Abstract
We designed this study to evaluate the effect of small-dose intravenous ketamine in combination with continuous femoral nerve block on postoperative pain and rehabilitation after total knee arthroplasty. Continuous femoral nerve block with ropivacaine was started before surgery and continued in the surgical ward for 48 h. Patients were randomly assigned to receive an initial bolus of 0.5 mg/kg ketamine followed by a continuous infusion of 3 μg·kg-1·min-1 during surgery and 1.5 μg·kg-1·min-1 for 48 h (Ketamine group) or an equal volume of saline (Control group). Additional postoperative analgesia was provided by patient-controlled intravenous morphine. Pain scores and morphine consumption were recorded over 48 hours. The maximal degree of active knee flexion tolerated was recorded daily until hospital discharge. Follow up was performed 6 weeks and 3 months after surgery. The Ketamine group required significantly less morphine than the Control group (45 ± 20 mg versus 69 ± 30 mg; P < 0.02). Patients in the Ketamine group reached 90° of active knee flexion more rapidly than those in the Control group (P < 0.02). Outcomes at 6 weeks and 3 months were similar in each group. These results confirm that ketamine is a useful analgesic adjuvant in perioperative multimodal analgesia with a positive impact on early knee mobilization.
Keywords: Anesthetic Technique: femoral nerve block; Pain: postoperative hyperalgesia, rehabilitation; Analgesics: ketamine, sufentanil, morphine; Anesthesia
INTRODUCTION
The concept of multimodal analgesia refers to the simultaneous use of multiple analgesic methods or drugs. Since acute pain is an integrated process that is mediated by activation of numerous biochemical and anatomical pathways (1), administration of analgesics acting on different targets is a rational postoperative analgesic strategy. Among the receptors implicated in the nociceptive transmission, the N-methyl-D-aspartate (NMDA) receptor play a critical role in neuronal plasticity leading to central sensitization and, therefore, in the intensity of perceived postoperative pain (2).
There is a growing body of evidence that ketamine, a non-competitive antagonist at NMDA receptors (3), can facilitate postoperative pain management (4). Ketamine also alleviates provoked pain by preventing postoperative hyperalgesia (5). Furthermore, a single intraoperative injection of 0.15 mg/kg ketamine improves passive knee mobilization 24 hours after arthroscopic anterior ligament repair (6) and improves postoperative functional outcome after outpatient knee arthroscopy (7).
Total knee arthroplasty generates substantial postoperative pain. Peripheral nerve blocks produce better analgesia than patient-controlled intravenous opioids, thereby accelerating rehabilitation (8,9). However, isolated femoral nerve blocks provide insufficient analgesia; patients given femoral nerve blocks alone thus ususally require rescue treatment with opioids (10,11). Combining a femoral nerve block with small-dose ketamine may be an alternative to concomittent opioid administration. However, the benefit of adjunctive small-dose ketamine in patients with peripheral nerve blocks has yet to be determined. We therefore tested the hypothesis that small-dose ketamine reduces postoperative pain and speeds rehabilitation after total knee arthroplasty in patients with a continuous femoral nerve block.
METHODS
With approval of the local ethics committee and informed consent, we studied ASA physical status I-III patients. All were scheduled to undergo elective total knee arthroplasty with general anesthesia. Exclusion criteria included age younger than 18 years or older than 80 years; weight exceeding 100 kg; inability to use patient-controlled analgesia (PCA); contraindications to continuous femoral nerve block (i.e., coagulation defects, infection at puncture site); previous total or unilateral knee arthroplasty; diabetes; severe respiratory insufficiency; renal impairment; psychiatric disorders; chronic opioid use; and history of chronic pain syndromes.
Previous studies (8,9) and our own experience indicate that PCA morphine use over 48 hours to be 67 ± 30 mg (mean ± SD) in patients having total knee arthroplasty and regional analgesia. Twenty patients per group thus provided an 80% power for detecting a 40% difference in morphine consumption at an alpha level of 0.05. We thus made an a priori decision to evaluate 20 patients per group.
Protocol
Patients were assigned randomly, in a double-blind fashion, to one of two groups (20 per group): a Control group and a Ketamine group. Before the study began, a random-number table was generated, specifying the group to which each patient would be assigned upon entry into the trial. For each patient, an envelope containing the group assignment was prepared, sealed, and sequentially numbered. On the morning of surgery, a nurse, not involved in the evaluation of the patient, opened the patient's envelope and prepared two syringes: one 5 ml syringe for the bolus dose and a 50 ml syringe for the continuous infusion, containing either saline or 10 mg/ml of ketamine. None of the other investigators involved in patient management and data collection was aware of the group assignment. In case of emergency, the anesthesiologist in charge of the patient had ready access to the information about the drugs given.
