Abstract
Context
Previous studies have suggested that subclinical abnormalities in TSH levels are associated with detrimental effects on the cardiovascular system.
Objective
To determine the relationship between baseline thyroid status and incident atrial fibrillation, incident cardiovascular disease, and mortality in older men and women not taking thyroid medication.
Design, Setting, and Patients
Participants were 3,233 US community-dwelling individuals aged 65 or over with baseline serum TSH levels who were enrolled in 1989–1990 in the Cardiovascular Health Study (CHS), a large, prospective cohort study.
Main Outcome Measures
Incident atrial fibrillation, coronary heart disease, cerebrovascular disease, cardiovascular death, and all-cause death assessed through June, 2002.
Results
Analyses are reported for four groups defined according to thyroid function test results: subclinical hyperthyroidism, euthyroidism, subclinical hypothyroidism, and overt hypothyroidism. Individuals with overt thyrotoxicosis were excluded due to small numbers. Eighty-two percent of participants were euthyroid, 15% had subclinical hypothyroidism, 1.6% were overtly hypothyroid, and 1.5% had subclinical hyperthyroidism. After exclusion of those with prevalent atrial fibrillation, individuals with subclinical hyperthyroidism had a greater incidence of atrial fibrillation compared to the euthyroid group (67 events vs. 31 events per 1,000 person-years; p<.001, and an adjusted hazard ratio [AHR] of 1.98; 95% confidence interval [CI] 1.29–3.03). No differences were seen between the subclinical hyperthyroidism group and euthyroid group for incident coronary heart disease, cerebrovascular disease, cardiovascular death, or all-cause death. Likewise, there were no differences between the subclinical hypothyroidism or overt hypothyroidism groups and the euthyroid group for cardiovascular outcomes or mortality. Specifically, individuals with subclinical hypothyroidism had an AHR of 1.07 (95% CI, 0.90–1.28) for incident coronary heart disease.
Conclusions
Our data show an association between subclinical hyperthyroidism and the development of atrial fibrillation, but do not support the hypothesis that unrecognized subclinical hyperthyroidism or subclinical hypothyroidism is associated with other cardiovascular disorders or mortality.
Keywords: Thyroid disease, cardiovascular disease, subclinical hyperthyroidism, subclinical hypothyroidism, cholesterol, atrial fibrillation, myocardial infarction, mortality, elderly, Cardiovascular Health Study
Cardiovascular diseases (CVD) are the most common cause of mortality, primarily affecting older adults. Heart disease causes nearly 700,000 deaths annually in the US.1 Although established risk factors explain most cardiac risks,2 significant attention has been focused on alternative biochemical markers to assist in identifying those at risk for a clinical cardiac event.3 Previous studies have suggested that abnormal levels of thyroid stimulating hormone (TSH) may represent a novel cardiac risk factor.4–8
Thyroid hormone excess and deficiency are common,9,10 as well as readily diagnosed and treated. Even mildly altered thyroid status reportedly affects serum cholesterol levels,11 heart rhythm12 and rate,13 ventricular function,14,15 risk of coronary artery disease,4,8,16 and cardiovascular mortality.5 However, the relationship between abnormal thyroid function and cardiovascular outcomes remains unclear, as prior studies reporting a relationship included individuals with overt thyroid disease when categorizing subclinical thyroid disease;12 included individuals taking thyroid hormone;8,17 inadequately adjusted for important confounders or initiation of thyroid hormone replacement;4,5,8,17 or used poorly characterized cardiovascular endpoints.4 Furthermore, no clinical trials have been performed to examine whether correcting thyroid dysfunction results in improvement of cardiovascular outcomes. This lack of experimental evaluation has resulted in continued controversy, as detailed in recommendations by both an expert panel and the US Preventive Services Task Force.18,19
Using data from a large cohort study representative of community-dwelling individuals sixty-five and older, we have tested the hypothesis that abnormal thyroid status is associated with increased cardiovascular risk and mortality in individuals with unrecognized thyroid dysfunction.
METHODS
Study Population
These analyses are based on data in the Cardiovascular Health Study (CHS).20 The CHS is a population-based, longitudinal study of risk factors for the development of CVD in 5,888 adults 65 years and older. Enrollment of an original cohort of 5,201 adults occurred between May 1989 and June 1990, and an additional cohort of 687, predominantly African Americans, was enrolled in 1992–1993. Eligible individuals were identified from an age- and gender-stratified random sample of the Medicare eligibility rosters in four US communities: Washington County, Maryland; Pittsburgh (Allegheny County), Pennsylvania; Sacramento County, California; and Forsyth County, North Carolina. To be eligible, individuals had to be non-institutionalized; expecting to remain in the area for the following three years; not under active treatment for cancer; not wheelchair-bound in the home; not requiring a proxy respondent at entry; and capable of providing consent. Household members of the sampled individual were recruited, if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
At the initial visit, a detailed medical history (including medication history), physical examination, and health status assessment that included any evidence of vascular disease were performed. Blood was drawn after a 12-hour fast and serum was frozen in −70° C freezers for future investigations.
Thyroid Hormone Analyses
Thyroid function tests were performed at the Nuclear Medicine In Vitro Laboratory of the Johns Hopkins Hospital from 1991 through 1993 on a sub-sample of individuals from the original cohort, selected according to availability of stored serum for analysis. Serum TSH concentration was measured using a chemiluminescent immunometric assay (LumaTag hTSH, Nichols Institute, San Juan Capistrano, CA) with a functional sensitivity of 0.008 mU/L. Of the 3,699 samples tested, there was sufficient serum for analysis in 3,678 (99%). Free thyroxine (free T4) concentrations were measured in individuals with serum TSH levels <0.10 or >4.50 mU/L, for the 95% of samples with sufficient serum for this additional test, which was performed with a direct, monoclonal antibody assay (Amerlex-MAB, Amersham International, UK) with a normal range of 0.7 to 1.7 ng/dL.
