Abstract
The cyclic production of estrogen and progesterone by the premenopausal ovary accounts for the steep rise in breast cancer risk in premenopausal women. These hormones are breast cell mitogens. By reducing exposure to these ovarian hormones, agonists of luteinizing hormone-releasing hormone (LHRH) given to suppress ovarian function may prove useful in cancer prevention. To prevent deleterious effects of hypoestrogenemia, the addition of low-dose hormone replacement to the LHRH agonist appears necessary. Pilot data with such an approach indicates it is feasible and reduces mammographic densities.
Keywords: breast cancer prevention, gonadotropin-releasing hormone agnonists, hormonal carcinogenesis, luteinizing hormone-releasing hormone agonists
Introduction
More than a decade ago Pike et al [1] first suggested a potential role for agonists of LHRH to prevent breast cancer. The rationale for considering LHRH agonists is due to their ability to suppress ovarian function and sex steroid production; the reduction in sex steroids is predicted to lead to the prevention of breast cancer.
Ovarian hormones
Ovarian hormones (estrogens and progestogens) are critical factors in the genesis of human breast cancer. During the premenopausal years breast cancer risk increases steeply, but after cessation of ovarian function (menopause) it increases at a much lower rate. Epidemiologic studies have clearly demonstrated that early menopause, whether natural or artificial (bilateral oophorectomy), substantially reduces breast cancer risk. Menopause before age 35 years is associated with a 60-75% reduction in breast cancer risk [2,3,4,5,6]. The calculated effect of an early oophorectomy on the age-incidence curve of breast cancer is given in Figure 1; age at menopause determines the transition point from the steeply rising premenopausal slope to the more gentle postmenopausal slope. The protective effect of oophorectomy on breast cancer risk has recently been shown in women carrying BRCA1 germline mutations. Bilateral prophylactic oophorectomy (done to prevent ovarian cancer) is associated with a reduction in breast cancer risk [7]. The magnitude of the protection reported by Rebbeck et al [7] is substantial (hazard ratio of 0.53) and increased with increasing duration of follow up after the prophylactic surgery.
Figure 1.
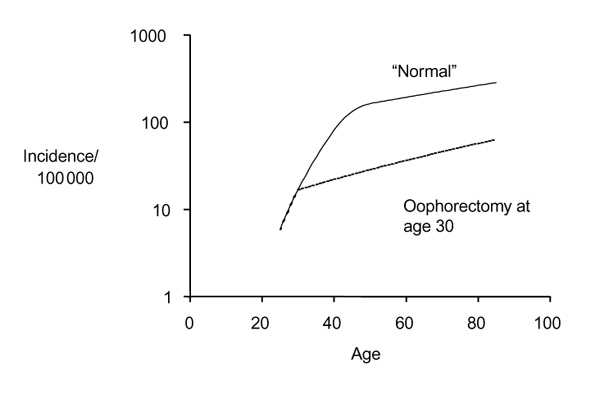
Effect of oophorectomy on the age-incidence curve for breast cancer. The calculations were made using the model described by Pike [12].
The effect of ovarian hormones on breast cancer risk is predictable in light of the effects of these hormones on breast epithelial cell proliferation. Cell proliferation is central to the process of carcinogenesis, and agents that increase cell proliferation increase the incidence of random mutations and hence cancer risk [8]. In the normal human breast epithelial cell, both estrogens and progestogens are mitogens (for review [9]), hence oophorectomy reduces cancer risk by eliminating the breast mitogen progesterone and reducing the estrogen levels.
Luteinizing hormone-releasing hormone
Native LHRH produced by the hypothalamus controls the secretion of follicle-stimulating hormone and luteinizing hormone by the pituitary gland, and hence gonadal steroid hormone production. Administration of potent synthetic agonists of LHRH to premenopausal women results in a transient rise in follicle-stimulating hormone/luteinizing hormone release, followed by a sustained suppression. The reduction in serum estradiol and serum progesterone to oophorectomized levels by LHRH agonists has been demonstrated in numerous reports [10], and led to their use in the treatment of hormone-responsive metastatic breast cancer in premenopausal women. Although the role of ovarian ablation in the management of early breast cancer remains unsettled, evidence [11] indicates that LHRH agonists may also prevent breast cancer recurrence in the adjuvant setting. Of particular interest is the large multicenter trial that evaluated the LHRH agonist goserelin in the adjuvant setting recently reported by Baum [11]. In that study a substantially reduced incidence of contralateral new primary breast tumors was reported.
