Abstract
We present the first study of the effects of monolayer shell physicochemical properties on the destruction of lipid-coated microbubbles during insonification with single, one-cycle pulses at 2.25 MHz and low-duty cycles. Shell cohesiveness was changed by varying phospholipid and emulsifier composition, and shell microstructure was controlled by postproduction processing. Individual microbubbles with initial resting diameters between 1 and 10 μm were isolated and recorded during pulsing with bright-field and fluorescence video microscopy. Microbubble destruction occurred through two modes: acoustic dissolution at 400 and 600 kPa and fragmentation at 800 kPa peak negative pressure. Lipid composition significantly impacted the acoustic dissolution rate, fragmentation propensity, and mechanism of excess lipid shedding. Less cohesive shells resulted in micron-scale or smaller particles of excess lipid material that shed either spontaneously or on the next pulse. Conversely, more cohesive shells resulted in the buildup of shell-associated lipid strands and globular aggregates of several microns in size; the latter showed a significant increase in total shell surface area and lability. Lipid-coated microbubbles were observed to reach a stable size over many pulses at intermediate acoustic pressures. Observations of shell microstructure between pulses allowed interpretation of the state of the shell during oscillation. We briefly discuss the implications of these results for therapeutic and diagnostic applications involving lipid-coated microbubbles as ultrasound contrast agents and drug/gene delivery vehicles.
I. Introduction
Destruction of lipid-coated microbubble contrast agents by ultrasound plays an important role in several medical applications, including the estimation of blood perfusion [1], vessel architecture mapping [2], and delivery of therapeutic agents [3]–[5]. Optical and acoustical methods have revealed some mechanisms of microbubble contrast agent destruction in terms of both ultrasound and shell parameters [2], [6]–[9]. For example, microbubbles coated with protein and polymer shells have been shown to exhibit sonic cracking, whereby disruption of the shell results in rapid static dissolution after the ultrasonic pulse due to high surface tension [2], [10], [11]. Polymer shells also have been shown to significantly dampen oscillations of the gas core and reduce echogenicity. Lipid-coated microbubbles, however, do not exhibit sonic cracking and readily expand and contract during the ultrasound pulse, making them highly echogenic and ideal for applications involving the detection of a nonlinear acoustic response. The lipid shell consists of a self-assembled monomolecular layer that is highly oriented due to hydrophobic forces and held together through physical associations (i.e., intermolecular dispersion and electrostatic forces), rather than covalent bonding or chain entanglement. Thus, the lipid shell rapidly reseals following dilatation or fracture to minimize surface tension and stabilize the gas core. Lipid-coated microbubbles have been shown to exhibit ultrasonic destruction by two main mechanisms that occur over different regimes of acoustic pressure: acoustic dissolution at low pressures, and fragmentation of the parent bubble into two or more daughter bubbles at high pressures. Recent experimental evidence has shown that the lipid shell is indeed a polycrystalline material [12], with lateral phase separation of the more highly ordered lipid into domains surrounded by a less ordered emulsifier-rich region [13], [14]. Composition and microstructure significantly affect the mechanical and transport properties of quasistatic microbubbles [12], [15]; presumably, differences in these parameters will influence the response of insonified microbubbles as well. Previous studies have shown that shell composition influences microbubble response over a sequence of short pulses, including a study incorporating a series of polymer-shelled microbubbles with different mechanical properties [11] and another comparing microbubbles coated with either cross-linked protein or phospholipid [2]. The purpose of the present study was to examine the effects of lipid shell composition and microstructure on microbubble response, particularly the stability of the echogenic gas core and release characteristics of excess shell material over a series of single, one-cycle pulses at 2.25 MHz with peak negative pressure ranging from 400 kPa to 800 kPa. We discuss the implications of our results for contrast-assisted ultrasound imaging and therapy.
Acoustic dissolution involves loss of gas molecules from the core to the local aqueous environment due to convection and diffusion during the pulse. Generally, acoustic dissolution occurs in the regime of driving pressures slightly lower than that required for fragmentation. Prior and subsequent to the pulse, the gas core is stabilized by the low surface tension and high gas transport resistance of the fully condensed lipid shell [16], [17]. During the pulse, however, the oscillating pressure field forces the bubble to experience drastic volumetric changes that create local concentration gradients, convection currents, and disruption of the monomolecular shell; each of these effects facilitates partial dissolution of the gas core. For polymer-shelled microbubbles, Leong-Poi et al. [11] showed that the rate of acoustic dissolution is dependent on the composition. For phospholipid-shelled microbubbles, we give experimental evidence here, for the first time, that composition affects acoustic dissolution for a given initial resting radius and set of ultrasound parameters.
The lipid monolayer shell of a resting microbubble is fully condensed due to persistent tension in the shell. Any gas loss due to acoustically driven dissolution must be accompanied by either a deviation from the spherical shape or the ejection of excess lipid material from the monolayer shell. Previous acoustic destruction experiments showed that the gas core remained spherical following the pulse [2], [6], [7]. Thus, excess lipid shedding is expected to accompany acoustic dissolution, most likely in the form of aggregate structures such as lamellar vesicles or micelles. Break-up of the lipid shell during insonification was observed previously [18]. Prior to the current study, however, the influence of the shell physicochemical properties on this shedding process was unknown. Knowledge of the size and shape of these shed lipid particles is important for understanding subsequent processes, e.g., potential bioeffects and the delivery kinetics of shell-associated therapeutic agents. In this study, we used fluorescence microscopy with a lipid probe to directly image the lipid-shedding process and, furthermore, to develop a relationship between the shedding mechanism and the lipid-shell physicochemical properties.
Recent studies using high-speed imaging systems have allowed direct observation of microbubble dynamics during insonification with high temporal resolution (50 ns) [6]. These experiments have allowed empirical fitting of modified Rayleigh-Plesset equations to model bubble oscillations and direct examination of fragmentation events [2], [7], [19]. High-speed camera observations on single microbubbles have allowed determination of the ultrasound parameters (namely, peak negative pressure, center frequency, phase angle, and pulse duration) that cause fragmentation in the experimental lipid-coated microbubble contrast agent MP1950 (kindly donated by Alexander L. Klibanov, University of Virginia, Charlottesville, VA) [8]. Properties of the microbubble itself also control the propensity for fragmentation; for example, fragmentation is strongly correlated to the resting radius. We show here that composition affects the probability of fragmentation for a given set of ultrasound parameters and initial resting radius.
