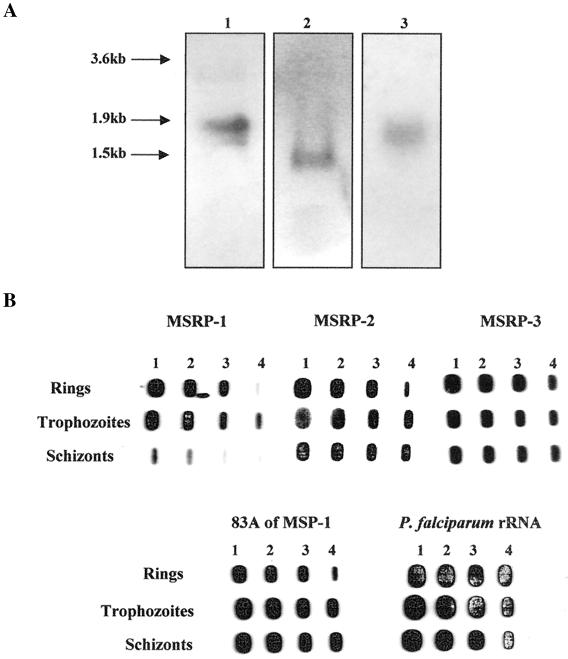
Due to the high degree of sequence conservation between the two P. yoelii gene products and several of the P. falciparum MSRPs, we have chosen to examine the expression and function of PfMSRP-1, PfMSRP-2, and PfMSRP-3 in greater detail. Northern blots were prepared from total RNA of mixed asynchronous cultures of P. falciparum strain 3D7 and examined. Figure 3A shows the approximate sizes of three P. falciparum transcripts. PfMSRP-1 was detected as a transcript of approximately 1.9 kb, PfMSRP-2 was detected as a transcript of approximately 1.5 kb, and PfMSRP-3 had a transcript of approximately 1.7 kb. RNA slot blots were prepared from asexual P. falciparum 3D7 parasites enriched for various stages. As a control, a probe corresponding to the amino-terminal end of PfMSP-1 demonstrated that MSP-1 was expressed in the three blood stages represented (see Fig. 3B). It was expressed at the lowest level during the ring stage of parasite development. The amount of RNA expression increased as the parasite developed into the trophozoite and was maintained in schizonts. Sequences from three of the P. falciparum open reading frames were used as probes to determine their patterns of expression in the various stages of parasite development. Expression of PfMSRP-2 and PfMSRP-3 followed the expression pattern of MSP-1 in P. falciparum. The expression of PfMSRP-2 was weakest in the ring stage but remained constant through the trophozoite and schizont stages. PfMSRP-3 displayed a pattern of expression similar to that of PfMSRP-2 with the exception of stronger RNA expression in the ring stage. PfMSRP-1 did not follow the same expression pattern as PfMSP-1, PfMSRP-2, or PfMSRP-3; rather, it was expressed at low levels in rings, increased in trophozoites, and then decreased in schizonts. The blot was also probed with a fragment specific for the small ribosomal subunit of P. falciparum to normalize the amount of RNA on the blots.
FIG. 3.
(A) Northern blot analysis of total RNA isolated from asynchronous cultures of P. falciparum strain 3D7. Blots were probed using PfMSRP-1 (lane 1), PfMSRP-2 (lane 2), and PfMSRP-3 (lane 3). (B) RNA slot blots containing total RNA isolated from synchronized cultures of P. falciparum strain 3D7 as described in Materials and Methods. The filters were probed with PfMSRP-1, PfMSRP-2, PfMSRP-3, and a probe corresponding to the 5′ region of MSP-1. The blot was also probed with a primer specific for the P. falciparum small ribosomal subunit to normalize loading. Lanes 1, 15.0 μg of RNA; lanes 2, 10.0 μg of RNA; lanes 3, 5.0 μg of RNA; lanes 4, 1.0 μg of RNA.
