Abstract
Yeasts respond to treatment with azoles and other sterol biosynthesis inhibitors by upregulating the expression of the ERG genes responsible for ergosterol production. Previous studies on Saccharomyces cerevisiae implicated the ROX1 repressor in ERG regulation. We report that ROX1 deletion resulted in 2.5- to 16-fold-lower susceptibilities to azoles and terbinafine. In untreated cultures, ERG11 was maximally expressed in mid-log phase and expression decreased in late log phase, while the inverse was observed for ROX1. In azole-treated cultures, ERG11 upregulation was preceded by a decrease in ROX1 RNA. These inverse correlations suggest that transcriptional regulation of ROX1 is an important determinant of ERG expression and hence of azole and terbinafine susceptibilities.
In fungi, the sterol biosynthesis pathway leads to the formation of ergosterol, with many steps in the pathway being essential (3, 16). Indeed, sterol biosynthesis inhibitors (SBIs) are widely used as antifungal agents in medicine and agriculture. The most important group of SBIs is the azoles, which target the ERG11-encoded enzyme lanosterol 14α-demethylase. As clinical use of these agents increased, so did the isolation of azole-resistant mutants, and one of the major resistance mechanisms involves constitutive ERG11 upregulation (18, 20, 24, 29). Furthermore, many strains of Candida albicans and related yeasts display “trailing” growth in azole susceptibility assays (21-23). One potential mechanism for trailing is ERG11 upregulation, and consistent with this idea, it has been shown that exposure of Candida species to SBIs upregulates the expression of ERG11 and other genes in the ergosterol biosynthesis pathway (4, 10). SBI-dependent ERG upregulation has also been demonstrated in the Saccharomyces cerevisiae genetic model (2, 6, 7, 13, 25, 26), and mutations that alter sterol biosynthesis have a similar effect (1, 2, 6, 8, 13, 19, 25, 26).
Previous studies examined regulatory elements within selected ERG promoters (1, 6, 25, 28) and the role of specific transcription factors in ERG expression (13, 28). ERG11 is positively regulated by the heme-activated transcription factor Hap1p and negatively regulated by the oxygen-responsive repressor Rox1p, while ERG9 is similarly regulated by these two factors along with Yap1p and Ino2p-Ino4p. ROX1 is autoregulated and Rox1p has a short half-life (<10 min), which are important characteristics as ROX1 overexpression may be lethal (5, 12, 30). Recently, DNA arrays have identified Rox1p-regulated genes under aerobic and anaerobic conditions (15, 27), confirming that selected ERG genes are regulated by this repressor. We show here that ROX1 is an important determinant of SBI susceptibility and that ROX1 and ERG expression are inversely correlated in response to growth phase and SBI treatment.
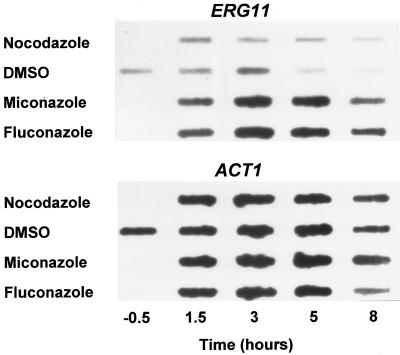
Initial studies employed two distinct azoles at concentrations three- to fivefold higher than the drugs' MICs. Log-phase cultures of S. cerevisiae W303-1A (MATaade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1) in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) at 30°C were exposed to fluconazole (20 μg/ml) or miconazole (0.3 μg/ml), and RNA levels were examined by slot blot hybridization as previously described (9). ERG11 expression was upregulated about twofold at 1.5 h and threefold at 3 h by these azoles, while a third drug with a different mechanism of action (the microtubule inhibitor nocodazole) had no effect (Fig. 1). By 5 h the control culture was in late log phase and ERG11 expression had noticeably declined; in contrast, ERG11 expression remained elevated in the presence of miconazole and fluconazole.
FIG. 1.
Azole-dependent upregulation of ERG11. RNA slot blot hybridization was used to examine ERG11 expression in strain W303-1A following treatment with nocodazole (3 μg/ml), fluconazole (20 μg/ml), miconazole (0.3 μg/ml), or dimethyl sulfide (DMSO; drug vehicle) for the times indicated in the figure. ACT1 expression was employed as a loading control. Cells were cultured in YPD medium. Probes were prepared from PCR products generated with the primers indicated in Table 1.