II. Methods
A. Microbubble Preparation
Acronyms of the lipid components used to coat microbubbles are defined in Table I. The phospholipids (DMPC, DSPC, and DBPC) and lipopolymer emulsifiers (DMPE-PEG2000 and DSPE-PEG2000) were purchased from Avanti (Alabaster, AL). The surfactant emulsifier PEG-40 stearate was purchased from Sigma (St. Louis, MO). The lipophilic probe DiI (Molecular Probes, Eugene, OR) was added for fluorescence microscopy experiments. Brewster angle microscopy experiments of lipid monolayers at the air-water interface have shown that lipophilic probes reveal intrinsic phase coexistence (e.g., DiI preferentially partitions into disordered monolayer phases) without significantly affecting phase behavior [20], [21]. Microbubble cocktails were prepared as previously described [22]. All microbubble cocktails contained 89 mol% phospholipid, 10 mol% emulsifier, and 1 mol% fluorescent probe as the shell materials and perfluorobutane (Syn-Quest, Alachua, FL) as the filling gas. Microbubble suspensions were formed by the shaking method [23]. Yield was measured with a Coulter Multisizer II (Fullerton, CA), and typical values were 108–1010 microbubbles/ml. Experiments were performed on single microbubbles composed of each emulsifier type and the three homologous phospholipids (for a total of six permutations) in order to understand the effects of each component. Microstructure was controlled for DSPC:DSPE-PEG2000-coated microbubbles by two methods: heating the bubble suspension 5°C above the phospholipid main phase transition temperature (Tm = 55°C for DSPC) to dissolve the crystalline domains followed by rapid quenching to room temperature to produce fine grains as described in [12], [13]; or annealing the polycrystalline shell by heating the microbubble suspension to 50°C for 5 minutes then cooling to room temperature three times to produce coarse grains as described in [12]. Following production, microbubbles were washed by a method similar to that described in [24] to remove fluorescent lipid debris, then they were diluted (103–106 microbubbles/ml) in purified water for experimentation. Single microbubbles ranging in diameter between 1 and 10 μm were abundant following heat treatment and washing.
TABLE I.
Lipid Components Used to Form Microbubble Shell.
| Abbreviation | Name | Alkyl chain length | Category |
|---|---|---|---|
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine | C14 | Phospholipid |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine | C18 | Phospholipid |
| DBPC | 1,2-dibehenoyl-sn-glycero-3-phosphocholine | C22 | Phospholipid |
| DMPE-PEG2000 | 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] | C14 | Lipopolymer emulsifier |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] | C18 | Lipopolymer emulsifier |
| PEG-40 stearate | Polyoxyethylene 40 stearate (Myrj 52) | C18 | Surfactant emulsifier |
B. Gas Core and Lipid Shell Visualization During Insonification
Single microbubbles with initial resting diameters between 1 and 10 μm were imaged as they were insonified with a single, one-cycle pulse at 2.25 MHz (rarefaction first). The experimental setup was similar to the configuration described in [22], in which single microbubbles were optically observed through an inverted microscope during insonification. Ultrasound was produced by a single element 2.25-MHz transducer with a 0.75-inch aperture and a 2.0-inch focus (V305, Panametrics, Waltham, MA). An arbitrary waveform generator (AWG2021, Tektronix, Inc., Beaverton, OR) and a radio-frequency amplifier (325LA, ENI, Rochester, NY) were used to drive the transducer. Acoustic pressure calibrations were performed using a needle hydrophone (PZT-Z44-0400, Specialty Engineering Associates, Soquel, CA) and a preamplifier (A17dB, Specialty Engineering Associates) connected to a digital oscilloscope (9350, LeCroy Corp., Chestnut Ridge, NY). A water-immersion 100x objective (Achroplan, Carl Zeiss Inc., Thornwood, NY) was used to achieve sufficient magnification to visualize the microbubble behavior. Microbubbles were easily visualized under bright field due to the difference in refractive index between gas and water. Error in bright-field diameter measurements was largest (±1 μm) at small diameters (D < 3 μm) due to the increased thermal movement of the smaller bubble, which caused it to move into and out of the optical focus. Lipid-shedding events were observed by tracking the location of the fluorescent lipid probe in dark field and intermittently reverting to bright field to track the location of the gas core (transitioning from dark to bright field took approximately 1–5 seconds). Images were recorded at 30 frames/second with a CCD camera (Dage-MTI CCD100, Michigan City, IN), digitized and analyzed using the software program NIH ImageJ (available at http://rsb.info.nih.gov/ij/). The rate of acoustic dissolution was defined as the average change in diameter per pulse and was determined by a linear, least-squares fit to the data. The fragmentation diameter was defined as the resting diameter immediately prior to the pulse in which bubble fragmentation occurred. Statistical comparisons between data sets were made using the Student t-test (2-tail) assuming unequal variances.
III. Results
A. Acoustic Dissolution
Acoustic dissolution was observed for microbubbles coated with each lipid composition when insonified at 400 and 600 kPa. Fig. 1 shows the change in size of a typical DSPC:DSPE-PEG2000-coated microbubble with an initial diameter of 9 μm over 35 single-cycle pulses at 400 kPa peak negative pressure (PNP) and 0.5 Hz pulse repetition frequency (PRF). The sigmoid shape of the diameter-pulse curve that was often observed for larger microbubbles could be categorized into three regimes: a small degree of acoustic dissolution at larger diameters; a linear regime, or constant rate of acoustic dissolution, with a high degree of acoustic dissolution at intermediate diameters; very low acoustic dissolution at a stable diameter. Interestingly, the stable diameter was observed previously over a few pulses for lipid-coated microbubbles under similar conditions of insonification [2]. Microbubbles with smaller initial diameters were observed to follow the same overall trend; intermediate-sized microbubbles shrank at a constant rate until they reached the stable diameter, but microbubbles with an initial resting diameter less than or equal to the stable diameter did not change in size over many pulses (>50 pulses). Microbubbles were never observed to grow during the sequence of pulses.
Fig. 1.
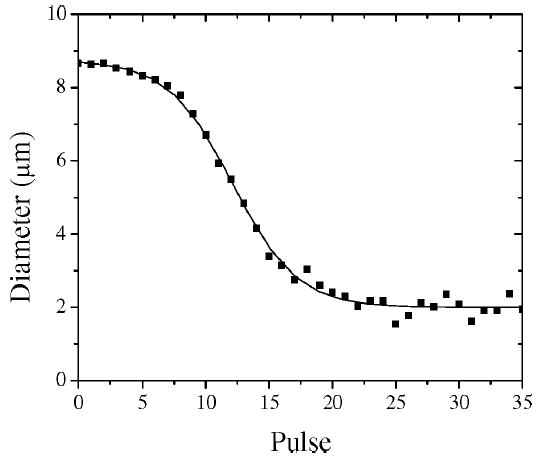
Typical pulse-diameter plot of a single microbubble (DSPC:DSPE-PEG2000) undergoing acoustic dissolution at 2.25 MHz, 400 kPa PNP, 0.5 Hz PRF. Measurements are shown as scatter, and sigmoid curve fit is shown as solid line. Sigmoid behavior exhibits the following three regimes: slow acoustic dissolution at large diameters; linear regime, with a constant acoustic dissolution rate at intermediate diameters; stable microbubble regime for which size does not change over many pulses.