All patients were premedicated with hydroxyzine 1-2 mg/kg orally, 1-2 hours before surgery. The patients were taken to a preoperative block room and vital signs were monitored. Midazolam (0.025 mg/kg IV) was given for sedation. A continuous femoral nerve block was performed using the landmarks suggested by Winnie et al (12). Patients were given 0.3 ml/kg ropivacaine 0.75% through a femoral catheter. Absence of sensory response to cold in the area of the femoral nerve confirmed that the catheter was properly positioned.
Anesthesia was subsequently induced with 3-5 mg/kg thiopental, 0.3 μg/kg sufentanil, and 0.5 mg/kg atracurium. The trachea was intubated, and controlled ventilation began. Anesthesia was maintained with sufentanil infused at a rate of 0.15 μg·kg-1·h-1 (which was stopped 30 min before skin closure) and sevoflurane (0.6 - 1.5 %) in a mixture of nitrous oxide (50%) with oxygen.
In patients assigned to ketamine, 0.05 ml/kg of the blinded test solution was given intravenously just after the induction of anesthesia. The initial bolus was followed by a maintenance intravenous infusion of 3 μg·kg–1·min–1 of ketamine that was continued until the patient emerged from anesthesia. Subsequently, the infusion rate was reduced to 1.5 μg·kg–1·min–1 and maintained for 48 hours. Patients allocated to the control group were given identical volumes of saline.
After extubation, patients were transferred to the postanesthesia care unit (PACU). The continuous femoral nerve block was maintained by a continuous infusion of 0.1 ml·kg–1·h–1 of 0.2% ropivacaine. Adequacy of the femoral nerve block was assessed daily by evaluating the sensory response to cold in the distribution of femoral nerve.
Pain was initially controlled in the PACU by titrating boluses of 3 mg morphine every 5 minutes until the VAS score (see below) was ≤ 30 mm. Titration was stopped if the sedation score (see below) was > 2 or the respiratory rate < 12 breaths per min. Additionally, patients were given access to a PCA device set to deliver 1-mg boluses of intravenous morphine with a lockout period of 5 minutes and no background infusion or limits. This PCA regimen was continued for 48 hours; no other analgesics were given.
Immediately after surgery, all patients started identical physical therapy regimens. During the initial 48-72 hours postoperatively, a continuous passive motion machine was used (Kinetec, Tournes, France), with a range of motion set at levels tolerated by the patient. From the day after surgery until hospital discharge, patients also performed assisted and active knee flexion and extension exercises against gravity.
After 48 hours, PCA and continuous infusion of ketamine or saline solution were discontinued and the femoral catheter was removed. After 48 hours, the analgesic regimen was standardized to two tablets of Di-antalvic (400 mg acetaminophen and 30 mg dextropropoxyphene; Aventis, Inc., Montrouge, France) every 6 hours; naproxen sodium, 550 mg twice daily. If additional analgesia was requested by the patient, subcutaneous morphine was given at 6-hour intervals. Upon discharge from the surgical ward, patients were admitted to a rehabilitation center.
Measurements
On the evening before surgery, patients were instructed about the use of visual analog rating scale (VAS) (0-100 mm; 0 = no pain, 100 = worst imaginable pain) and the PCA system (Graseby 3300 PCAS, Watford, UK). Pain was measured with VAS before and after mobilization. The maximal degree of active knee flexion tolerated by each patient was recorded every day until hospital discharge.
The time that elapsed between the end of surgery and the patient's first request for analgesic medication was recorded. PCA morphine requirements, pain intensity, heart rate, arterial blood pressure, respiratory rate and sedation score were recorded hourly for 4 hours, and then every 4 hours for 48 hours.
Potential side effects of ketamine and opioids were recorded, including nausea, vomiting, pruritus, dysphoria (including hallucinations and dreams), and diplopia. Sedation was monitored using the following 4-point rating scale: 0 = patient fully awake; 1 = patient somnolent and responsive to verbal commands; 2 = patient somnolent and responsive to tactile stimulation; 3 = patient asleep and responsive to painful stimulation.
After 48 hours, patients rated their global satisfaction on a 5-point verbal rating scale (0 = very dissatisfied, 1 = dissatisfied, 2 = neutral, 3 = satisfied, 4 = very satisfied). The number of postoperative days required to obtain 90° of active knee flexion, and the duration of hospital stay were recorded. The surgeons had a follow up visit at 6 weeks and 3 months after the procedure and determined the maximal amplitude of knee flexion.