Participants from the CHS in whom thyroid function testing was performed were more likely to be female than those in the untested group. The mean ages of the tested and untested groups were not different, nor were there differences in race, income, thyroid medication use, or prevalent CVD.
Classification by Thyroid Status
Study participants were classified into one of the following five groups based on their thyroid function tests:
Overt thyrotoxicosis was defined as a TSH concentration <0.10 mU/L with an elevated free thyroxine level (n=2).
Subclinical hyperthyroidism was defined as a TSH concentration ≥ 0.10 and <0.45 mU/L (n=40), or <0.10 U/L with a normal free T4 concentration (n=7).
Euthyroidism was defined as a normal TSH concentration (0.45 to 4.50 mU/L) (n=2,639).
Subclinical hypothyroidism was defined as a TSH concentration >4.50 and <20 mU/L with a normal free T4 concentration (n=496).
Overt hypothyroidism was defined as a TSH ≥ 20 mU/L (n=33) or TSH concentration >4.50 mU/L with a free T4 concentration level below normal (<0.70 ng/dL) (n=18).
Because our primary study question pertained to unrecognized thyroid function testing abnormalities, participants taking thyroid hormone preparations at baseline were excluded from the above categorization (n=339), as were individuals taking other medications that could affect thyroid testing, including antithyroid drugs and corticosteroids (n=78). No patients were taking amiodarone at the baseline examination. One participants whose testing suggested nonthyroidal illness (a low TSH and low free T4) was excluded. Those with definite (n=2) or possible (n=2) overt thyrotoxicosis were also excluded due to the small number in this category.
Ascertainment of Events
The events studied were atrial fibrillation, coronary heart disease, cerebrovascular disease, and death (cardiovascular and all-cause). Atrial fibrillation at baseline was self-reported or determined with a twelve-lead resting electrocardiogram (ECG) or Holter monitor. Incident atrial fibrillation was determined from self-report, annual ECG, or hospital discharge codes 427.3, 427.31, or 427.32. Coronary heart disease was defined by the occurrence of angina, myocardial infarction, coronary angioplasty, or bypass surgery. Cerebrovascular disease was defined as a cerebrovascular accident or a transient ischemic attack. Subclinical CVD, which has been shown to be an independent predictor of CVD in the CHS population,21 was defined as any one of the following: ankle arm index ≤0.9, common or internal carotid wall thickness >80th percentile, carotid stenosis >25%, major ECG abnormalities, Rose questionnaire claudication- or angina-positive, or abnormal ejection fraction or wall motion on echocardiogram, in the absence of clinical CVD. Cardiovascular deaths were defined as those from atherosclerosis (including peripheral vascular disease), coronary heart disease, cerebrovascular events, and other cardiovascular causes.
Participants were contacted semi-annually regarding hospitalizations or new occurrences of cardiovascular events. The full details of the surveillance and ascertainment of events in the CHS have been published previously.22 Provisional diagnoses of coronary heart disease and cerebrovascular disease were reviewed and adjudicated at periodic meetings of the study's morbidity and mortality subcommittee, including investigators from each field center and the coordinating center. All outcomes were adjudicated except atrial fibrillation. Information about deaths was obtained from reviews of medical records, death certificates, autopsy reports, and coroners’ reports. Ascertainment of mortality in the CHS is 100 percent. The incident events in this report occurred after baseline and through June 30, 2002, with a mean duration of follow-up of 12.5 years.
Assessment of Covariates
Thyroid and lipid-lowering medication use was assessed annually via medication bottle examination. Information on race was collected in CHS to examine racial differences in CVD risk. Race was self-described as white, black, American Indian/Alaskan native, Asian/Pacific Islander, or other after reviewing a card that displayed these options. Fasting total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured directly and standardized according to CDC guidelines, with low-density lipoprotein (LDL) cholesterol calculated according to the Friedewald equation.23 Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or self-report of hypertension and antihypertensive medication use. Diabetes was defined as a fasting glucose ≥126 mg/dL, or use of insulin or an oral hypoglycemic medication. Impaired fasting glucose was defined as a fasting glucose ≥100 mg/dL. C-reactive protein was measured using an enzyme-linked immunosorbent assay (CHS Blood Laboratory, Colchester, VT).
Statistical Analysis
Study participants’ baseline characteristics were summarized according to thyroid status and compared against those in the euthyroid group using a t-test or Chi-Square test as appropriate. Incidence rates of cardiovascular and total mortality and first occurrence of coronary heart disease or cerebrovascular disease were calculated by dividing the total number of each event by person-years at risk among participants without clinical CVD or atrial fibrillation at baseline. Incidence rates of atrial fibrillation were calculated similarly, excluding only participants with atrial fibrillation at baseline from the risk set. Kaplan-Meier analysis was used to study the cumulative incidence of atrial fibrillation, cerebrovascular disease, coronary heart disease, and mortality by thyroid category across the 13 years of follow-up.
Multivariable Cox regression models were used to estimate the hazard ratio of each thyroid disease group compared to the euthyroid group, adjusting for other baseline risk factors or potential confounders and thyroid medication use during follow-up. Participants who died or were lost to follow-up before having an event were censored at the date of death or last contact. Both incident and recurrent coronary heart disease and cerebrovascular events were considered, but only incident atrial fibrillation was modeled. Models were originally stratified by CVD status at baseline and by sex. Results were consistent across strata and, when combined, statistical tests for interactions between thyroid group and sex or thyroid group and baseline CVD were not significant. Final models included men and women, and participants with and without CVD at baseline. Models were done in stages, adjusting first for age, sex, disease status at baseline and thyroid medication use during follow-up as a time-dependent covariate. For atrial fibrillation, the second and final stage of analysis added black race, left atrial size, systolic blood pressure, fasting glucose, valvular disease history, and diuretics or beta-adrenergic blocking agent use, factors that have previously been shown to predict atrial fibrillation in CHS.24 For other outcomes, a second adjustment stage added black race, smoking status (never, former or current) and diabetes. CVD risk factors that could have been mediated via thyroid dysfunction were added in the final adjustment stage, and included LDL cholesterol, lipid-lowering medications, hypertension, body mass index, and C-reactive protein. Results from the second and final models were compared to assess for overadjustment in the final model. Separate models that incorporated thyroid hormone use as a time-dependent covariate and that censored at the time of thyroid hormone use were examined, with similar results; the model incorporating the time-dependent covariate is shown. All analyses were done using SPSS for Windows, version 13 and STATA version 9.