Fig. 2 shows selected diameter-pulse plots for typical DMPC:DMPE-PEG2000-coated and DBPC:DSPE-PEG2000-coated microbubbles insonified with multiple, single-cycle pulses at 400 kPa PNP and 0.5 Hz PRF in order to illustrate the impact of phospholipid composition on the acoustic dissolution rate and stable diameter (large diameter regime not shown). The acoustic dissolution rate for each microbubble was determined by fitting a linear, least-squares trendline through the linear portion. In general, increasing the acyl chain length of the main phospholipid component caused the resulting microbubble to be more stable against acoustic dissolution. The stable diameter was determined by taking the average value of the measurements in the small diameter plateau region.
Fig. 2.
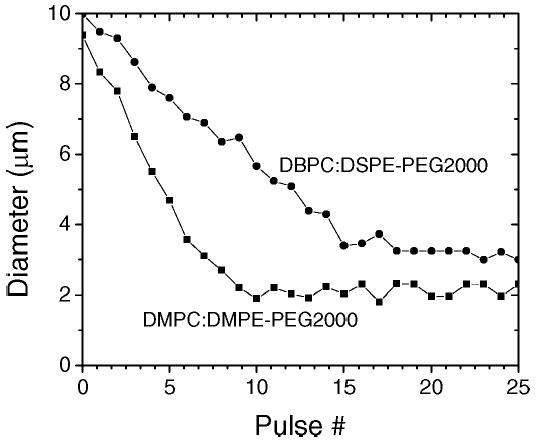
Composition dependence of pulse-diameter plots. Data is shown for single microbubbles coated with DMPC:DMPE-PEG2000 (▪) and DBPC:DSPE-PEG2000 (•).
Fig. 3 shows the values of the stable diameter and the linear acoustic dissolution rate (micrometers/pulse) for each microbubble as a function of the initial resting diameter prior to the first pulse (D0) for the three phospholipid species with the lipopolymer emulsifier. The statistical mean and standard deviation values of these parameters are listed in Table II for each of the tested phospholipid:emulsifier combinations and postprocessing conditions. The rate of acoustic dissolution in the linear regime decreased monotonically with increasing phospholipid acyl chain length (p < 0.0001). The stable diameter changed between DMPC and DSPC (p < 0.0001), but it remained unchanged within experimental error between DSPC and DBPC. Changing microstructure (i.e., coarse versus fine domains) or emulsifier type for a given phospholipid acyl chain length did not significantly affect the acoustic dissolution parameters.
Fig. 3.
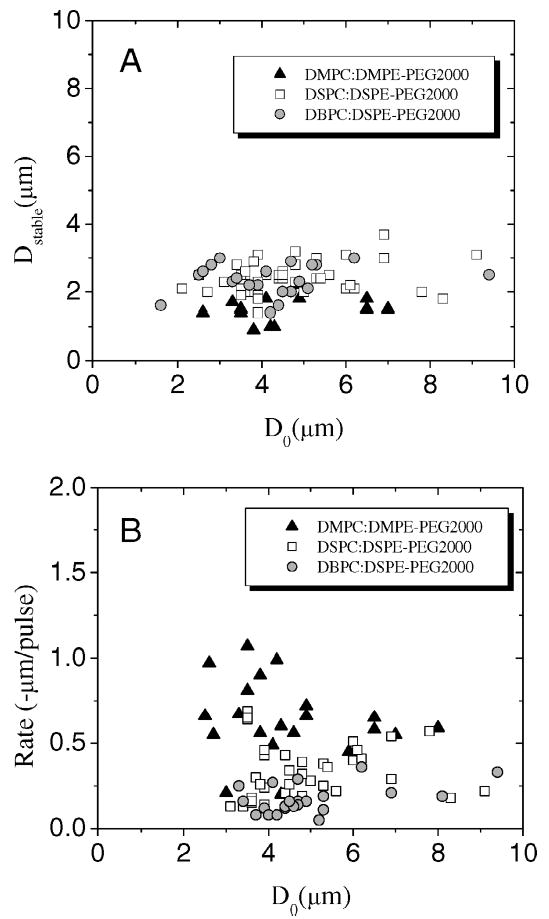
Composition and initial resting diameter dependence of acoustic dissolution parameters at 2.25 MHz, 400 kPa PNP, 0.5 Hz PRF. (a) Stable diameter and (b) acoustic dissolution rate of linear regime versus initial resting diameter. Data shown for microbubbles coated with the three phospholipid chain lengths with the lipopolymer emulsifier (i.e., DMPC:DMPE-PEG2000, DSPC:DSPE-PEG2000, and DBPC:DSPE-PEG2000).
TABLE II.
Experimentally Determined Acoustic Dissolution and Fragmentation Parameters.
| Phospholipid | Emulsifier | Post-production processing | Dissolution rate at 400 kPa PNP (μm/pulse)mean ± S.D. | Stable diameter at 400 kPa PNP (μm) mean ± S.D. | Fragmentation diameter ratio at 800 kPa PNP mean ± S.D. |
|---|---|---|---|---|---|
| DMPC | DMPE-PEG2000 | None | −0.6 ± 0.2 | 1.5 ± 0.4 | 0.9 ± 0.1 |
| PEG-40 stearate | None | −0.7 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.1 | |
| DSPC | DSPE-PEG2000 | None | −0.3 ± 0.2 | 2.4 ± 0.5 | 0.8 ± 0.2 |
| DSPE-PEG2000 | Rapid quench | −0.3 ± 0.2 | 2.1 ± 0.3 | — | |
| DSPE-PEG2000 | Anneal | −0.3 ± 0.1 | 2.6 ± 0.4 | — | |
| PEG-40 stearate | None | −0.3 ± 0.1 | 1.7 ± 0.3 | 0.7 ± 0.2 | |
| DBPC | DSPE-PEG2000 | None | −0.2 ± 0.1 | 2.4 ± 0.5 | 0.7 ± 0.1 |
| PEG-40 stearate | None | −0.3 ± 0.2 | 2.4 ± 0.5 | 0.6 ± 0.2 |
B. Fragmentation
Fig. 4 shows experimental data for the fragmentation diameter (Dfrag), defined as the microbubble resting diameter immediately prior to the pulse that results in fragmentation, for microbubbles coated with each phospholipid:lipopolymer composition that was insonified with single, one-cycle pulses at 800 kPa PNP and 0.5 Hz PRF. Fragmentation was observed as small microbubbles were expelled from the parent microbubble subsequent to the pulse (note that the optical resolution of our system was ~0.3 μm). Larger bubbles generally exhibited acoustic dissolution during the first few pulses prior to fragmentation (i.e., Dfrag < D0). The ratio of the fragmentation diameter to the initial resting diameter is a measure of the propensity for fragmentation (listed in column 6 of Table II). The propensity for fragmentation over the range of initial diameters tested decreased monotonically with increasing phospholipid acyl chain length (p < 0.0001), but it remained unchanged with varying emulsifier type for a given phospholipid.