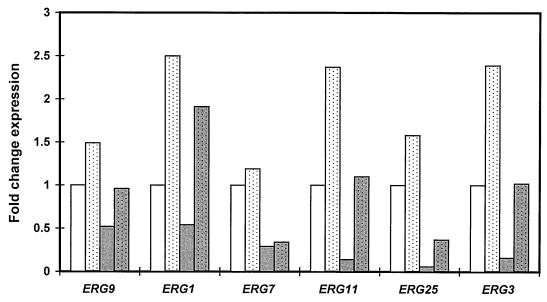
To examine potential mechanisms for ERG regulation, two S. cerevisiae strains with ROX1 deletions were studied. Strain YJN433 (derived from W303-1A and with the same genotype) was transformed with a PCR product generated with the primers ROX1ΔF and ROX1ΔR (Table 1) and the template pFA6a-His5MX6 (17). Transformants were selected on His− DOB medium (Bio 101, Carlsbad, Calif.), and PCR was used to confirm ROX1 deletion. In mid-log-phase cultures, the expression of ERG1, ERG11, and ERG3 as measured by RNA hybridization and densitometric analysis increased 2.3- to 2.5-fold in the rox1Δ strain compared to that in the YJN433 parent strain (Fig. 2). The expression of ERG9 and ERG25 also modestly increased, while ERG7 expression was essentially unchanged. In late-log-phase cultures, the expression of all ERG genes examined decreased relative to that in mid-log-phase cultures (Fig. 2). ROX1 deletion again resulted in the increased expression of all but one of these ERG genes; indeed, the increase was proportionately greater than that observed in mid-log-phase cultures. Specifically, ERG1, ERG11, ERG3, and ERG25 expression increased 3.4- to 7.9-fold, while ERG7 expression was essentially unchanged. S. cerevisiae strain RZ53-6 (MATatrp1-289 ura3-52 leu2-3,112 ade1-100) and its rox1::LEU2 derivative (obtained from R. Zitomer [5]) similarly demonstrated increased expression of the ERG genes noted above (1.7- to 2.2-fold in mid-log-phase cultures, 2.4- to 4.9-fold in late-log-phase cultures), again with the exception of ERG7 (data not shown).
TABLE 1.
Primers used in this studya
| Gene | GenBank accession no. | Position (bp) | Primer pair | Sequence (5′ to 3′) |
|---|---|---|---|---|
| ACT1 | L00026 | 1297-1811 | ACT2000F | ACCGAAGCTCCAATGAATCCAAAATCC |
| ACT2516R | GTTTGGTCAATACCAGCAGCTTCCAAA | |||
| ROX1 | X60458 | 545-1295 | ROX1F | CAATCAACAATGAATCCTAAATC |
| ROX1R | TTACCGGTGTTTGACTGCTG | |||
| ROX1, His5+ | X60458 | 503-1701 | ROX1ΔF | AGAAAATACTAATACTTCACACAAAAGAAACGCAGTAGACAATCAACGGATCCCCGGGTTAATTAA |
| ROX1ΔR | ATAATATATATAACGGAAAGAAGAAATGGAAAAAAAAAATCATTTCGGATGAATTCGAGCTCGTTTACAC | |||
| ERG11 | M18109 | 1762-2266 | ERG11F | ATTGGTATTCTTATGGGTGGTCAACATAC |
| ERG11R | CCCAATACATCTATGTCTACCACCACC | |||
| ERG1 | M64994 | 1085-1747 | ERG1F | TTGACAATTAGTTGTGATGGTAT |
| ERG1R | CTTTGGAAATATTTGAAACAACC | |||
| ERG3 | M62623 | 1275-1793 | ERG3F | CCWMTTTGAAAAACCAAATG |
| ERG3R | GAATTGACCGTAGTTGTAGTTGAA | |||
| ERG7 | U04841 | 2424-2988 | ERG7F | TATCCATACGTGGAATGTAC |
| ERG7R | TGTATAWACCTAATGCCTTAAT | |||
| ERG9 | X59959 | 778-1231 | ERG9F | AAAATGGGTAATGGTATGGC |
| ERG9R | CTTGYGGAATYGCACAAAAT |
For each gene, the GenBank accession number and the corresponding termini (base pair position) of the PCR product are indicated. Nucleotides that are complementary to the regions flanking the His5+ gene of plasmid pFA6a-His5MX6 are underlined. ROX1ΔF corresponds to the ROX1 sequence immediately upstream of the start codon; ROX1ΔR overlaps the stop codon.