RESULTS
Baseline characteristics
At study entry, 2,639 individuals (82%) were euthyroid. Subclinical hypothyroidism had the highest prevalence of any thyroid testing abnormality (n=496; 15%), with fewer participants displaying results consistent with overt hypothyroidism (n=51; 1.6%) or subclinical hyperthyroidism (n=47; 1.5%). Ages were similar across all thyroid status categories in these elderly subjects, with a mean age of 72.7 years (Table 1). Women were more likely to have subclinical thyroid dysfunction than men, achieving statistical significance for the comparison between the subclinical hypothyroid and euthyroid groups.
Table 1.
Baseline Characteristics By Thyroid Status in the Cardiovascular Health Study*
| Total (n=3233) | Subclinical Hyperthyroid (n=47) | Euthyroid (n=2639) | Subclinical Hypothyroid (n=496) | Hypothyroid (n=51) | |
|---|---|---|---|---|---|
| TSH (mU/L) | 3.27 ± 4.26 | 0.25 ± 0.13 | 2.20 ± 0.99 | 6.67 ± 2.54 | 28.1 ± 15.7 |
| Free T4 (ng/dL) | 1.26 ± 0.24 | 0.99 ± 0.16 | 0.63 ± 0.19 | ||
| Age, y† | 72.7 ± 5.6 | 73.9 ± 6.8 | 72.6 ± 5.6 | 73.2‡± 5.6 | 73.1 ± 6.0 |
| Female | 1926 (59.6) | 32 (68.1) | 1543 (58.5) | 321 (64.7)‡ | 30 (58.8) |
| White Race | 3064 (94.8) | 45 (95.7) | 2495 (94.5) | 476 (96.0) | 48 (94.1) |
| BMI (kg/m2) | 26.2 ± 4.4 | 27.5‡± 5.4 | 26.2 ± 4.4 | 26.4 ± 4.8 | 27.0 ± 4.5 |
| Current or former smoking | 1661 (51.4) | 25 (53.2) | 1377 (52.2) | 235 (47.5) | 24 (47.1) |
| Cholesterol | |||||
| Total (mg/dL) | 215 ± 39 | 203‡± 41 | 215 ± 38 | 214 ± 41 | 228‡± 54 |
| LDL (mg/dL) | 133 ± 35 | 121‡± 30 | 133 ± 35 | 132 ± 36 | 148‡± 53 |
| HDL (mg/dL) | 54 ± 16 | 52 ± 16 | 55 ± 16 | 54 ± 16 | 53 ± 13 |
| Lipid-lowering medication | 166 (5.1) | 2 (4.3) | 141 (5.3) | 15 (3.0)‡ | 8 (15.7)‡ |
| Hypertension | 1823 (56.6) | 32 (68.1) | 1496 (56.9) | 266 (53.7) | 29 (56.9) |
| Diabetes status | |||||
| Normal | 1590 (49.2) | 25 (53.2) | 1274 (48.3) | 262 (52.8) | 29 (56.9) |
| IFG | 1188 (36.9) | 11 (23.4) | 1002 (38.0) | 161 (32.5) | 14 (27.5) |
| Diabetes | 454 (14.0) | 11 (23.4) | 362 (13.7) | 73 (14.7) | 8 (15.7) |
| Fasting glucose (mg/L) | 109 ± 33 | 111 ± 39 | 109 ± 33 | 108 ± 32 | 109 ± 30 |
| Fasting insulin§ (IU/mL) | 13.3 ± 24 | 16.1‡ ± 79 | 13.3 ± 24 | 13.4 ± 16 | 12.8 ± 9 |
| Estrogen use (women only) | 227 (11.8) | 4 (12.5) | 182 (11.8) | 40 (12.5) | 1 (3.3) |
| CRP§ (mg/L) | 1.80 ± 6.3 | 2.15 ± 7.8 | 1.77 ± 5.8 | 1.92 ± 7.1 | 2.10 ± 15.9 |
| Lp(a)§ (mg/dL) | 31.1 ± 57 | 27.6 ± 50 | 31.0 ± 51 | 31.0 ± 82 | 37.1 ± 56 |
Abbreviations: BMI, body mass index, defined as weight in kilograms divided by the square of height in meters; LDL, low-density lipoprotein; HDL, high-density lipoprotein; IFG, impaired fasting glucose; CRP, C-reactive protein; Lp(a), lipoprotein(a).
See Methods for thyroid category definitions.
Data are presented as mean ± SD or as number (percentage) unless otherwise indicated.
P<0.05 for comparison with euthyroid category
Geometric mean
Examination of cardiovascular risk factors showed that study participants with subclinical hyperthyroidism had a higher BMI than euthyroid subjects, along with higher fasting insulin levels and a non-statistically significant higher prevalence of hypertension. Among those not using lipid-lowering medications, serum total LDL and cholesterol levels were lowest in the subclinical hyperthyroidism group and highest in the overt hypothyroidism group; furthermore, those with undetected overt hypothyroidism used lipid-lowering medication the most. However, lipid levels did not differ between the subclinical hypothyroid and euthyroid groups, and those with subclinical hypothyroidism had a slightly lower rate of lipid-lowering medication use.
Prevalent CVD by thyroid status
There was no difference in atrial fibrillation, coronary heart disease, cerebrovascular disease, or subclinical CVD at baseline between the euthyroid category and any of the three thyroid dysfunction categories (Table 2).