Use of LHRH in premenopausal women is predictably associated with hypoestrogenic symptoms, including hot flushes, vaginal dryness, and sleep disturbances. A loss of bone mineral density (BMD) has been seen in the majority of studies that involved protracted (6 months) LHRH agonist treatment. Because oophorectomy at a young age is associated with an increased risk of cardiovascular disease, the long-term use of a LHRH agonist is of concern. Although the side effects and risks associated with hypoestrogenemia are acceptable in the setting of metastatic breast cancer and in the adjuvant treatment of early breast cancer, such effects may not be acceptable to women who are only at risk for the development of the disease. A LHRH agonist given at a dose sufficient to suppress ovarian function to postmenopausal levels should achieve a major reduction in a woman's lifetime breast cancer risk, but the benefit will only occur if the agent is continued for prolonged periods of time.
In an effort to minimize the deleterious effects of hypoestrogenemia the addition of other agents, including bisphosphonates, selective estrogen receptor modulators, and low-dose add-back sex steroids, is under consideration or study. The rationale for bisphosphonates and selective estrogen receptor modulators is related to their protective effect on BMD. The tolerance of women to hypoestrogenic symptoms remains to be evaluated, however.
Pike et al [1] suggested that the addition of low-dose hormone replacement therapy (HRT) would reduce the hypoestrogenic effects of the LHRH agonist, while preserving the major reduction in cancer risk. The combined LHRH agonist and low-dose hormone replacement results in a reduction in estrogen exposure by 60% and in progestogen exposure by 75% when compared to premenopausal hormone levels. Because the add-back low-dose hormone replacement should permit long-term use, protracted reductions in hormone exposure is possible. Table 1 shows that lifetime breast cancer risk is predicted to be reduced by almost one-third if used for 5 years and by more than 50% if used for 10 years. These figures for breast cancer are calculated from a mathematical model [1,12,13], and include an effect of progestogen that is consistent with that reported from recent studies of HRT on breast cancer risk [14].
Table 1.
Predicted reduction in lifetime risk of cancer with the prototype contraceptive
| Duration of regimen (years) | |||
| Cancer type | 5 | 10 | 15 |
| Breast | 31% | 53% | 70% |
| Endometrium | 18% | 33% | 45% |
| Ovary | 41% | 67% | 84% |
The calculations were made using the model described by Pike [12], and were based on using the regimen at any time after the first full-term pregnancy and before age 40 years.
Clinical effects of luteinizing hormone-releasing hormone agonists
In a pilot study designed to determine the effects of an LHRH agonist plus low-dose replacement therapy, bone metabolism, lipoprotein metabolism, the endometrium, and menopausal symptoms were evaluated in women predisposed to familial breast cancer [15,16]. The regimen tested included a depot LHRH agonist administered monthly, low-dose estrogen with conjugated estrogens 0.625-0.9mg for 6 days each week, and the progestogen medroxyprogesterone acetate 10mg for 14 days every 112 days (4 months). Subsequently, the effects of replacing ovarian androgen (which is also suppressed by LHRH agonists) with the add-back hormone regimen were evaluated. Subjects were premenopausal women, aged 25-40 years, with one of the following breast cancer risk factors: lobular carcinoma in situ, mother and sister with breast cancer (at least one premenopausal), or a mother or sister with bilateral premenopausal breast cancer. Twenty-one individuals were entered and were randomized on a 2:1 basis to a treatment group and a control group.
Overall the regimen was well tolerated [16]. A questionnaire assessed frequency and intensity of possible symptoms of menopausal distress and premenstrual syndrome. Symptoms of menopausal distress were infrequent, and the treated individuals had a decrease in luteal phase or premenstrual syndrome symptoms of 'abdominal bloating or fullness'; 'abdominal cramps or pain'; 'breast swelling'; 'breast pain or tenderness'; 'anxious, tense, or nervous'; 'irritable, angry, impatient'; and 'mood swings' [16].
During the first year of the study, a small but significant 2.9% reduction in lumbar spine BMD was noted in the treated group. As a result the study was modified to replace ovarian androgens suppressed by the LHRH using methyltestosterone. The mean change in BMD in the lumbar spine after the addition of the androgen is depicted in Table 2. These results suggest that the addition of the androgen may have an effect on maintenance of BMD.