FIG. 2.
ROX1 deletion results in increased expression of multiple ERG genes. RNA slot blot hybridization was used to measure the expression of the indicated ERG genes in strains YJN433 (solid bars) and YJN433 rox1Δ (stippled bars). RNA was extracted from mid-log-phase cultures (2 × 107 cells/ml) (unshaded bars) or late-log-phase cultures (1 × 108 cells/ml) (shaded bars). Autoradiographs were densitometrically scanned; values shown represent the fold change in RNA relative to the levels in mid-log YJN433 cultures, with normalization to ACT1 RNA levels.
Since ROX1 deletion resulted in the increased expression of multiple ERG genes, it was of interest to test the effects of this deletion on susceptibility to SBI antifungals that target ergosterol biosynthesis. Indeed, in both strain backgrounds described above, ROX1 deletion resulted in decreased susceptibilities to azoles and the Erg1p-targeted allylamine terbinafine (Table 2). Specifically, for the rox1Δ derivatives, 50% inhibitory concentrations (IC50s) of fluconazole, itraconazole, and miconazole increased an average of 5.6-, 16-, and 2.5-fold, respectively, and the IC50 of terbinafine increased an average of 6.5-fold. For comparison, the RZ53-6 strains were tested for sensitivity to the microtubule inhibitor nocodazole and the protein synthesis inhibitor cycloheximide; there were no significant differences associated with ROX1 deletion (data not shown).
TABLE 2.
SBI susceptibilities of S. cerevisiae strains and rox1Δ derivativesa
| Inhibitor | IC50 (μg/ml)
|
|||
|---|---|---|---|---|
| RZ53-6
|
YJN433
|
|||
| ROX1 | rox1Δ | ROX1 | rox1Δ | |
| Fluconazole | 6.2 | 23 | 1.1 | 8.3 |
| Itraconazole | 0.30 | 5.1 | 0.34 | 5.2 |
| Miconazole | 0.17 | 0.40 | 0.09 | 0.23 |
| Terbinafine | 3.0 | 11 | 0.06 | 0.56 |
Susceptibilities were determined by serial dilution in 96-well plates as previously described (10), except that YPD medium at 30°C was employed. IC50s were estimated by extrapolation from the results for the two wells spanning the 50% growth point (control absorbance × 0.5).
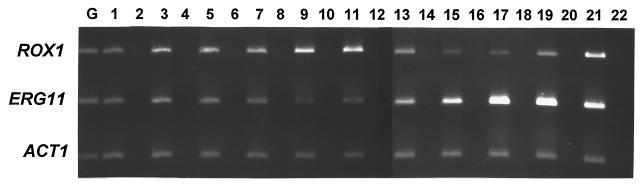
The data above indicate that ERG transcription and SBI susceptibility are regulated by ROX1. It is likely that ROX1 itself is transcriptionally regulated, since Rox1p has a short half-life (<10 min) and the ROX1 promoter includes known regulatory elements (30, 31). Reverse transcriptase PCR (RT-PCR) analysis (10) of an untreated S. cerevisiae W303-1A culture making a transition from mid-log to late log phase demonstrated that ROX1 expression increased as ERG11 expression decreased (Fig. 3, lanes 1 to 11). Specifically, the ratio of ERG11 RNA to ROX1 RNA (determined by densitometric analysis of the RT-PCR products) decreased 2-fold after 1 h but ≥10-fold after 2 or 5 h of incubation. This inverse correlation suggests that transcriptional regulation of ROX1 mediates the transcriptional regulation of ERG genes.
FIG. 3.
ERG11 expression is inversely correlated with ROX1 expression. RT-PCR (odd-numbered lanes) was used to examine ROX1, ERG11, and ACT1 expression in untreated (lanes 3 to 11) or fluconazole-treated (9 μg/ml) (lanes 13 to 21) cultures of strain W303-1A. Log-phase cultures (3 × 107 cells per ml) were sampled after incubation for 0 h (lane 1), 0.25 h (lanes 3 and 13), 0.5 h (lanes 5 and 15), 1 h (lanes 7 and 17), 2 h (lanes 9 and 19), or 5 h (lanes 11 and 21). Control reactions which lacked RT (even-numbered lanes) confirmed that the observed bands were not due to genomic DNA contamination. Lane G is a positive control from a reaction mixture containing genomic DNA. Amplification was for 23 (ROX1 and ERG11) or 25 (ACT1) cycles, which was within the logarithmic range. Primers are indicated in Table 1.