P. falciparum MSRPs localize to the parasite membrane.
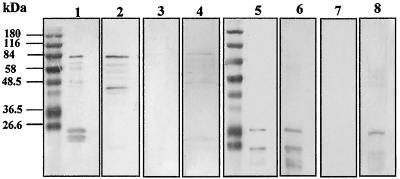
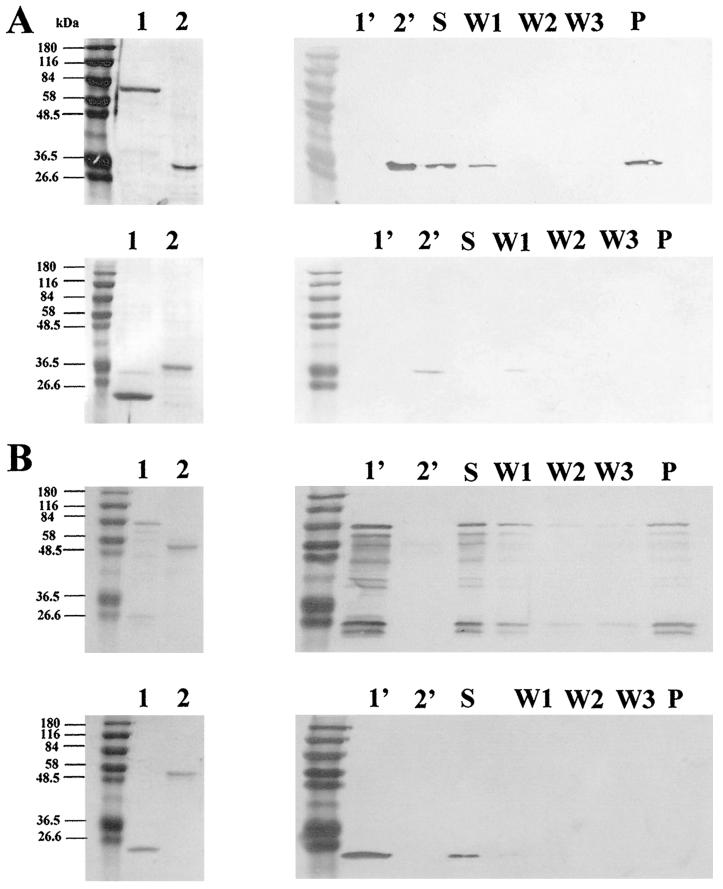
The amino-terminal portion of the 83-kDa fragment of MSP-1 in P. falciparum and two of the P. falciparum gene products (PfMSRP-1 and PfMSRP-2) were expressed as fusion proteins in E. coli. PfMSRP-1 was also used in a DNA-based immunization. PfMSRP-1 and the Pf83a portion of MSP-1 were expressed as GST fusions, and PfMSRP-2 was expressed as a His tag fusion. The proteins were purified and used to immunize BALB/cByJ mice (PfMSRP-1 and PfMSRP-2) or guinea pigs (PfMSP-1). The antisera produced in response to the immunizations with PfMSRP-1 and PfMSRP-2 recognized the recombinant fusion proteins and did not show cross-reactivity (Fig. 4). As depicted in Fig. 4, lanes 1 to 4, PfMSRP-1, produced as a GST fusion, reacts with anti-GST antisera and anti-PfMSRP-1 sera but fails to react with anti-PfMSRP-2 antisera. Similarly, Fig. 4, lanes 5 to 8, shows that the converse is also true, in that PfMSRP-2, produced as a His tag fusion, reacts with a mouse anti-His monoclonal antibody and anti-PfMSRP-2 antisera but fails to react with anti-PfMSRP-1 antisera. Both recombinant proteins failed to react with mouse preimmune antisera (data not shown). Lanes 4 and 8 are Coomassie stains of the individual proteins.
FIG. 4.
Lack of cross-reactivity of antisera directed against recombinant PfMSRP-1 and PfMSRP-2. A 50.0-ng portion of PfMSRP-1 is run on each blot in lanes 1 to 4, and 50.0 ng of PfMSRP-2 is run on each of the blots in lanes 5 to 8. Lane 1, polyclonal mouse anti-GST IgG antibody as a positive control; lanes 2 and 7, mouse anti-PfMSRP-1; lanes 3 and 6, mouse anti-PfMSRP-2; lane 4, Coomassie stain of recombinant PfMSRP-1 used in the studies; lane 5, monoclonal mouse anti-His antibody; lane 8, Coomassie stain of recombinant PfMSRP-2 used in the studies. PfMSRP-1 and PfMSRP-2 did not show any reactivity when incubated with preimmune mouse serum or with the goat anti-mouse horseradish peroxidase-conjugated secondary antibody alone. The same molecular weight marker is run in lanes 1 and 5.