Fig. 4.
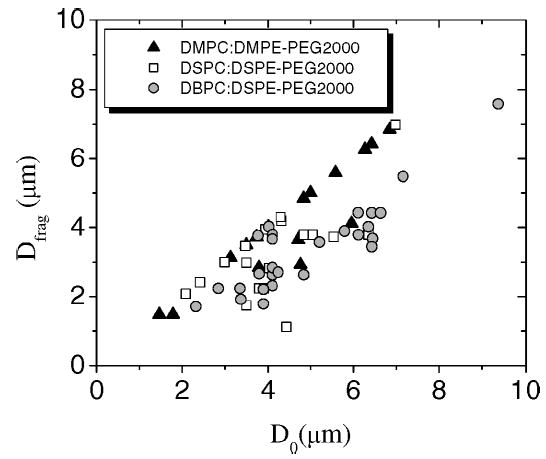
Composition and initial resting diameter dependence of fragmentation propensity at 2.25 MHz, 800 kPa PNP, 0.5 Hz PRF. Fragmentation diameter (Dfrag), defined as the resting diameter immediately prior to the pulse that induces fragmentation to form two or more daughter bubbles, is shown as a function of the initial resting diameter (D0) for individual microbubbles coated with the three phospholipid chain lengths and the lipopolymer emulsifier (i.e., DMPC:DMPE-PEG2000, DSPC:DSPE-PEG2000, and DBPC:DSPE-PEG2000).
C. Lipid Shedding
A reduction in surface area is the natural consequence of the irreversible gas loss from the echogenic core experienced during acoustic dissolution. Lipid shedding entails the expulsion of phospholipid:emulsifier material in excess of that required to complete the fully condensed monolayer shell around the resulting partially deflated gas core. This phenomenon requires the transition of shell material from the two-dimensional (2-D), fully condensed monolayer state to the 3-D collapse state and subsequent detachment from the monolayer shell. Herein, we describe the different lipid shedding mechanisms during intermittent and 0.5 Hz PRF insonification (single, one-cycle pulses; 400 kPa; 2.25 MHz) that were observed for the tested shell compositions and relate these mechanisms to shell cohesiveness.
Fig. 5 shows sequential fluorescent images of typical microbubbles that illustrate the composition dependence of the different lipid-shedding mechanisms during intermittent insonification. Row a shows a typical microbubble coated with DMPC:DMPE-PEG2000 experiencing acoustic dissolution with no observable shedding or increase in fluorescence intensity on the shell, perhaps indicating that the lipid material is being shed as diffuse, submicron particles (i.e., as micelles and vesicles) during each pulse. Row b shows a typical microbubble coated with DSPC:DSPE-PEG2000 experiencing the formation of a bud during the pulse that spontaneously detaches from the microbubble shell and diffuses away under Brownian motion prior to the next pulse. Row c shows a microbubble coated with the same phospholipid but a different emulsifier (i.e., DSPC:PEG-40 stearate); shell material builds up in the form of lipid strings and globular aggregates that intermittently detach from the shell and diffuse away over a series of pulses. Shown in row d is a typical microbubble coated with DBPC:PEG-40 stearate that exhibits extended buildup of shell material while the gas core is completely diminished due to acoustic dissolution. Imaging in bright-field mode showed that the gas core of each bubble was spherical subsequent to each pulse throughout the entire experiment; insonification did not produce elongated, crumpled, or folded shapes of the gas core as was observed in previous static dissolution studies in degassed media [15]. It is possible, however, that aspherical bubble morphologies were present during the pulse, as was observed previously [25].
Fig. 5.
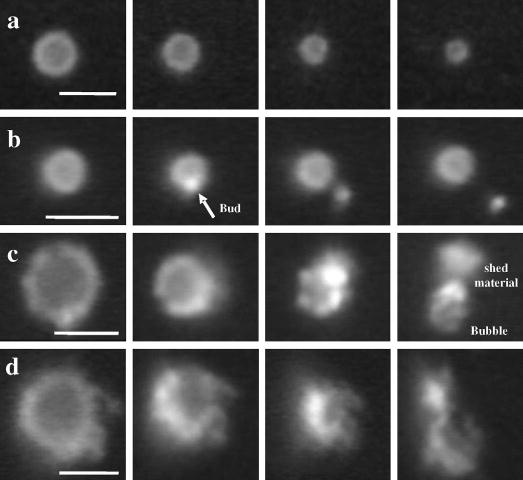
Sequential fluorescent video micrographs showing lipid shedding mechanisms as a function of lipid shell composition during intermittent, single-cycle pulsing at 2.25 MHz, 400 kPa PNP: (a) DMPC:DMPE-PEG2000-coated microbubbles shed excess material, probably as diffuse submicron particles, without an observable buildup of collapse lipid on the shell. (b) DSPC:DSPE-PEG2000-coated microbubbles form micron-sized buds that spontaneously shed under thermal motion or due to ultrasound on a subsequent pulse. (c) DSPC:PEG-40 stearate-coated microbubbles exhibit buds that grow during pulsing and detach less readily, significantly increasing the bubble surface area. (d) DBPC:PEG-40 stearate-coated microbubbles exhibit buds and strings that build up to form large lipid particles several microns in diameter after the gas core has been completely eliminated. Scale bars represent 5 μm.
The lipid-shedding behavior observed for each composition during insonification at 0.5 Hz PRF was similar to that observed during intermittent pulsing. Interestingly, DMPC-coated microbubbles exhibited two modes of lipid shedding that depended on the diameter of the gas core prior to the pulse (Dgas). The formation of micron-scale fluorescent buds were observed at smaller diameters (Dgas < 5 μm), but no discernable lipid buildup or aggregate formation was observed at larger diameters (Dgas > 5 μm). The latter case probably involved the shedding of diffuse, submicron particles. The formation of lipid buds following each pulse was observed for all other lipid-shell compositions, independent of microbubble size. However, the number of pulses between initial bud formation and detachment, which is a measure of the buildup of 3-D collapsed lipid-shell material, varied significantly as a function of shell composition. For example, 1-2 μm fluorescent buds often formed then detached either spontaneously or due to the next pulse for the soft-shell microbubbles (DMPC:DMPE-PEG2000, DMPC:PEG-40 stearate, and DSPC:DSPE-PEG2000). In contrast, the initial buds grew into larger collapse structures (up to 5 μm in length), such as fluorescent strings and globules, during multiple pulses prior to detachment for the stiff-shell microbubbles (DSPC:PEG-40 stearate, DBPC:DSPE-PEG2000, and DBPC:PEG-40 stearate). Detachment in the latter case was facilitated by the ultrasound pulse, presumably due to oscillation of the gas core. Each fluorescent lipid collapse structure was eventually shed after many pulses, even for the stiffest shell composition, resulting in a “clean” shell without excessive buildup once a microbubble had been insonified at the stable diameter for several pulses.
