Abstract
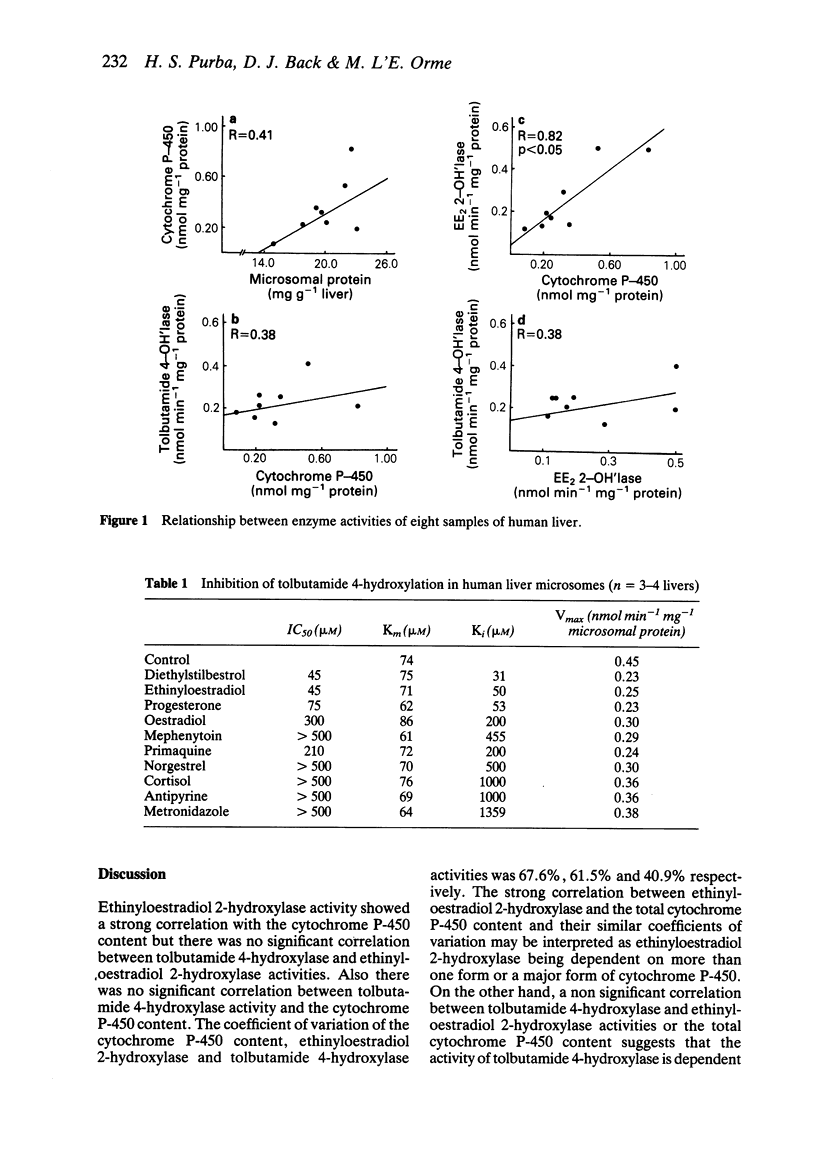
Eight samples of human liver have been characterised for microsomal protein content, cytochrome P-450 content, tolbutamide 4-hydroxylase and ethinyloestradiol 2-hydroxylase activities. Cytochrome P-450 content correlated significantly with ethinyloestradiol 2-hydroxylase activity but not with tolbutamide 4-hydroxylase activity. There was no significant correlation between ethinyloestradiol 2-hydroxylase and tolbutamide 4-hydroxylase activities. The maximum tolbutamide 4-hydroxylase activity was 0.45 nmol min-1 mg-1 microsomal protein, with a Km value of 74 microM. A number of compounds were tested for their ability to inhibit tolbutamide metabolism. All the compounds showing inhibition were either non-competitive or mixed non-competitive inhibitors of tolbutamide 4-hydroxylation. These studies suggest that tolbutamide is metabolised by an isozyme of cytochrome P-450 which appears to be distinct from those isozymes metabolising many other drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. B., Park B. K., Tjia J. F., Newby S. N. Sulphaphenazole and drug oxidation in man. Br J Clin Pharmacol. 1983 Oct;16(4):460–461. doi: 10.1111/j.1365-2125.1983.tb02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back D. J., Sutcliffe F., Tjia J. F. Tolbutamide as a model drug for the study of enzyme induction and enzyme inhibition in the rat. Br J Pharmacol. 1984 Mar;81(3):557–562. doi: 10.1111/j.1476-5381.1984.tb10109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis A. R., Hampden C. E., Murray S., Beaune P., Davies D. S. Attempts to phenotype human liver samples in vitro for debrisoquine 4-hydroxylase activity. Br J Clin Pharmacol. 1985 Jun;19(6):721–729. doi: 10.1111/j.1365-2125.1985.tb02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN L. K., HANSEN J. M., KRISTENSEN M. SULPHAPHENAZOLE-INDUCED HYPOGLYCAEMIC ATTACKS IN TOLBUTAMIDE-TREATED DIABETICS. Lancet. 1963 Dec 21;2(7321):1298–1301. doi: 10.1016/s0140-6736(63)90847-x. [DOI] [PubMed] [Google Scholar]
- Darby F. J., Grundy R. K., Evans D. A. Apparent Michaelis constants for the metabolism of (ureyl- 14 C)tolbutamide by human liver microsomal preparations. Biochem Pharmacol. 1972 Feb 1;21(3):407–414. doi: 10.1016/0006-2952(72)90352-8. [DOI] [PubMed] [Google Scholar]