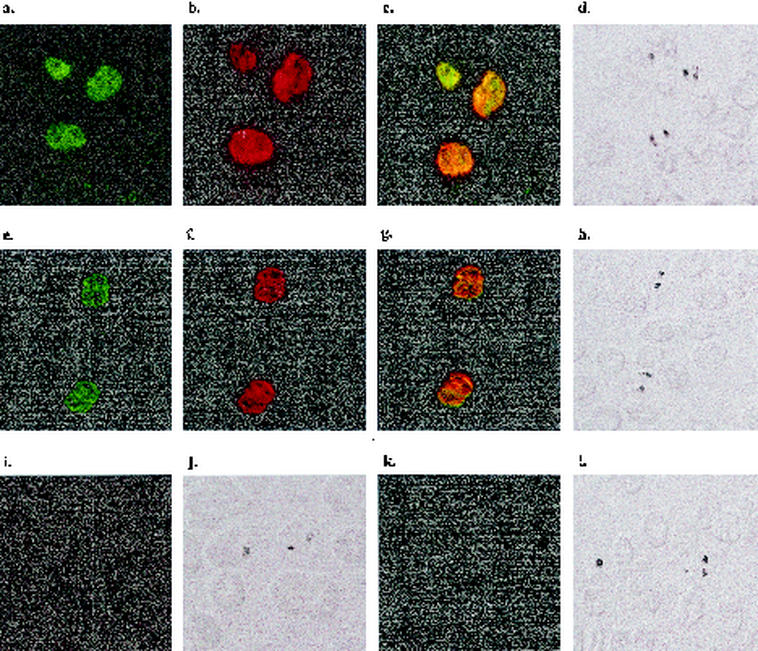
Localization of PfMSRP-1 or PfMSRP-2 and the amino-terminal portion of PfMSP-1 in parasitized erythrocytes was assessed using indirect immunofluorescence. PfMSRP-1 and PfMSRP-2 were localized to the membranes of trophozoites (Fig. 5a and e). This is similar to the pattern observed with the amino-terminal portion of PfMSP-1 (Fig. 5b and f). Figure 5c shows the overlay and colocalization of PfMSRP-1 with the amino-terminal portion of PfMSP-1, while Fig. 5g shows the overlay and colocalization of PfMSRP-2 and PfMSP-1. No fluorescent signal was obtained with preimmune guinea pig serum (Fig. 6i and j) or normal mouse serum (Fig. 6k and l). This result suggests that both molecules are expressed and are present on the surfaces of late-stage intraerythrocytic parasites at the same relative time as PfMSP-1.
FIG. 5.
Colocalization of PfMSRP-1 and PfMSRP-2 with P. falciparum MSP-1 as demonstrated by immunofluorescence of thin blood smears of asynchronous cultures of P. falciparum 3D7 prepared as described in Materials and Methods. (a) Slides were incubated with a mouse anti-PfMSRP-1 serum and a fluorescein isothiocyanate-labeled rabbit anti-mouse IgG secondary antibody. (b and f) Slides were incubated with a guinea pig anti-Pf83a serum and a rhodamine-labeled donkey anti-guinea pig IgG secondary antibody. (c) Overlay of panels a and b. (d) Bright-field image of panels a, b, and c. (e) Slides were incubated with a mouse anti-PfMSRP-2 serum and a fluorescein isothiocyanate-labeled rabbit anti-mouse IgG secondary antibody. (g) Overlay of panels e and f. (h) Bright-field image of panels e, f, and g. (i) Slides were incubated with a preimmune guinea pig serum and a rhodamine-labeled donkey anti-guinea pig IgG secondary antibody. (j) Bright-field image of panel i. (k) Slides were incubated with normal mouse serum and a fluorescein isothiocyanate-labeled rabbit anti-mouse IgG secondary antibody. (l) Bright field image of panel k.
FIG. 6.
In vitro protein binding assay of Pf MSP-1 with PfMSRP-1 and PfMSRP-2. (A) (Top) Coomassie gel (lane 1, GST-PfMSP-1; lane 2, His-PfMSRP-2) and Western blot analysis of fractions from a binding reaction using a mouse anti-His antibody (lane 1′, GST-PfMSP-1; lane 2′, His-PfMSRP-2; lane S, supernatant; lanes W1 to W3, washes 1 to 3; lane P, eluted fraction). (Bottom) Coomassie gel (lane 1, GST alone; lane 2, His-PfMSRP-2) and Western blot analysis of fractions (designated as explained above) from a binding reaction using a mouse anti-His antibody. (B) (Top) Coomassie gel (lane 1, GST-PfMSRP-1; lane 2, His-PfMSP-1) and Western blot analysis of fractions (designated as explained above) from a binding reaction using a mouse anti-GST antibody. (Bottom) Coomassie gel (lane 1, GST alone; lane 2, His-PfMSP-1) and Western blot analysis of fractions (designated as explained above) from a binding reaction using a mouse anti-GST antibody.