Table 2.
Prevalence of Cardiovascular Diseases According to Thyroid Status*
| Prevalent CVD | Subclinical Hyperthyroid (n=47) No. (%) | Euthyroid (n=2639) No. (%) | Subclinical Hypothyroid (n=496) No. (%) | Hypothyroid (n=51) No. (%) |
|---|---|---|---|---|
| Atrial fibrillation | 4 (8.5) | 137 (5.2) | 24 (4.8) | 2 (3.9) |
| Coronary heart disease | 11 (23.4) | 489 (18.5) | 98 (19.8) | 12 (23.5) |
| Cerebrovascular disease | 2 (4.3) | 154 (5.8) | 25 (5.0) | 4 (7.8) |
| Subclinical CVD† | 17 (36.2) | 949 (36.0) | 184 (38.1) | 14 (27.5) |
See Methods for thyroid category definitions.
Without Clinical CVD.
Thyroid medication use during the study
Thyroid medication use was available throughout follow-up. Seven participants in the subclinical hyperthyroidism group started thyroid hormone therapy during the follow-up period, 91 in the euthyroid group, 142 in the subclinical hypothyroid group, and 31 in the hypothyroid group. Due to the potential effect of thyroid hormone initiation on subsequent cardiovascular risk, thyroid hormone medication use was included in all adjusted analyses.
Incident atrial fibrillation by thyroid status
After excluding those with prevalent atrial fibrillation, individuals with subclinical hyperthyroidism had a greater incidence of atrial fibrillation over the 13-year follow-up than the euthyroid group, with 67 vs. 31 events per 1000 person-years; (p<.001) (Figure and Table 3). This effect persisted after sequential adjustment for other risk factors for atrial fibrillation. As shown in Table 3, after adjustment for age, sex, clinical CVD at baseline, subsequent thyroid medication use, and other known risk factors for atrial fibrillation, participants with subclinical hyperthyroidism had nearly twice the risk of developing atrial fibrillation (hazard ratio 1.98; 95% CI, 1.29–3.03).
Figure.
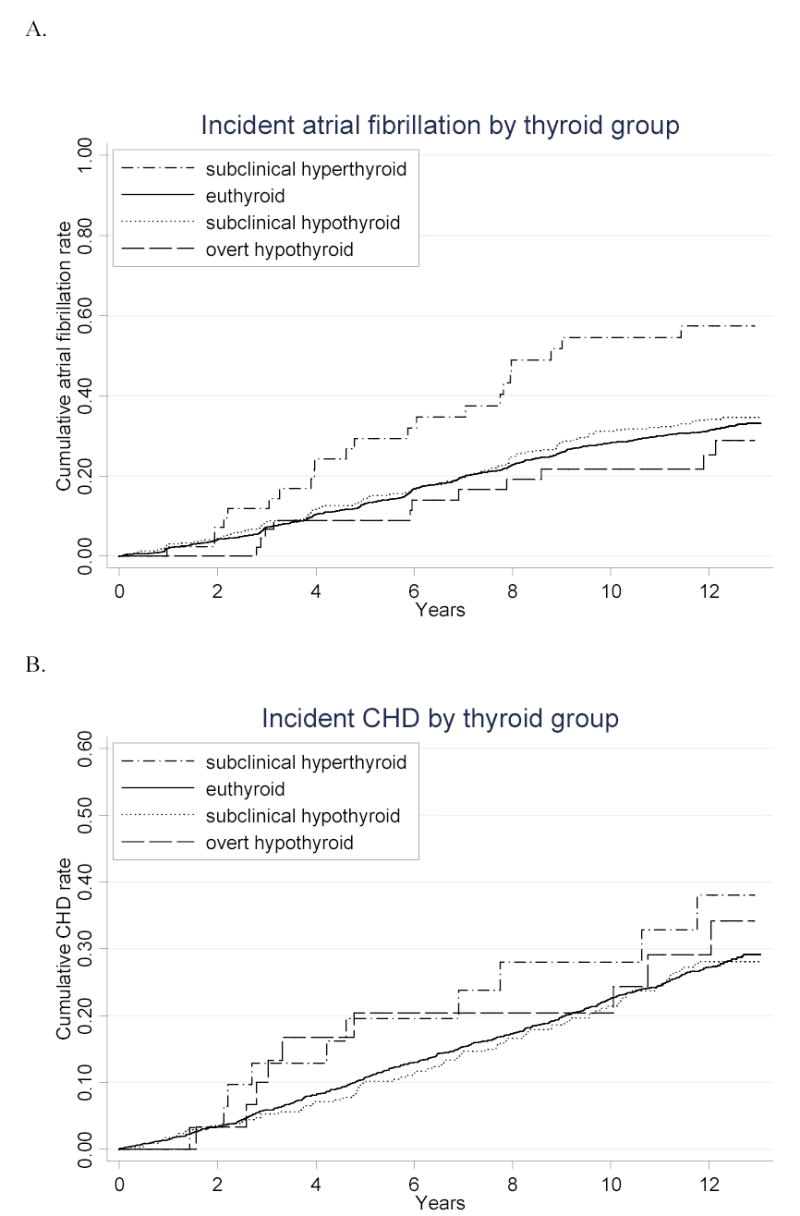
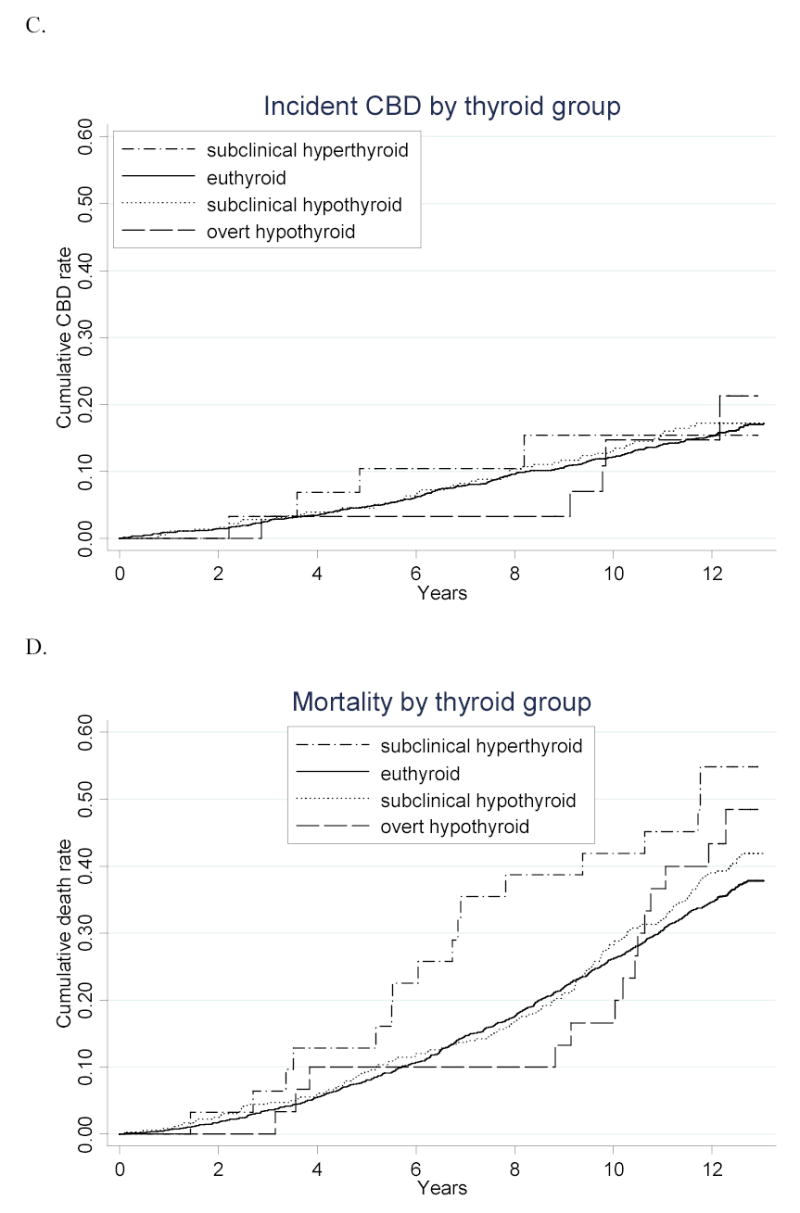
Cumulative incidence of A. atrial fibrillation, B. coronary heart disease, C. cerebrovascular disease, and D. death from all causes over the 13 years of follow-up, according to thyroid status. Number at risk for atrial fibrillation plot: n=47 for subclinical hyperthyroid, 2502 for euthyroid, 472 for subclinical hypothyroid, and 49 for hypothyroid. Number at risk for coronary heart disease, cerebrovascular disease, and mortality plots: n=31 for subclinical hyperthyroid, 1838 for euthyroid, 347 for subclinical hypothyroid, and 30 for hypothyroid. P<.001 for comparison of atrial fibrillation incidence between subclinical hyperthyroid and euthyroid groups. P=.02 for comparison of mortality between subclinical hyperthyroid and euthyroid groups. All other comparisons are not statistically significant.
Table 3.