Consistent with results of previous studies, treatment of S. cerevisiae with fluconazole upregulated ERG11 expression (Fig. 3, lanes 1 and 13 to 21). This increase was maximal after 1 to 2 h of treatment. Conversely, fluconazole treatment resulted in decreased ROX1 expression that appeared to precede (minimum at 30 min) this increase in ERG11 RNA. Consequently, the ratio of ERG11 RNA to ROX1 RNA increased ninefold by 1 h after treatment. (By 5 h, however, the fluconazole-treated culture had resumed growth and entered late log phase, resulting once again in increased ROX1 expression and decreased ERG11 expression.) Similar results were obtained in RNA hybridization studies of strain YJN433 (data not shown).
The SBI-dependent ERG upregulation demonstrated here and previously is predicted to reduce SBI susceptibility, just as constitutive ERG upregulation (due to currently uncharacterized mutations) contributes to SBI resistance in many clinical isolates. Understanding the mechanism behind this SBI response could lead to much needed improvements in antifungal therapy and a greater understanding of resistance mechanisms. Since disruption of ergosterol biosynthesis by SBI treatment or genetic lesion at any of several different steps in the pathway results in the upregulation of multiple ERG genes, there is likely to be a common mechanism for their transcriptional control. The data presented here, combined with those from previous studies, indicate that the repressor Rox1p is a promising candidate. Potential Rox1p binding sites (31) can be identified upstream of most ERG promoters; specifically, 17 of 22 ERG promoters but only 5 of 22 randomly selected non-ERG promoters include at least one copy (allowing for two mismatches) of the YYYATTGTTCTC consensus binding site (unpublished data).
A C. albicans gene, RFG1, with limited homology to ROX1 was recently reported; however, its deletion did not alter the expression of oxygen-regulated genes but rather blocked hypha-to-yeast morphogenesis (11, 14). C. albicans may therefore regulate its ERG genes by mechanisms that are at least partially distinct from those employed by S. cerevisiae. Other clinically important species, such as Candida glabrata, are more closely related to S. cerevisiae and even more problematic in terms of azole resistance. Examining the role of ROX1 homologs in these species would therefore be of interest.
Acknowledgments
This study was funded by National Institutes of Health grants AI46768 and AI47718 to T.D.E. and HL67401 to J.T.N.
We thank R. Zitomer for providing strains.
REFERENCES
- 1.Arthington-Skaggs, B. A., D. N. Crowell, H. Yang, S. L. Sturley, and M. Bard. 1996. Positive and negative regulation of a sterol biosynthetic gene (ERG3) in the post-squalene portion of the yeast ergosterol pathway. FEBS Lett. 392:161-165. [DOI] [PubMed] [Google Scholar]
- 2.Bammert, G. F., and J. M. Fostel. 2000. Genome-wide expression patterns in Saccharomyces cerevisiae: comparison of drug treatments and genetic alterations affecting biosynthesis of ergosterol. Antimicrob. Agents Chemother. 44:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daum, G., N. D. Lees, M. Bard, and R. Dickson. 1998. Biochemistry, cell biology and molecular biology of lipids in Saccharomyces cerevisiae. Yeast 14:1471-1510. [DOI] [PubMed] [Google Scholar]
- 4.De Backer, M. D., T. Ilyina, X.-J. Ma, S. Vandoninck, W. H. M. L. Luyten, and H. Vanden Bossche. 2001. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chemother. 45:1660-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deckert, J., R. Perin, B. Balasubramanian, and R. S. Zitomer. 1995. Multiple elements and auto-repression regulate ROX1, a repressor of hypoxic genes in Saccharomyces cerevisiae. Genetics 139:1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimster-Denk, D., and J. Rine. 1996. Transcriptional regulation of a sterol-biosynthetic enzyme by sterol levels in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:3981-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimster-Denk, D., J. Rine, J. Phillips, S. Scherer, P. Cundiff, K. DeBord, D. Gilliland, S. Hickman, A. Jarvis, L. Tong, and M. Ashby. 1999. Comprehensive evaluation of isoprenoid biosynthesis regulation in Saccharomyces cerevisiae utilizing the Genome Reporter Matrix™. J. Lipid Res. 40:850-860. [PubMed] [Google Scholar]