Table 2.
Annualized change In BMD
| Group | Lumbar spine | Femoral neck |
| Control | 0.4% | 0.2% |
| Treated with CE + MPA | -2.9%† | -2.2%‡ |
| Treated with CE + MT + MPA | 0% | 1.6% |
BMD was measured using quantitative digital radiography (Hologic Inc, Waltham, MA, USA). CE, conjugated estrogens; MPA, medroxyprogesterone acetate; MT, methyltestosterone. †P= 0.001, ‡P = 0.006.
The initial regimen was associated with favorable effects on the lipids during the months when medroxyprogesterone acetate was not administered. The addition of the methyltestosterone eliminated the beneficial effect of the regimen on lipoproteins. However, the changes in cholesterol (compared with baseline values) were not different from those in the control individuals. Oral methyltestosterone is not considered an optimal method for replacement of ovarian androgens.
Scheduled bleeding occurred after most progestogen administrations. Unscheduled bleeding or spotting occurred infrequently, as depicted in Table 3. No endometrial hyperplasia was identified in the endometrial biopsies performed at cycle 13 and cycle 25. Recovery of menses was timed from the date of injection of the last dose of depot LHRH. The mean time was 5 months and median 3.9 months, although 16 months passed before return of menses in one individual.
Table 3.
Unscheduled bleeding and spotting
| Cycle number | Number of days/ 28-day cycle (mean [standard error]) | Percentage of days |
| 1-4 | 0.92 (0.28) | 3.3 |
| 5-8 | 1.4 (0.37) | 4.9 |
| 9-12 | 1.0 (0.38) | 3.7 |
| 13-16 | 0.74 (0.38) | 2.6 |
| 17-20 | 1.4 (0.45) | 4.9 |
| 21-24 | 1.9 (0.91) | 6.8 |
In the pilot study changes in mammographic densities were measured in women on the treatment regimen and in the control women. Because mammographic classification schemes are unable to distinguish fine changes that do not cause a change in category, the study directly measured the changes in mammographic densities. The radiologists were masked both as to whether the mammograms were from treated or control individuals and whether they were baseline or follow-up studies [17]. Figure 2 shows the substantial improvement overall in the treated group. The reduced estrogen and progestogen exposures achieved by the regimen resulted in significant reductions in follow-up mammographic densities. Epidemiologic studies [18,19,20,21] have consistently found that increased mammographic densities are associated with greater risk independent of other breast cancer risk factors. The significant mammographic changes further support the evidence that an LHRH agonist and low-dose add-back HRT may contribute significantly to breast cancer reduction; a second study in high-risk women is ongoing.
Figure 2.
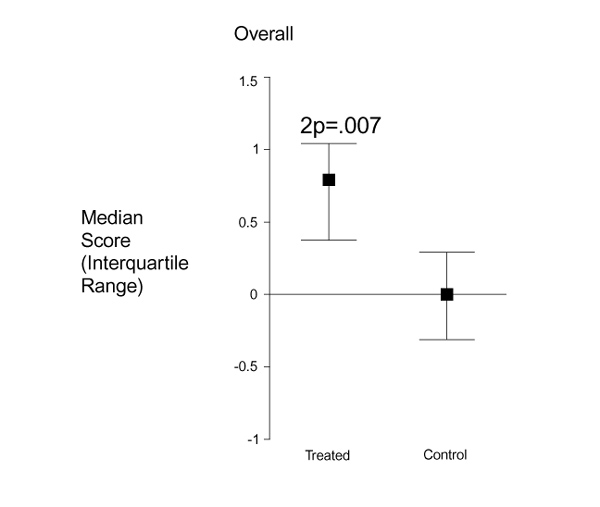
Mammographic changes in treated and control individuals. Values are expressed as median change from baseline and interquartile range.
Conclusion
The risk of breast cancer rises steeply during the premenopausal years. This is, in all likelihood, a result of stochaistic mutations associated with ovarian hormone-driven repetitive breast epithelial cell proliferation. A regimen to reduce premenopausal exposure to estrogen and progesterone based on suppression of ovarian function by an agonist of LHRH and replacement of low-dose hormones would be expected to reduce breast cancer risk. A pilot trial of such an approach demonstrated both its feasibility and a beneficial reduction in mammographic density. Studies are in progress to improve the acceptability of the regimen and to test its effects on the mammogram in high-risk women.