Incidence of Atrial Fibrillation in Those Without Atrial Fibrillation at Baseline, According to Thyroid Status*
| Subclinical Hyperthyroid | Euthyroid | Subclinical Hypothyroid | Hypothyroid | |
|---|---|---|---|---|
| No. at risk | 43 | 2502 | 472 | 49 |
| No. of events | 22 | 703 | 142 | 11 |
| Incidence (per 1000 person-years) | 67.0 (44–102)† | 31.0 (28.8–33.4) | 33.6 (28.5–39.6) | 25.2 (13.9–45.5) |
| Model 1 | 2.18 (1.42–3.33) | 1.0 | 1.11 (0.92–1.34) | 0.88 (0.48–1.63) |
| Model 2 | 1.98 (1.29–3.03) | 1.0 | 1.13 (0.94–1.36) | 0.96 (0.52–1.79) |
Model 1 is adjusted for age, sex, clinical CVD at baseline, and thyroid medication use during follow-up.
Model 2 is additionally adjusted for left atrial size, systolic blood pressure, fasting glucose, history of valvular disease, and use of diuretics or beta blockers.
See Methods for thyroid category definitions.
P<.001 for comparison with euthyroid category; p=.001 after adjustment for age and sex.
We subsequently repeated these analyses, limiting it to those with a TSH of 0.1 to 0.44 mU/L (n=40). The incidence rate in this subgroup was 59 per thousand person-years (p=.007 compared to euthyroid group). After adjustment for age, sex, baseline clinical cardiovascular disease, and thyroid hormone use during follow-up, the hazard ratio was 1.85 (95% CI 1.1–3.0).
Incident and recurrent cardiovascular events by thyroid status
There were no differences in the incidence of coronary heart disease, cerebrovascular disease, cardiovascular death, or all-cause death between the euthyroid and subclinical or overt hypothyroid groups (Figure and Table 4). There was a statistically significant increase in mortality in the subclinical hyperthyroid group (58.1 vs. 34.2 events per 1000 person-years; p=.02), which disappeared after adjustment for age and sex (p=.29). We subsequently evaluated the relationship between each thyroid group and each cardiovascular outcome using various modeling strategies in adjusted analyses. No thyroid category was statistically significantly different from the euthyroid category; thus, only our crude and final models are displayed in Table 5. After adjustment, those with subclinical hypothyroidism had a hazard ratio of 1.07 (95% CI, 0.90–1.28) for coronary heart disease. All-cause death was not increased in subclinical hyperthyroidism (HR 1.08; 95% CI, 0.72–1.62) or subclinical hypothyroidism (HR 1.14; 95% CI, 0.98–1.32). Estimates for each of the covariates included in the final model for coronary heart disease validate increased risk from age, male sex, diabetes, LDL cholesterol, hypertension, CRP, and baseline CVD in our study population, while showing no appreciable increase in risk from any of the thyroid categories.