In vitro binding of PfMSRP-1 and Pf83a.
Although MSP-7 was previously found in the shed MSP-1 complex, the molecular details of the protein-protein interactions were not clear. Using the yeast two-hybrid system, we have demonstrated the direct interaction between two P. yoelii gene products and the amino-terminal end of the 83-kDa fragment of MSP-1. As discussed above, these molecules appear to be members of multigene families in both species. For further characterization of the interaction between P. falciparum family members and MSP-1, we expressed PfMSRP-1, PfMSRP-2, and the amino-terminal portion of PfMSP-1 in E. coli and used the purified molecules in an in vitro binding experiment. For these experiments we expressed the fragment of PfMSP-1 both as a GST-MSP-1 fusion and as a His tag-MSP-1 fusion. Purified GST-PfMSP-1 or purified GST was mixed with purified His-tagged PfMSRP-2 as described in Materials and Methods. As seen in Fig. 6A, the His-tagged PfMSRP-2 molecule was specifically “pulled down” when incubated with GST-PfMSP-1 but was absent in the control experiment using GST alone, suggesting a specific protein-protein interaction between this fragment of MSP-1 and PfMSRP-2. Similarly, in vitro binding experiments using purified GST-PfMSRP-1 and His-tagged PfMSP-1, depicted in Fig. 6B, demonstrated that these two molecules specifically interact.
DISCUSSION
Although MSP-1 has attracted significant attention as a major blood-stage vaccine candidate, very little is known about the biological role(s) of the molecule during the erythrocytic stages of infection. MSP-1 undergoes a number of proteolytic cleavages, upon merozoite egress from the infected red blood cell, to produce proteolytic fragments of 83, 30, 38, and 42 kDa. We have initiated a series of experiments, utilizing the yeast two-hybrid system, to dissect protein-protein interactions involving these regions of MSP-1. In this study, we used the amino-terminal portion of the 83-kDa fragment of P. yoelii MSP-1 to screen a blood-stage cDNA library in an activation domain vector. DNA sequence analysis of the initial 12 positive interacting clones revealed the isolation of two distinct but related gene products. Analysis of the full-length sequences of these genes obtained from the TIGR P. yoelii genome database revealed that the gene products are 25% identical and 43% similar and are separated from each other by approximately 900 bp on chromosome 13 (see Fig. 1A). Holder and coworkers previously identified one of the sequences as a homolog of P. falciparum MSP-7, a protein that copurifies with the shed MSP-1 complex. Both of the P. yoelii sequences, which we have termed PyMSP-7 and PyMSRP-2, are expressed at significant levels in the sequenced P. yoelii EST Library (31 and 20 expression tags, respectively).
BLAST searches of the EST P. yoelii library have revealed the partial sequence of a third related molecule (which we have designated PyMSRP-1), which maps to the extreme end of the same contig (see Fig. 1A), but additional genomic sequences of this region are not available at the TIGR database. Experiments are now under way to isolate additional flanking sequences in order to determine the complete sequence of this gene and to evaluate its interaction with PyMSP-1. The possible presence of additional members of the MSRP family, as seen in P. falciparum, will also be revealed by these experiments.
Searches of the P. falciparum genome database revealed the presence of a related multigene family, including PfMSP-7 (24). Similar to the observation in P. yoelii, these genes were all present on the same chromosome 13 contig (see Fig. 2A). A comprehensive pairwise comparison is presented in Table 1; it shows significant homology, but with various degrees of identity and similarity and BLAST scores. We have examined the expression patterns of several family members and have found the mRNAs to be present when the mRNA for MSP-1 is expressed. Several of the genes are reiterated in the P. falciparum EST collection. Microarray analysis using asynchronous mixed blood-stage cultures of P. falciparum strains 3D7 and HB3 suggests that the six genes are expressed at various degrees in these RNA samples (J. Morrisey and A. Vaidya, unpublished data). This finding suggests that the expression of these genes varies both qualitatively and quantitatively during the blood stages of parasite infection.