Fig. 6 is a schematic representation showing the progression of the possible lipid-shedding mechanisms with increasing shell cohesiveness, which includes the following: the expulsion of diffuse, submicron particles, budding with spontaneous shedding due to thermal motion or ultrasound-assisted shedding due to oscillation during the next pulse, buildup of excess lipid with intermittent, ultrasound-assisted shedding, and extended buildup of excess lipid with occasional ultrasound-assisted shedding. The amount of lipid material that accumulated on the shell with each subsequent pulse increased with the acyl chain length of the main phospholipid component (Table III). Thus, the size and type of the particle that was shed due to acoustic dissolution depended on shell composition and, in the case of DMPC as the main lipid component, microbubble size.
Fig. 6.

Schematic representation showing the progression of possible excess lipid shedding mechanisms during acoustic dissolution with increasing cohesiveness of the phospholipid/emulsifier monolayer shell. Arrows designate a change in shell composition that leads to greater cohesiveness (e.g., increasing phospholipid acyl-chain length).
TABLE III.
Experimentally Determined Lipid-Shedding Parameters.
| Shell composition | Shed particle type | Pulses between shedding events mean ± S.D. |
|---|---|---|
| DMPC:DMPE-PEG2000 | Submicron (Dbub > 5 μm), 1 μm buds (Dbub < 5 μm) | 1 ± 1 |
| DMPC:PEG-40 stearate | Submicron (Dbub > 5 μm), 1–2 μm buds (Dbub < 5 μm) | 2 ± 2 |
| DSPC:DSPE-PEG2000 | 1–2 μm buds | 2 ± 2 |
| DSPC:PEG-40 stearate | 1–3 μm buds/strings | 3 ± 3 |
| DBPC:DSPE-PEG2000 | 1–5 μm buds/strings/globules | 8 ± 2 |
| DBPC:PEG-40 stearate | 1–5 μm buds/strings/globules | 9 ± 5 |
D. Effect of Microstructure
Changing lipid-domain size for a given shell composition and initial resting diameter did not significantly affect the overall mechanism of lipid shedding (Table II). For example, DSPC:DSPE-PEG2000-coated microbubbles with large domains (produced by annealing) exhibited the same type of budding with spontaneous shedding as microbubbles of the same composition with small domains produced by rapid quenching. However, observations of microbubbles with large domains did yield some insight into the shell behavior during insonification. Fig. 7 shows sequential images of a typical microbubble exhibiting large domains as it experiences acoustic dissolution over a series of single-cycle pulses (600 kPa PNP; 0.5 Hz PRF). The lobed shapes of the dark, condensed phase domains were conserved over several pulses. The shell morphology changed as the resting diameter becomes small (D < 5 μm), eventually resulting in more uniform fluorescence at a lower intensity. Patches of bright fluorescence intensity often were observed in the intervening fluorescent areas; buds of excess lipid material were observed to nucleate from these patches in the interdomain regions.
Fig. 7.
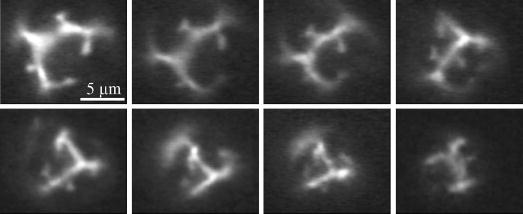
Effect of acoustic dissolution on lipid-shell microstructure (DSPC:DSPE-PEG2000; 2.25 MHz, 600 kPa PNP, intermittent, single-cycle pulsing). Sequential images are shown in chronological order going from left to right then top to bottom. Condensed lipid-domain shapes are conserved after several pulses, and bud formation occurs in interdomain areas as evident from patches of high fluorescence intensity. Scale bar represents 5 μm.
Domain morphology was conserved after bubble coalescence and fragmentation as well. Fig. 8 shows a typical case of two microbubbles with large domains coming together under secondary radiation force, then coalescing. The presence of the large domains is clearly evident on the resulting bubble. Dark domains also were observed on the daughter bubbles following fragmentation of a microbubble exhibiting heterogeneous shell morphology. These results indicate that the condensed-phase, lipid domains remain intact during the shell fracture and resealing events that occur during fragmentation and coalescence.
Fig. 8.
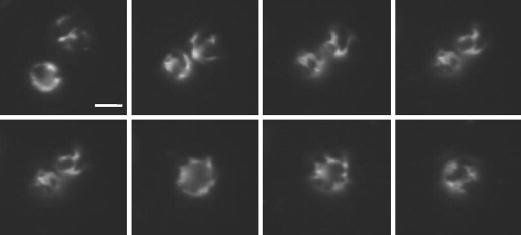
Effect of ultrasound-induced coalescence on lipid-shell microstructure (DSPC:DSPE-PEG2000; 2.25 MHz, 400 kPa PNP, 0.5 Hz PRF). Sequential images are shown in chronological order going from left to right then top to bottom. Microbubbles approach each other due to secondary radiation force then coalesce. The presence of condensed-phase domains persist on the fused microbubble. Scale bar represents 5 μm.
IV. Discussion
Previous studies using ultra-high speed cameras to image single, lipid-coated microbubbles during the ultrasound pulse have permitted direct observation of microbubble dynamics [2], [6]–[10], [25]. Results from these experiments have allowed the empirical determination of ultrasound parameter thresholds that lead to microbubble destruction and observations on the morphology of the gas core during compression and rarefaction. However, due to the low light intensity of fluorescent emissions from the microbubble shell, it has not yet been possible to use these high-speed camera techniques to image the dynamics of the microbubble shell during ultrasound insonification. Results from the present study, which examine the state of the shell between pulses, allow interpretation of the state of the shell during the pulse. Furthermore, these results show that lipid shell composition plays a significant role in the shell dynamics, particularly with respect to destruction events such as acoustic dissolution and fragmentation. What follows is a discussion of the impact of lipid shell microstructure and composition on the fate of the shell during destructive insonification.