Acknowledgement
Darcy Spicer and Malcolm Pike have a substantial interest in Balance Pharmaceuticals, Inc.
References
- Pike MC, Ross RK, Lobo RA, Key TJA, Potts M, Henderson BE. LHRH agonists and the prevention of breast and ovarian cancer. . Br J Cancer. 1989;60:142–148. doi: 10.1038/bjc.1989.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld A. The relationship of cancer of the female breast to artificial menopause and marital status. Cancer. 1956;9:927–934. doi: 10.1002/1097-0142(195609/10)9:5<927::aid-cncr2820090510>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Wynder EL. A study of the epidemiology of cancer of the breast II. The influence of hysterectomy. Cancer. 1962;15:28–38. doi: 10.1002/1097-0142(196201/02)15:1<28::aid-cncr2820150105>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315–329. [PubMed] [Google Scholar]
- Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst. 1972;48:605–613. [PubMed] [Google Scholar]
- Kelsey J. A review of the epidemiology of human breast cancer. Epidemiol Rev. 1979;1:74–109. doi: 10.1093/oxfordjournals.epirev.a036215. [DOI] [PubMed] [Google Scholar]
- Rebbeck R, Levin A, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475–1479. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- Ames BN, Gold LS. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990;249:970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation and breast cancer risk. . Epidemiol Rev. 1993;15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- Kaufman M, Jonat W, Kleeberg U, et al. Goserelin, a depot gonadotrophin-releasing hormone agonist in the treatment of premenopausal patients with metastatic breast cancer. J Clin Oncol . 1989;7:1113–1119. doi: 10.1200/JCO.1989.7.8.1113. [DOI] [PubMed] [Google Scholar]
- Baum M. Adjuvant treatment of premenopausal breast cancer with zoladex and tamoxifen [abstract]. Breast Cancer Res Treat. 1999;57:30. [Google Scholar]
- Pike MC. Age-related factors in cancer of the breast, ovary and endometrium. J Chron Dis. 1987;40:59–69. doi: 10.1016/s0021-9681(87)80009-7. [DOI] [PubMed] [Google Scholar]
- Spicer D, Shoupe D, Pike M. Gonadotropin-releasing hormone agonist plus add-back sex steroids to reduce risk of breast cancer [letter; comment]. J Natl Cancer Inst. 1991;83 doi: 10.1093/jnci/83.23.1763. [DOI] [PubMed] [Google Scholar]
- Ross RK, Paganini-Hill A, Wan PC, Pike MC. Estrogen versus estrogen-progestin hormone replacement therapy: effect on breast cancer risk. J Natl Cancer Inst. 2000;92:328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- Spicer DV, Shoupe D, Pike M. GnRH agonists as contraceptive agents: predicted significantly reduced risk of breast cancer. . Contraception. 1991;44:289–310. doi: 10.1016/0010-7824(91)90019-c. [DOI] [PubMed] [Google Scholar]
- Spicer DV, Pike MC, Pike A, Rude R, Shoupe D, Richardson J. Pilot trial of a gonadotropin hormone agonist with replacement hormones as a prototype contraceptive to prevent breast cancer. Contraception. 1993;47:427–444. doi: 10.1016/0010-7824(93)90095-o. [DOI] [PubMed] [Google Scholar]
- Spicer D, Ursin G, Parisky Y, et al. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J Natl Cancer Inst. 1994;86:431–436. doi: 10.1093/jnci/86.6.431. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Szklo M. Mammographic parenchymal patterns and breast cancer risk. Epidemiol Rev. 1987;9:146–174. doi: 10.1093/oxfordjournals.epirev.a036300. [DOI] [PubMed] [Google Scholar]
- Brisson J, Morrison AS, Khalid N. Mammographic parenchymal features and breast cancer in the brest cancer detection demonstration project. J Natl Cancer Inst. 1988;80:1532–1540. doi: 10.1093/jnci/80.19.1534. [DOI] [PubMed] [Google Scholar]
- Warner E, Lockwood G, Tritchler D, Boyd NF. The risk of breast cancer associated with mammographic parenchymal patterns: a meta-analysis of the published literature to examine the effect of method of classification. . Cancer Detect Prev. 1992;16:67–72. [PubMed] [Google Scholar]
- Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev. 1993;15:196–208. doi: 10.1093/oxfordjournals.epirev.a036105. [DOI] [PubMed] [Google Scholar]