Using the yeast two-hybrid system, we have shown that the carboxy-terminal regions of both P. yoelii gene products (amino acids 127 to 408 of PyMSRP-1 and amino acids 113 to 323 of PyMSP-7) are required for interaction with the amino terminal half of the 83-kDa fragment of MSP-1. This is consistent with proposed amino-terminal processing of P. falciparum MSP-7 in the shed complex with MSP-1 and MSP-6 (24). Furthermore, we have demonstrated that the proline- and serine-rich carboxy-terminal extension of PyMSRP-2 is not required for the interaction with PyMSP-1 (data not shown). We have also demonstrated by an in vitro binding assay that PfMSRP-1 and PfMSRP-2 interact directly with MSP-1. All of the P. falciparum gene products are predicted to contain an amino-terminal signal sequence. Using specific antisera directed against PfMSRP-1 and PfMSRP-2, we have shown that these molecules are expressed on the surfaces of late-stage intraerythrocytic parasites and appear to colocalize with MSP-1. Thus, it is unclear why only PfMSP-7 was isolated biochemically in the shed MSP-1 complex (24), especially since other, related molecules are expressed at the same particular stages of the parasite life cycle. Preliminary data using [35S]-labeled parasite cultures suggest that at least PfMSRP-2 can be immunoprecipitated from the cleared culture supernatant (K. Mello, T. M. Daly, and L. W. Bergman, unpublished data). Holder and coworkers (30) have isolated an additional molecule, termed PfMSP-6, as part of the shed PfMSP-1 complex. PfMSP-6 is related to and encoded close to PfMSP-3, which is also a member of a multigene family. It is noteworthy that BLAST searches of the P. yoelii genome or EST database revealed no significant matches to PfMSP-6 or PfMSP-3. Therefore, it is unclear whether the shed MSP-1 complex of P. yoelii lacks this molecule or differs in composition from that of P. falciparum. The biological role of these sequences is presently unclear and will await further biochemical analysis and genetic analysis using gene knockout technology.
While MSP-1 presumably is involved in the process of host red cell invasion, the specifics of such interactions in the biology of the parasite remain largely a mystery. MSP-1 is synthesized in late-stage intraerythrocytic parasites and inserted into the plasma membrane, only to be proteolytically processed upon merozoite release from the red cell. Despite the proteolytic processing, the molecule remains in a complex. Previous work from our laboratory has shown a protein-protein interaction between the 42-kDa region and the 38-kDa region which spans a proteolytic cleavage site. We have suggested that this interaction is critical for stabilizing the MSP-1 complex. Previous work by others (22, 24, 27, 30) and the work presented in this study demonstrate that MSP-1 associates with additional molecules (members of a multigene family) via direct protein-protein interactions. Genetic approaches to the study of the MSRP molecules, such as gene knock-out experiments, may be difficult due to the possible redundancy of function, in that one molecule may substitute for another in its absence, and given the essential nature of MSP-1 (6). At present the precise roles of the MSRP family members are unclear. We are currently investigating whether other family members also bind MSP-1 and whether the MSRP molecules have a role in multiple parasite stages. It is possible that these molecules may play a role in the invasion process or possibly as molecular chaperones for the MSP-1 molecule. The yeast two-hybrid system has allowed the study of this complex MSP-1 molecule by defining both intra- and intermolecular protein-protein interactions. By studying these interactions, we can hope to develop a better understanding of the biological role of MSP-1 as well as approaches to disrupting the crucial function of the molecule.
Acknowledgments
This research was supported by NIH grant AI48226 to L.W.B.
We thank the scientists and funding agencies comprising the international Malaria Genome Project for making sequence data from the genome of P. falciparum (3D7) public prior to publication of the completed sequence. The Sanger Centre (Hinton, United Kingdom) provided sequence for chromosomes 1, 3 to 9, and 13, with financial support from the Wellcome Trust. A consortium composed of TIGR and the Naval Medical Research Center, sequenced chromosomes 2, 10, 11, and 14, and the genome and ESTs of P. yoelii with support from NIAID/NIH, the Burroughs Wellcome Fund, and the Department of Defense. The Stanford Genome Technology Center sequenced chromosome 12, with support from the Burroughs Wellcome Fund. The Plasmodium Genome Database is a collaborative effort of investigators at the University of Pennsylvania and Monash University (Melbourne, Australia), supported by the Burroughs Wellcome Fund.