Table 4.
Incidence of Cardiovascular Events and Mortality (per 1000 person-years) in Those Without Any CVD at Baseline, According to Thyroid Status*
| Subclinical Hyperthyroid | Euthyroid | Subclinical Hypothyroid | Hypothyroid | |
|---|---|---|---|---|
| No. at risk | 31 | 1838 | 347 | 30 |
| Coronary Heart Disease | ||||
| No. of events | 10 | 462 | 85 | 9 |
| Incidence | 37.4 (20.1–69.5) | 25.9 (23.6–28.3) | 25.1 (20.3–31.1) | 32.0 (16.6–61.5) |
| Cerebrovascular Disease | ||||
| No. of events | 4 | 261 | 50 | 5 |
| Incidence | 14.4 (5.4–38.3) | 13.8 (12.2–15.6) | 14.1 (10.7–18.6) | 15.6 (6.5–37.4) |
| Death from Cardiovascular Causes | ||||
| No. of deaths | 6 | 228 | 47 | 5 |
| Incidence | 20.5 (9.2–45.6) | 11.5 (10.1–13.1) | 12.7 (9.5–16.9) | 15.3 (6.4–36.8) |
| Death From All Causes | ||||
| No. of deaths | 17 | 678 | 138 | 14 |
| Incidence | 58.1 (36.1–93.4)† | 34.2 (31.8–36.9) | 37.2 (31.5–43.9) | 42.9 (25.4–72.5) |
See Methods for thyroid category definitions.
P=.017 for comparison with euthyroid category; p=.29 after adjustment for age and sex.
Table 5.
Hazard Ratios for Events and Death, According to Thyroid Status*
| Subclinical Hyperthyroid (n=47) | Euthyroid (n=2639) | Subclinical Hypothyroid (n=496) | Hypothyroid (n=51) | |
|---|---|---|---|---|
| Coronary Heart Disease | ||||
| Model 1 | 1.18 (0.74–1.88) | 1.0 | 1.04 (0.87–1.23) | 0.97 (0.59–1.59) |
| Model 2 | 1.04 (0.64–1.69) | 1.0 | 1.07 (0.90–1.28) | 0.93 (0.57–1.53) |
| Cerebrovascular Disease | ||||
| Model 1 | 0.82 (0.39–1.73) | 1.0 | 0.99 (0.78–1.26) | 0.87 (0.42–1.78) |
| Model 2 | 0.70 (0.31–1.57) | 1.0 | 1.01 (0.79–1.29) | 0.86 (0.42–1.76) |
| Death From Vascular Causes | ||||
| Model 1 | 1.02 (0.53–1.98) | 1.0 | 1.14 (0.91–1.43) | 0.88 (0.44–1.73) |
| Model 2 | 0.94 (0.49–1.83) | 1.0 | 1.16 (0.92–1.46) | 0.97 (0.49–1.92) |
| Death From All Causes | ||||
| Model 1 | 1.13 (0.76–1.70) | 1.0 | 1.10 (0.95–1.27) | 1.26 (0.84–1.89) |
| Model 2 | 1.08 (0.72–1.62) | 1.0 | 1.14 (0.98–1.32) | 1.43 (0.95–2.14) |
Model 1 is adjusted for age, sex, clinical CVD at baseline, atrial fibrillation at baseline, and thyroid medication use during follow-up.
Model 2 is additionally adjusted for race, smoking status, diabetes, LDL cholesterol, use of lipid-lowering medications, hypertension, body mass index, and C-reactive protein.
See Methods for thyroid category definitions.
COMMENT
We report an independent association of subclinical hyperthyroidism with incident atrial fibrillation, but not with other clinical cardiovascular conditions or mortality, in a large, population-based cohort designed to examine cardiovascular risk factors in men and women over 65. We also found no relationship between subclinical hypothyroidism or overt hypothyroidism and prevalent or incident atherosclerotic disease, cardiovascular mortality, or all-cause mortality, though relationships between traditional cardiovascular risk factors and CVD were confirmed in our models.
Prevalence of Endogenous Thyroid Dysfunction
Excluding those taking thyroid hormone preparations, the prevalence of subclinical hyperthyroidism in our cohort was 1.5%, a figure lower than two published reports in older people.12,25 The prevalence of subclinical hypothyroidism was 15%, which is comparable to estimates from several community-based cohorts,26–28 and somewhat higher than others.4,25,29–31 The discrepancies seen in these prevalence rates likely reflect differences in definitions of subclinical thyroid disease and the health status of participants among cohorts.
Subclinical Hyperthyroidism
Our findings of increased risk of atrial fibrillation concur with a cross-sectional study32 and with prospective results from the Framingham Heart Study, in which individuals with TSH values ≤ 0.1 mU/L who were not receiving thyroid hormone therapy had an adjusted relative risk of 3.8 (95% CI 1.7–8.3) for developing atrial fibrillation and those with TSH values between 0.1 and 0.4 mU/L had an adjusted relative risk of 1.6 (1.0–2.5).12 Individuals with elevated thyroxine levels, indicating overt hyperthyroidism, were included in their category of TSH values <0.1 mU/L, which could have led to an overestimate of the effect of subclinical hyperthyroidism. Our results clearly show a relationship between low TSH levels and atrial fibrillation incidence in older individuals with endogenous subclinical hyperthyroidism, including those with TSH levels of 0.1 to 0.44 mU/L.