Our results clearly show that physicochemical properties of the lipid shell have a substantial Effect on the microbubble response to insonification. As shown in Figs. 2, 3, and 4 and Tables II and III, the acyl chain length of the main component, phospholipid, influenced the rate of acoustic dissolution, the fragmentation propensity, and the excess lipid shedding mechanism for the ultrasound parameters tested in the present study. The Effect of composition on these properties followed the principle first proposed by Kim et al. [12] and Kim [26] for lipid-coated microbubbles, that is, increasing the number of methylene groups per hydrocarbon chain increases the attractive intermolecular dispersion forces between constituent lipids in the monolayer shell and thus increases the overall cohesiveness of the monolayer. This increase in cohesiveness clearly results in greater stability for a microbubble experiencing oscillations due to insonification. Possible mechanisms leading to the increase in stability against acoustic dissolution include: decreased gas permeability of the shell restricting gas escape, and increased shell stiffness resisting collapse and thus increasing the minimum diameter experienced during compression. The monolayer is fractured and reformed during fragmentation, a process that probably is strongly dependent on monolayer cohesiveness. A similar phenomenon occurs during the process of lipid shedding. Excess lipid is likely ejected from the monolayer shell through the formation of collapse folds which grow into lamellar extensions that protrude into the surrounding medium, as was observed previously for similar lipid monolayer systems at the air-water interface [27]. Lipid shedding involves the detachment of the folded extension—or vesicle—from the monolayer shell, and thus the cohesive forces that resist monolayer fracture must be overcome. This explains the tendency of microbubbles coated with longer acyl chain phospholipids to exhibit material buildup on their shells during insonification (Fig. 5). Emulsifier type, however, does not follow this trend. The double-chain lipopolymer emulsifier should experience more intermolecular dispersion forces and thus should lead to a more cohesive shell than the single-chain, surfactant emulsifier. The result that PEG-40 stearate yielded significantly more material buildup and less shedding during insonification when mixed with each phospholipid indicates that factors other than dispersion forces between hydrophobic moieties may be playing a role. Regardless, these results clearly show that lipid-shell composition can be varied with ultrasonic preconditioning to modulate the surface characteristics of the microbubble shell.
The presence of a stable diameter at the low acoustic pressure of 400 kPa PNP was intriguing (Figs. 1 and 2). Observations of the gas core in bright field and the “clean” shell after multiple pulses at the stable diameter in dark field indicate that the particle is essentially a monolayer-coated bubble that is acoustically active. Earlier results have shown that the relative expansion (i.e., the ratio of the maximum diameter achieved during rarefaction to the initial resting diameter) increases significantly with decreasing initial resting diameter [2], [6]–[8]. Accordingly, relative compression—which is dominated by the inertial collapse of the gas core following rarefaction—should be greater for smaller bubbles. These factors indicate that acoustic dissolution should accelerate with the decreasing resting radius during pulsing. However, our results clearly show the existence of a stable resting diameter equal to 2 ± 1 μm for these lipid-coated microbubbles (Figs. 1–3 and Table II). Microbubbles with an initial resting diameter larger than this value experience acoustic dissolution until they reach the stable diameter, at which point they do not change in size over many pulses; but those with a smaller initial resting diameter do not experience any observable acoustic dissolution (note that our optical resolution was limited to 1 μm). The latter observation indicates that the stable diameter is not due to ultrasound conditioning of the lipid shell (as might be inferred from the observations of excess lipid shell buildup) because microbubbles with smaller initial diameters would not have experienced any prior pulsing and thus would be expected to experience acoustic dissolution. Interestingly, the stable diameter is close to the estimated linear resonance diameter of 3 μm calculated for an unshelled microbubble [19]. It is unclear if resonance itself minimizes acoustic dissolution, or if this result is merely a coincidence. Another possible explanation for the existence of the stable diameter is the rectified diffusion threshold, a phenomenon widely cited in the experimental and theoretical descriptions of steady-state sonoluminescent bubbles [28]. Microbubbles with diameters smaller than this threshold are predicted to grow until they reach this threshold (although they would rapidly dissolve back to the initial resting diameter following pulsing due to the inability of new lipid to adsorb and minimize surface tension), and microbubbles with larger diameters are predicted to shrink until they reach this threshold. A lack of mechanical parameters describing the dynamic dilatational and shear visco-elastic behavior of lipid monolayers at ultrasonic frequencies and analytical expressions that include such physical constraints precludes direct calculation of the rectified diffusion threshold diameter for the given ultrasound parameters. Regardless of the mechanism responsible for this interesting phenomenon, the existence of the observed stable diameter likely will be useful in designing acoustic preconditioning techniques to modify contrast agent size distribution. It also is useful to emphasize the unique ability of the lipid monolayer shell to maintain its integrity over many cycles of high lateral stretching and compression. Future work in our lab will include investigations into the effects of ultrasound parameters such as pressure, frequency, phase angle, and PRF—in concert with shell parameters such as composition and microstructure—on the stable diameter of single microbubbles and populations.
Observations of insonified, lipid-coated microbubbles with large condensed lipid domains in the shell yield some important insights into the fate of the shell during expansion and compression. Previous work by our group involving experimental observations of microbubble behavior during insonification has revealed the extent to which the microbubble expands and contracts during rarefaction and compression [19]. A modified Herring equation with viscous and elastic shell terms, which successfully modeled the transient radial oscillations of an insonified microbubble of similar composition and with similar ultrasound parameters (2.4 MHz, 360 kPa, single, one-cycle pulse, 180° phase angle) as the present study, predicted that for a 2.0-μm microbubble the diameter will expand by a factor of approximately 2.5 during rarefaction and contract by a factor of approximately 2 during compression; the surface area is predicted to increase roughly by a factor of 6 and contract roughly by a factor of 4, respectively, under these conditions. Thus, the shell experiences drastic dilatational deformation during each pulse.
As shown in Figs. 7 and 8, the persistence of the dark domains and the conservation of their shape indicate that they probably do not completely dissolve into the expanded phase region during rarefaction or fracture during compression. More evidence that planar lipid domains persist during rarefaction and fold during compression was recently reported for microbubbles undergoing relatively slow dynamics due to changes in hydrostatic pressure [29]. A recent study by Postema et al. [30] of ultrasound-induced coalescence of lipid-coated microbubbles indicates that the shell ruptures during rarefaction such that it exposes patches of bare air-water interface. Likewise, the expanded phase region is not preferentially expelled into the surrounding medium such that a dark shell remains comprising only condensed phase domains. Thus, the heterogeneous shell morphology is hypothesized to be present during all states of oscillation in any given pulse. A possible picture of the shell during each stage of the pulse emerges, in which lipid domains persist as islands on the bubble surface as the gas core expands, then fold and straighten out—like an accordion—as the shell collapses and expands back to the resting radius. The decrease in domain size, slow change in domain morphology, and eventual conversion to a uniformly fluorescent shell over many pulses, however, does indicate that some dissolution of the domains into the interdomain area might occur with each pulse, and that the remixing of the shell components increases as the bubble resting diameter decreases (i.e., the driving force and kinetics for domain dissolution increase).