REFERENCES
- 1.Balter, M. 2000. Malaria. Can W. H. O. roll back malaria? Science 290:430. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, M. J., J. A. Chappel, S. Shai, and A. A. Holder. 1993. A conserved parasite serine protease processes the Plasmodium falciparum merozoite surface protein-1. Mol. Biochem. Parasitol. 62:103-114. [DOI] [PubMed] [Google Scholar]
- 3.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien, C. T., P. L. Bartel, R. Sternglanz, and S. Fields. 1991. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl. Acad. Sci. USA 88:9578-9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, J. 1993. Merozoite surface antigen of Plasmodium. Parasitol. Today 9:50-54. [DOI] [PubMed] [Google Scholar]
- 6.Cowman, A. F., D. L. Baldi, J. Healer, K. E. Mills, R. A. O'Donnell, M. B. Reed, T. Triglia, M. E. Wickham, and B. S. Crabb. 2000. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Lett. 476:84-88. [DOI] [PubMed] [Google Scholar]
- 7.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein-1 induces a protective response in mice. Infect. Immun. 61:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 9.Daly, T. M., C. A. Long, and L. W. Bergman. 2001. Interaction between two domains of the P. yoelii MSP-1 protein detected using the yeast two-hybrid system. Mol. Biochem. Parasitol. 117:27-35. [DOI] [PubMed] [Google Scholar]
- 10.Etlinger, H. M., P. Caspers, H. Matile, H.-J. Schoenfeld, D. Stueber, and B. Takacs. 1991. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect. Immun. 59:3498-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-250. [DOI] [PubMed] [Google Scholar]
- 12.Good, M. F., D. C. Kaslow, and L. H. Miller. 1998. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 16:57-87. [DOI] [PubMed] [Google Scholar]
- 13.Holder, A. A., et al. 1987. Processing the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology 94:199-208. [DOI] [PubMed] [Google Scholar]
- 14.Holder, A. A., and R. R. Freeman. 1981. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 294:361-366. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 17.Lechner, M. S., G. E. Begg, D. W. Speicher, and F. J. Rauscher III. 2000. Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol. Cell. Biol. 20:6449-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis, A. P. 1989. Cloning and analysis of the gene encoding 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol. Biochem. Parasitol. 36:271-282. [DOI] [PubMed] [Google Scholar]
- 19.Long, C. A. 1993. Immunity to blood stages of malaria. Curr. Opin. Immunol. 5:548-556. [DOI] [PubMed] [Google Scholar]
- 20.Luban, J., and S. P. Goff. 1995. The yeast two hybrid system for studying protein-protein interactions. Curr. Opin. Biotechnol. 6:59-67. [DOI] [PubMed] [Google Scholar]
- 21.Lyon, J. A., R. H. Geller, J. D. Haynes, J. D. Chulay, and J. L. Weber. 1986. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc. Natl. Acad. Sci. USA 83:2989-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride, J. S., and H. G. Heidrich. 1987. Fragments of the polymorphic Mr 185,000glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, H., J. Engelbrecht, and G. Brunaks von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 24.Pachebat, J. A., I. T. Ling, M. Grainer, C. Trucco, S. Howell, D. F. Reyes, R. Gunaratne, and A. A. Holder. 2001. The 22-kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol. Biochem. Parasitol. 117:83-89. [DOI] [PubMed] [Google Scholar]
- 25.Pan, W., R. Tolle, and H. Bujard. 1995. A direct and rapid sequencing strategy for the Plasmodium falciparum antigen gene gp190 (MSA-1). Mol. Biochem. Parasitol. 73:241-244. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui, W. A., L. Q. Tam, K. J. Kramer, G. S. Hui, K. M. Yamaga, S. P. Chang, E. B. Chan, and S. C. Kan. 1987. Merozoite surface coat precursor completely protects Aotus monkeys against Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 84:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stafford, H. L., B. Gunder, A. Harris, H. Heidrich, A. A. Holder, and M. J. Blackman. 1996. A 22-kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 80:159-169. [DOI] [PubMed] [Google Scholar]
- 28.Tian, J. H., S. Kumar, D. C. Kaslow, and L. H. Miller. 1997. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein-1 of Plasmodium yoelii. Infect. Immun. 65:3032-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 30.Trucco, C., D. Fernander-Reyes, S. Howell, W. H. Stafford, T. J. Scott-Finnigan, M. Grainger, S. A. Ogun, W. R. Taylor, and A. A. Holder. 2001. The merozoite surface protein 6 gene encodes for a 36-kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112:91-101. [DOI] [PubMed] [Google Scholar]