We found no relationship between subclinical hyperthyroidism and atherosclerotic CVD, cardiovascular mortality, or all-cause mortality between those with subclinical hyperthyroidism and euthyroid individuals. In contrast, Parle et al. reported a higher cardiovascular mortality rate in those with TSH <0.5 mU/L in comparison to the remainder of their cohort and the mortality rate from circulatory disease in England and Wales.5 However, their analyses are limited by a less rigorous definition of subclinical hyperthyroidism, so that those with other causes of low TSH may have been included in their low TSH category, and by minimal adjustment for other covariates associated with cardiovascular mortality, excepting age and sex.
Subclinical Hypothyroidism
We found no association between subclinical hypothyroidism and atherosclerotic disease, either prevalent or incident. Multiple prior studies have examined the subclinical hypothyroidism-CVD relationship. They have shown subclinical hypothyroidism to either increase4,6,8 or have no effect5,17,33 on CVD risk, though one has shown decreased cardiovascular and all-cause mortality,34 and each has had serious design limitations not present in our study, fueling the controversy rather than providing evidence to resolve it.
Cross-sectional associations between subclinical hypothyroidism and CVD4,6,8 have only held up longitudinally in one study, the Busselton Health Study.8 Though the Busselton Health Study has twenty years of follow-up, data were not collected specifically on thyroid hormone replacement; cardiovascular events were collected by record linkage, rather than prospective follow-up and adjudication; and LDL cholesterol concentrations were not available for adjusted analyses. Interestingly, the separation in CVD risk between the subclinical hypothyroid and euthyroid groups did not occur until ten years of follow-up. In our study, there is a suggestion of an increase in all-cause mortality in the subclinical hypothyroid group at ten years, which is not paralleled in the atrial fibrillation, coronary heart disease, and cerebrovascular disease curves, which are nearly indistinguishable from the euthyroid group over the entire study follow-up. The late increase in all-cause mortality either reflects an increase in non-cardiovascular causes or is simply due to chance.
Other studies that have shown no association between subclinical hypothyroidism and cardiovascular risk have been questioned due to shorter follow-up5,17 or the inclusion of individuals who subsequently initiated thyroid hormone therapy, which theoretically could have attenuated their cardiovascular risk.33 During the course of our study, 27% of those in the subclinical hypothyroid group initiated thyroid hormone replacement. We saw no evidence of an effect on cardiovascular risk, and our thyroid hormone replacement covariate was not statistically significant for any of the outcomes studied, suggesting that thyroid hormone initiation has no effect on cardiovascular risk.
Thyroid Status and Atherosclerotic Risk Factors
Our results suggest a dose-response effect between TSH and serum total cholesterol levels, with the lowest levels of cholesterol present in those with subclinical hyperthyroidism and highest in those with overt hypothyroidism, a previously reported effect.31,35 In our cohort, individuals with hypothyroidism had the highest levels of serum total and LDL cholesterol, and took lipid-lowering medications at three times the rate of the euthyroid population. This finding highlights the need to investigate secondary causes of hypercholesterolemia before initiation of lipid-lowering medications, as recommended by the National Cholesterol Education Program.36
Earlier observational studies examining the relationship between subclinical hypothyroidism and cholesterol levels yielded conflicting results.37 In our study, serum cholesterol concentrations were similar between euthyroid and untreated subclinically hypothyroid individuals. Similarly, there were no differences in LP(a), CRP, or fasting insulin and glucose concentrations between euthyroid and subclinically hypothyroid subjects.
Strengths and Weaknesses
A major strength of our study is the use of a large, population-based cohort of older men and women, designed to examine cardiovascular risk factors, with an average of 12.5 years of follow-up data for events. The prevalent and incident disease assignments were made using objective information collected during examination and review of hospital and physicians’ records.20 Laboratory assays were performed without knowledge of CVD status. Further, we excluded individuals taking thyroid medication or with other conditions that could affect thyroid function testing at baseline, and incorporated thyroid medication use over time as a time-dependent covariate to examine risk of endogenous thyroid dysfunction.
We performed analyses using multiple models: stratified by sex and prevalent CVD, adjusting for incident thyroid hormone use in several ways (none, censoring at the time of thyroid hormone initiation, or used as a time-dependent covariate), and adjusting for cardiovascular risk factors in a stepwise manner to avoid overadjustment. We showed an independent effect of subclinical hyperthyroidism on incident atrial fibrillation and of traditional cardiovascular risk factors on incident CVD. The presence of these positive findings and the thoroughness of our modeling strategies suggest that it is unlikely that we failed to detect a cardiovascular risk factor of consequence in the remainder of our analyses. Post-hoc calculations showed adequate power to detect meaningful differences between the subclinical hypothyroid and euthyroid groups for each outcome; specifically, our study had adequate power to detect a HR ≥ 1.30 for coronary heart disease and HR≥ 1.26 for all-cause death.
We are limited in looking at thyroid function testing abnormalities to one point in time. Thus, we are unable to comment on the relationship between persistent thyroid abnormalities and CVD and mortality. In addition, the number of individuals with subclinical hyperthyroidism or overt hypothyroidism is small in our study, limiting our power to detect an effect of either of these types of thyroid dysfunction on CVD outcomes or mortality.
Clinical Implications
Thyroid testing abnormalities are quite common in older women and men without known thyroid dysfunction. While the US Preventive Services Task Force and an expert panel do not recommend generalized screening for thyroid disease,18,38 the American College of Physicians currently advises screening women over 50 for unsuspected but symptomatic thyroid disease,39 and the American Thyroid Association recommends screening adults every five years beginning at age 35.40 Our analyses do not support screening older individuals solely to prevent atrial fibrillation, with an estimated number needed to screen of 2,500 older individuals to find one case of atrial fibrillation associated with subclinical hyperthyroidism. Our findings do suggest that if endogenous subclinical hyperthyroidism is detected, older individuals may benefit from treatment to prevent atrial fibrillation. An expert panel has recommended consideration of treatment for those with endogenous subclinical hyperthyroidism and TSH levels that are below 0.1 mU/L, with insufficient evidence to treat those with TSH 0.1–0.45 mU/L.18 Our data support treatment of all older individuals with subclinical hyperthyroidism, even those with mild decreases in TSH (0.1–0.44 mU/L). Our analyses do not support screening older individuals for thyroid disease in order to prevent CVD, and, although our data are observational, they do not support treatment of individuals with subclinical hypothyroidism to prevent cardiovascular events.