The lipid shedding mechanisms described here provide insight into how composition might be used in microbubble targeting and drug delivery. For targeting, the shell surface area of the microbubble for a given gas volume is an important consideration because it dictates the architecture and total number of ligands available for binding. Rychak et al. [31] recently showed that microbubbles which were partially deflated by subjecting them to positive pressure in order to achieve an increased surface area to gas volume ratio bind more efficiently than their fully inflated counterparts. Composition provides another tool for manipulating total surface area. For example, using long-acyl chain phospholipids with the surfactant emulsifier may increase binding efficiency due to the increased surface area and deformability of the built-up shell material. Furthermore, the shedding mechanism plays an important role in the delivery of shell-associated therapeutic agents to the site of interest. Short-acyl chain phospholipids and the lipopolymer emulsifier are more suitable for applications requiring rapid release throughout the target volume, but long-acyl chain phospholipids and the surfactant emulsifier are more suitable for applications that require concentration of drug to particular cells amid nontarget cells.
V. Conclusions
The current work presents a phenomenological study of the role of shell composition in modulating the acoustic response of lipid-coated microbubbles. We investigated stability and lipid-shedding behavior for agents composed of a homologous series of saturated phospholipids and surfactant versus lipopolymer emulsifier type. Our results clearly show that composition plays a role; increasing shell cohesiveness through choosing longer phospholipid acyl chain lengths decreased the rate of acoustic dissolution and propensity for fragmentation. Interestingly, we found that microbubbles were capable of reaching a stable diameter over many pulses. Future work in our lab will entail experiments that probe the influence of resonance and frequency on the stable diameter. Microstructure—that is, the size of crystalline domains within the monomolecular shell—did not significantly affect either microbubble stability or lipid-shedding behavior during insonification under the conditions tested. However, domain visualization during a sequence of pulses allowed some insight into the state of the shell during the rarefaction and compression regimes of the pulse, as well as the role of the resting diameter. Future work will entail probing these physicochemical effects over a range of ultrasonic frequencies. In general, these results show that lipid-shell composition should be an important consideration in the design of ultrasound contrast agents and ultrasound-assisted drug delivery vehicles.

Mark Andrew Borden (M’04) received the B.S. degree in chemical engineering in 1999 from the University of Arizona, Tucson, and the Ph.D. degree in chemical engineering in 2003 from the University of California, Davis. He conducted undergraduate research on competitive protein adsorption in glass capillaries and peptide affinity to immobilized-metal-ion chromatography columns, and he designed and tested an actuator to accurately control surface tension-driven sample aspiration into glass capillaries. His doctoral research involved production and characterization of diblock copolymer bilayer vesicles and modeling and characterization of the gas transport properties, such as oxygen permeability and surface morphology of lipid-coated microbubbles. He is currently a project scientist in the Department of Biomedical Engineering and Fellow in the Professors for the Future Program at the University of California, Davis.
His current research interests include investigation of the physical properties of condensed multicomponent lipid monolayers and engineering-improved ultrasound contrast agents for applications in molecular imaging and drug delivery.

Paul Alexander Dayton graduated from Villanova University, Villanova, PA, in 1995 with B.S. degrees in physics and comprehensive science, and a minor in chemistry. As an undergraduate, he conducted research in surface science with the Physics Department and pulse sequence programming for nuclear magnetic resonance spectrometer in the Chemistry Department. He received his M.E. degree in electrical engineering and his Ph.D. degree in biomedical engineering from the University of Virginia, Charlottesville, VA, in 1998 and 2001, respectively. His research involved the study of ultrasound contrast agents. Dr. Dayton conducted post-doctoral research at the University of California, Davis, and is now an assistant research professor at the same institution.
His current research interests include targeted imaging and drug delivery.

Katherine Whittaker Ferrara (S’82–M’87–SM’99) received the B.S. and M.S. degrees in electrical engineering in 1982 and 1983, respectively, from the California State University, Sacramento, and the Ph.D. degree in electrical engineering and computer science in 1989 from the University of California, Davis.
From 1983 to 1988, she worked for Sound Imaging, Inc., Folsom, CA, and for General Electric Medical Systems, Rancho Cordova, CA, in the areas of magnetic resonance and ultrasound imaging. From 1989–1993, she was an associate professor in the Department of Electrical Engineering at California State University, Sacramento. From 1993–1995, she was a principal member of the research staff at the Riverside Research Institute, New York, NY. From 1995–1999, she was an associate professor at the University of Virginia, Charlottesville, VA.
She is currently a Professor in the Department of Biomedical Engineering, University of California, Davis, having served as the founding chair of the department from 1999 to 2004. She served as a regular member of the Diagnostic Radiology Study Section (2000–2004), is a Fellow of the Acoustical Society of America, and serves on the Editorial Board of Annual Reviews in Biomedical Engineering.

Charles Caskey received his B.S. degree in electrical and computer engineering from the University of Texas, Austin, where he did undergraduate research in elasticity imaging. He is currently pursuing a Ph.D. degree in biomedical engineering at the University of California, Davis.
His current research interests include improving vascular permeability with ultrasound contrast agents.

Dustin E. Kruse (S’99–M’04) received a B.A. degree in physics from the State University of New York College at Geneseo in 1996, the M.E. degree in electrical engineering from the University of Virginia, Charlottesville, VA, in 1999, and the Ph.D. degree in biomedical engineering in 2004, also from the University of Virginia.
His graduate work involved the development and application of high-frequency ultrasound to image blood flow in the microcirculation. His research interests include velocity estimation, ultrasound instrumentation, and contrast-assisted imaging. He is a member of Sigma Pi Sigma.

Shukui Zhao (S’02) received the B.S. degree in material science and engineering in 1998 and the M.S. degree in biomedical engineering in 2001, both from Sichuan University, Chengdu, China, and the M.S. degree in electrical engineering in 2003 from St. Cloud State University, St. Cloud, MN. He is currently pursuing a Ph.D. degree in biomedical engineering in the Department of Biomedical Engineering at the University of California, Davis.
His current research interests include targeted imaging with ultrasound contrast agent and ultrasound radiation force for enhanced targeting efficiency.
Acknowledgments
The authors would like to thank the following grant sources for funding: NIH CA 76062 and NIH CA 103828. A portion of this investigation was presented at the 2004 IEEE UFFC meeting in Montreal, Canada.