- Davies D. S., Kahn G. C., Murray S., Brodie M. J., Boobis A. R. Evidence for an enzymatic defect in the 4-hydroxylation of debrisoquine by human liver. Br J Clin Pharmacol. 1981 Jan;11(1):89–91. doi: 10.1111/j.1365-2125.1981.tb01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer P., Balant L., Courvoisier F., Kupfer A., Kubli A., Gorgia A., Fabre J. The genetic control of bufuralol metabolism in man. Eur J Drug Metab Pharmacokinet. 1982 Jan-Mar;7(1):73–77. doi: 10.1007/BF03189547. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M. Defective oxidation of drugs: pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1982 Jan-Feb;7(1):1–22. doi: 10.2165/00003088-198207010-00001. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Distlerath L. M., Reilly P. E., Wolff T., Shimada T., Umbenhauer D. R., Martin M. V. Human-liver cytochromes P-450 involved in polymorphisms of drug oxidation. Xenobiotica. 1986 May;16(5):367–378. doi: 10.3109/00498258609050245. [DOI] [PubMed] [Google Scholar]
- Hansen J. M., Christensen L. K. Drug interactions with oral sulphonylurea hypoglycaemic drugs. Drugs. 1977 Jan;13(1):24–34. doi: 10.2165/00003495-197713010-00003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minder E. I., Meier P. J., Müller H. K., Minder C., Meyer U. A. Bufuralol metabolism in human liver: a sensitive probe for the debrisoquine-type polymorphism of drug oxidation. Eur J Clin Invest. 1984 Jun;14(3):184–189. doi: 10.1111/j.1365-2362.1984.tb01121.x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Pond S. M., Birkett D. J., Wade D. N. Mechanisms of inhibition of tolbutamide metabolism: phenylbutazone, oxyphenbutazone, sulfaphenazole. Clin Pharmacol Ther. 1977 Nov;22(5 Pt 1):573–579. doi: 10.1002/cpt1977225part1573. [DOI] [PubMed] [Google Scholar]
- Purba H. S., Back D. J., Breckenridge A. M. Inhibition of estrogen 2-hydroxylase. J Steroid Biochem. 1986 May;24(5):1091–1093. doi: 10.1016/0022-4731(86)90365-1. [DOI] [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Poffenbarger P. L. Tolbutamide pharmacogenetics and the UGDP controversy. JAMA. 1979 Jul 6;242(1):45–48. [PubMed] [Google Scholar]
- Spina E., Birgersson C., von Bahr C., Ericsson O., Mellström B., Steiner E., Sjöqvist F. Phenotypic consistency in hydroxylation of desmethylimipramine and debrisoquine in healthy subjects and in human liver microsomes. Clin Pharmacol Ther. 1984 Nov;36(5):677–682. doi: 10.1038/clpt.1984.239. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Ikeda G. J. The metabolic fate of tolbutamide in man and in the rat. J Med Chem. 1966 Jul;9(4):507–510. doi: 10.1021/jm00322a014. [DOI] [PubMed] [Google Scholar]