Footnotes
Author Contributions: Dr. Arnold had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Funding/Support: This study was supported by an American Heart Association (AHA) Grant-in-Aid (to Dr. Fried) with funding from July 1991 to June 1993. The research reported in this article was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, and N01 HC-15103 from the National Heart, Lung, and Blood Institute (NHLBI). A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org. Dr. Cappola is supported by K23-AG19161 from the National Institutes of Aging (NIA).
Role of the Sponsor: This study was funded through contracts with the National Heart, Lung, and Blood Institute (NHLBI) and included substantial NHLBI involvement in study design and oversight. A member of the NHLBI serves on the executive committee of the study, and NHLBI reviews all manuscripts prior to publication.
References
- 1.Hoyert, DL, Kung HC, and Smith, BL. Deaths: Preliminary Data for 2003. National vital statistics reports. 53[15]. 2005. Hyattsville, Maryland, National Center for Health Statistics. [PubMed]
- 2.Magnus P, Beaglehole R. The real contribution of the major risk factors to the coronary epidemics: time to end the “only-50%” myth. Arch Intern Med. 2001;161:2657–2660. doi: 10.1001/archinte.161.22.2657. [DOI] [PubMed] [Google Scholar]
- 3.Pahor M, Elam MB, Garrison RJ, Kritchevsky SB, Applegate WB. Emerging noninvasive biochemical measures to predict cardiovascular risk. Arch Intern Med. 1999;159:237–245. doi: 10.1001/archinte.159.3.237. [DOI] [PubMed] [Google Scholar]
- 4.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358:861–865. doi: 10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- 6.Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab. 2004;89:3365–3370. doi: 10.1210/jc.2003-031089. [DOI] [PubMed] [Google Scholar]
- 7.Kvetny J, Heldgaard PE, Bladbjerg EM, Gram J. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol (Oxf) 2004;61:232–238. doi: 10.1111/j.1365-2265.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 8.Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165:2467–2472. doi: 10.1001/archinte.165.21.2467. [DOI] [PubMed] [Google Scholar]
- 9.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 10.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 11.Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 12.Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 13.Bell GM, Sawers JS, Forfar JC, Doig A, Toft AD. The effect of minor increments in plasma thyroxine on heart rate and urinary sodium excretion. Clin Endocrinol (Oxf) 1983;18:511–516. doi: 10.1111/j.1365-2265.1983.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 14.Biondi B, Palmieri EA, Fazio S, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab. 2000;85:4701–4705. doi: 10.1210/jcem.85.12.7085. [DOI] [PubMed] [Google Scholar]
- 15.Biondi B, Fazio S, Palmieri EA, et al. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J Clin Endocrinol Metab. 1999;84:2064–2067. doi: 10.1210/jcem.84.6.5733. [DOI] [PubMed] [Google Scholar]
- 16.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 17.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 18.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 19.Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:128–141. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Kuller LH, Shemanski L, Psaty BM, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 22.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 25.Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 1991;34:77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 26.Lindeman RD, Schade DS, LaRue A, et al. Subclinical hypothyroidism in a biethnic, urban community. J Am Geriatr Soc. 1999;47:703–709. doi: 10.1111/j.1532-5415.1999.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal MJ, Hunt WC, Garry PJ, Goodwin JS. Thyroid failure in the elderly. Microsomal antibodies as discriminant for therapy. JAMA. 1987;258:209–213. [PubMed] [Google Scholar]
- 28.Sawin CT, Chopra D, Azizi F, Mannix JE, Bacharach P. The aging thyroid. Increased prevalence of elevated serum thyrotropin levels in the elderly. JAMA. 1979;242:247–250. doi: 10.1001/jama.242.3.247. [DOI] [PubMed] [Google Scholar]
- 29.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 30.Bagchi N, Brown TR, Parish RF. Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med. 1990;150:785–787. [PubMed] [Google Scholar]
- 31.Kanaya AM, Harris F, Volpato S, Perez-Stable EJ, Harris T, Bauer DC. Association between thyroid dysfunction and total cholesterol level in an older biracial population: the health, aging and body composition study. Arch Intern Med. 2002;162:773–779. doi: 10.1001/archinte.162.7.773. [DOI] [PubMed] [Google Scholar]
- 32.Auer J, Scheibner P, Mische T, Langsteger W, Eber O, Eber B. Subclinical hyperthyroidism as a risk factor for atrial fibrillation. Am Heart J. 2001;142:838–842. doi: 10.1067/mhj.2001.119370. [DOI] [PubMed] [Google Scholar]
- 33.Vanderpump MP, Tunbridge WM, French JM, et al. The development of ischemic heart disease in relation to autoimmune thyroid disease in a 20-year follow-up study of an English community. Thyroid. 1996;6:155–160. doi: 10.1089/thy.1996.6.155. [DOI] [PubMed] [Google Scholar]
- 34.Gussekloo J, Van EE, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 35.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 36.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 37.Chu JW, Crapo LM. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab. 2001;86:4591–4599. doi: 10.1210/jcem.86.10.7961. [DOI] [PubMed] [Google Scholar]
- 38.Screening for thyroid disease: recommendation statement. Ann Intern Med. 2004;140:125–127. doi: 10.7326/0003-4819-140-2-200401200-00014. [DOI] [PubMed] [Google Scholar]
- 39.Clinical guideline, part 1. Screening for thyroid disease. American College of Physicians. Ann Intern Med. 1998;129:141–143. [PubMed] [Google Scholar]
- 40.Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160:1573–1575. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]