References
- 1.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. “Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion,”. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 2.Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. “Mechanisms of contrast agent destruction,”. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2001;48:232–248. doi: 10.1109/58.896136. [DOI] [PubMed] [Google Scholar]
- 3.Lindner JR. “Microbubbles in medical imaging: Current applications and future directions,”. Nature Rev Drug Discovery. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 4.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. “Therapeutic applications of lipid-coated microbubbles,”. Adv Drug Delivery Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. “A method for radiation-force localized drug delivery using gas-filled lipospheres,”. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2004;51:822–831. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- 6.Dayton PA, Morgan KE, Klibanov AL, Branden-burger GH, Ferrara KW. “Optical and acoustical observations of the effects of ultrasound on contrast agents,”. IEEE Trans Ultrason, Ferroelect, Freq Contr. 1999;46:220–232. doi: 10.1109/58.741536. [DOI] [PubMed] [Google Scholar]
- 7.Chomas JE, Dayton PA, May D, Allen J, Klibanov A, Ferrara K. “Optical observation of contrast agent destruction,”. Appl Phys Lett. 2000;77:1056–1058. [Google Scholar]
- 8.Chomas JE, Dayton P, May D, Ferrara K. “Threshold of fragmentation for ultrasonic contrast agents,”. J Biomed Opt. 2001;6:141–150. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- 9.Postema M, Bouakaz A, Chin CT, de Jong N. “Simulations and measurements of optical images of insonified ultrasound contrast microbubbles,”. IEEE Trans Ultrason, Ferro-elect, Freq Contr. 2003;50:523–536. [Google Scholar]
- 10.Bloch SH, Wan M, Dayton PA, Ferrara KW. “Optical observation of lipid- and polymer-shelled ultrasound microbubble contrast agents,”. Appl Phys Lett. 2004;84:631–633. [Google Scholar]
- 11.Leong-Poi H, Song J, Rim SJ, Christiansen J, Kaul S, Lindner JR. “Influence of microbubble shell properties on ultrasound signal: Implications for low-power perfusion imaging,”. J Amer Soc Echocardiogr. 2002;15:1269–1276. doi: 10.1067/mje.2002.124516. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Costello MJ, Duncan PB, Needham D. “Mechanical properties and microstructure of polycrystalline phospholipid monolayer shells: Novel solid microparticles,”. Langmuir. 2003;19:8455–8466. [Google Scholar]
- 13.Borden MA, Pu G, Runner GJ, Longo ML. “Surface phase behavior and microstructure of lipid/PEG-emulsifier monolayer-coated microbubbles,”. Colloids Surfaces B-Biointerfaces. 2004;35:209–223. doi: 10.1016/j.colsurfb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.M. A. Borden, P. Dayton, S. Zhao, and K. Ferrara, “Physicochemical properties of the microbubble lipid shell,” presented at IEEE Ultrason. Symp., Montreal, Quebec, Canada, 2004.
- 15.Borden MA, Longo ML. “Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: Effect of lipid hydrophobic chain length,”. Langmuir. 2002;18:9225–9233. [Google Scholar]
- 16.Duncan PB, Needham D. “Test of the Epstein-Plesset model for gas microparticle dissolution in aqueous media: Effect of surface tension and gas undersaturation in solution,”. Langmuir. 2004;20:2567–2578. doi: 10.1021/la034930i. [DOI] [PubMed] [Google Scholar]
- 17.Borden MA, Longo ML. “Oxygen permeability of fully condensed lipid monolayers,”. J Phys Chem B. 2004;108:6009–6016. [Google Scholar]
- 18.Klibanov AL, Hughes MS, Wojdyla JK, Wible JH, Brandenburger GH. “Destruction of contrast agent microbubbles in the ultrasound field: The fate of the microbubble shell and the importance of the bubble gas content,”. Academic Radiol. 2002;9:S41–S45. doi: 10.1016/s1076-6332(03)80393-8. [DOI] [PubMed] [Google Scholar]
- 19.Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibanov AL, Ferrara KW. “Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size,”. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2000;47:1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- 20.Nag K, Keough KMW. “Epifluorescence microscopic studies of monolayers containing mixtures of dioleoyl- and dipalmitoylphosphatidylcholines,”. Biophys J. 1993;65:1019–1026. doi: 10.1016/S0006-3495(93)81155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klopfer KJ, Vanderlick TK. “Isotherms of dipalmitoylphosphatidylcholine (DPPC) monolayers: Features revealed and features obscured,”. J Colloid Interface Sci. 1996;182:220–229. [Google Scholar]
- 22.Zhao S, Borden MA, Bloch S, Kruse D, Ferrara KW, Dayton PA. “Radiation-force assisted targeting facilitates ultrasonic molecular imaging,”. Molecular Imag. 2004;3:135–148. doi: 10.1162/1535350042380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.E. C. Unger, T. A. Fritz, T. Matsunaga, Ramaswami, Varadarajan, D. Yellowhair, Wu, and Guanli, “Methods of preparing gas-filled liposomes,” U.S. Patent 5,935,553, Nov. 1999.
- 24.Takalkar AM, Klibanov AL, Rychak JJ, Lindner JR, Ley K. “Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow,”. J Controlled Release. 2004;96:473–482. doi: 10.1016/j.jconrel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.M. Versluis, S. M. van der Meer, D. Lohse, P. Palanchon, D. Goertz, C. T. Chin, and N. de Jong, “Microbubble surface modes,” presented at Proc. IEEE Ultrason. Symp., Montreal, Quebec, Canada, 2004.
- 26.D. H. Kim, “Mechanical properties, microstructure, and specific adhesion of phospholipid monolayer-coated microbubbles,” Ph.D. dissertation, Duke University, Durham, NC, 1999.
- 27.Ridsdale RA, Palaniyar N, Possmayer F, Harauz G. “Formation of folds and vesicles by dipalmitoylphosphatidyl-choline monolayers spread in excess,”. J Membrane Biol. 2001;180:21–32. doi: 10.1007/s002320010055. [DOI] [PubMed] [Google Scholar]
- 28.Louisnard O, Gomez F. “Growth by rectified diffusion of strongly acoustically forced gas bubbles in nearly saturated liquids,”. Phys Rev E. 2003;67(1–12):036610. doi: 10.1103/PhysRevE.67.036610. [DOI] [PubMed] [Google Scholar]
- 29.Klibanov A, Rychak JJ, Hughes MS, Cantrell GL, Wible JH. “Monolayer-coated gas-filled microbubbles: Shell behavior in the pressure field,”. Biophys J. 2005;88:p. 207A. [Google Scholar]
- 30.Postema M, Marmottant P, Lancee CT, Hilgenfeldt S, De Jong N. “Ultrasound-induced microbubble coalescence,”. Ultrasound Med Biol. 2004;30:1337–1344. doi: 10.1016/j.ultrasmedbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Rychak JJ, Ley K, Klibanov A. “Shape modification enhances the attachment efficiency of P-selectin targeted ultrasound contrast microbubbles,”. Biophys J. 2004;86:p. 520a. [Google Scholar]