- 8.Geber, A., C. A. Hitchcock, J. E. Swartz, F. S. Pullen, K. E. Marsden, K. J. Kwon-Chung, and J. E. Bennett. 1995. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal sensitivity. Antimicrob. Agents Chemother. 39:2708-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry, K. W., M. C. Cruz, S. K. Katiyar, and T. D. Edlind. 1999. Antagonism of azole activity against Candida albicans following induction of multidrug resistance genes by selected antimicrobial agents. Antimicrob. Agents Chemother. 43:1968-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry, K. W., J. T. Nickels, and T. D. Edlind. 2000. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 44:2693-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadosh, D., and A. D. Johnson. 2001. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell. Biol. 21:2496-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastaniotis, A. J., and R. S. Zitomer. 2000. Rox1 mediated repression. Oxygen dependent repression in yeast. Adv. Exp. Med. Biol. 475:185-195. [PubMed] [Google Scholar]
- 13.Kennedy, M. A., R. Barbuch, and M. Bard. 1999. Transcriptional regulation of the squalene synthase gene (ERG9) in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1445:110-122. [DOI] [PubMed] [Google Scholar]
- 14.Khalaf, R. A., and R. S. Zitomer. 2001. The DNA binding protein Rfg1 is a repressor of filamentation in Candida albicans. Genetics 157:1503-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwast, K. E., L.-C. Lai, N. Menda, D. T. James III, S. Aref, and P. V. Burke. 2002. Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J. Bacteriol. 184:250-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lees, N. D., M. Bard, and D. R. Kirsch. 1999. Biochemistry and molecular biology of sterol synthesis in Saccharomyces cerevisiae. Crit. Rev. Biochem. Mol. Biol. 34:33-47. [PubMed] [Google Scholar]
- 17.Longtine, M. S., A. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 18.Lupetti, A., R. Danesi, M. Campa, M. Del Tacca, and S. Kelly. 2002. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8:76-81. [DOI] [PubMed] [Google Scholar]
- 19.M'Baya, B., M. Fegueur, M. Servouse, and F. Karst. 1989. Regulation of squalene synthetase and squalene epoxidase activities in Saccharomyces cerevisiae. Lipids 24:1020-1023. [DOI] [PubMed] [Google Scholar]
- 20.Perea, S., J. L. López-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillán, M. Martínez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhnke, M., A. Schmidt-Westhausen, E. Engelmann, and M. Trautmann. 1996. Comparative evaluation of three antifungal susceptibility test methods for Candida albicans isolates and correlation with response to fluconazole therapy. J. Clin. Microbiol. 34:3208-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 25.Smith, S. J., J. H. Crowley, and L. W. Parks. 1996. Transcriptional regulation by ergosterol in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 16:5427-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soustre, I., P.-H. Dupuy, S. Silve, F. Karst, and G. Loison. 2000. Sterol metabolism and ERG2 gene regulation in the yeast Saccharomyces cerevisiae. FEBS Lett. 470:102-106. [DOI] [PubMed] [Google Scholar]
- 27.Ter Linde, J. J. M., and H. Y. Steensma. 2002. A microarray-assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae. Yeast 19:825-840. [DOI] [PubMed] [Google Scholar]
- 28.Turi, T. G., and J. C. Loper. 1992. Multiple regulatory elements control expression of the gene encoding the Saccharomyces cerevisiae cytochrome P450, lanosterol 14α-demethylase (ERG11). J. Biol. Chem. 267:2046-2056. [PubMed] [Google Scholar]
- 29.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitomer, R. S., M. P. Limback, A. M. Rodriguez-Torres, B. Balasubramanian, J. Deckert, and P. M. Snow. 1997. Approaches to the study of Rox1 repression of the hypoxic genes in the yeast Saccharomyces cerevisiae. Methods 11:279-288. [DOI] [PubMed] [Google Scholar]
- 31.Zitomer, R. S., and C. V. Lowry. 1992. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol. Rev. 56:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]