Statistical analysis
Morphometric and demographic characteristics of the patients, clinical variables, cumulative and hourly doses of morphine over 48 hours, and amplitude of knee flexion in the ketamine and controls groups were compared with unpaired, two-tailed Student's t tests. VAS pain intensity scores were analyzed by two-way repeated-measures analysis of variance. Since maximal amplitude of knee flexion and morphine consumption did not follow a normal distribution, Mann-Whitney U-tests were usedd to compare these two outcomes. The number of days required to obtain 90° of active knee flexion in each group was compared with a log-rank test. Chi-square tests were used to compare the incidence of side effects and global satisfaction. Results are expressed as means ± SDs; P < 0.05 was considered statistically significant.
RESULTS
Forty-two patients were randomized. However, one in each group was excluded from the study. One excluded patient had a postoperative hematoma that led to reoperation; the other had continuous femoral nerve block failure. Twenty patients in each group thus completed the study. The two groups were comparable with respect to demographic data, duration of surgery, and the intraoperative dose of sufentanil (Table 1).
Table 1.
Patient Characteristics and Intraoperative Data.
| Control (n = 20) | Ketamine (n = 20) | |
|---|---|---|
| All values, except for male/female ratio, are mean ± SD | ||
| Gender (M/F) | 7/13 | 6/14 |
| Age (yr) | 69 ± 6 | 68 ± 8 |
| Weight (kg) | 74 ± 14 | 71 ± 10 |
| Height (cm) | 166 ± 6 | 165 ± 7 |
| Duration of surgery (min) | 115 ± 26 | 115 ± 27 |
| Intraoperative sufentanil (μg/kg) | 0.64 ± 0.2 | 0.59 ± 0.2 |
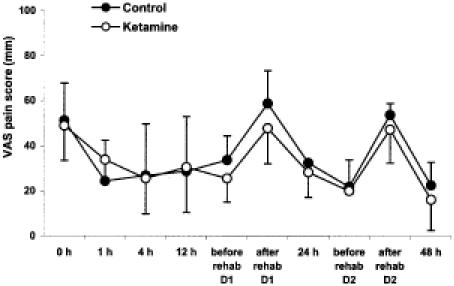
There were no statistically significant differences between the two groups in the VAS score at rest and after mobilization either during the first 48 hours or at any time thereafter until discharge. Pain intensity was greatest at the first evaluation in the PACU and after mobilization (Fig 1).
Fig. 1.
Visual analog pain scores (VAS) during the initial 48 postoperative hours and before and after rehabilitative therapy on days 1 and 2. Results are presented as means ± SDs.
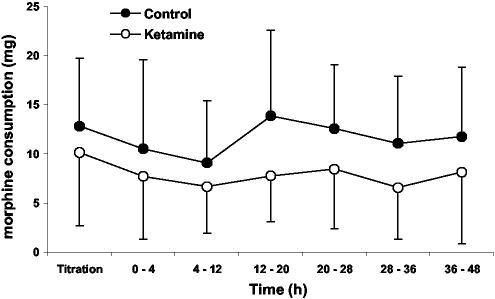
The delay before the first request for analgesics in the PACU was similar in the groups (Control group: 9 ± 7 min; Ketamine group: 10 ± 7 min). Morphine requirements in the PACU were also similar in each group: 13 ± 7 mg in the control patients and 10 ± 7 mg in the ketamine patients (P=0.26). However, cumulative morphine consumption over 48 postoperative h was significantly greater in the control patients than in those given ketamine (69 ± 30 mg versus 45 ± 20 mg; P < 0.02). Incremental morphine consumption during the first postoperative 48 h was also less in the Ketamine than in the Control patients (Fig. 2; P < 0.01). From 48 hours until hospital discharge, supplemental morphine consumption was similar in the two groups.
Fig. 2.
Incremental postoperative morphine consumption during the initial 48 postoperative hours was significantly less in patients given ketamine than in those given saline (P < 0.01). Results are presented as means ± SDs.
Preoperative knee flexion was comparable in the two groups; active knee flexion was also similar after six weeks (Control group: 102 ± 14°; Ketamine group: 104 ± 14°) and 3 months (Control group: 106 ± 16°; Ketamine group: 112 ± 11°). However, maximal active knee flexion was significantly greater in the Ketamine than in the Control group during the first seven postoperative days (Fig. 3; P < 0.02). The time required to reach 90° of active knee flexion was significantly shorter in the Ketamine than in the Control group (Fig. 4; P < 0.03).
Fig. 3.
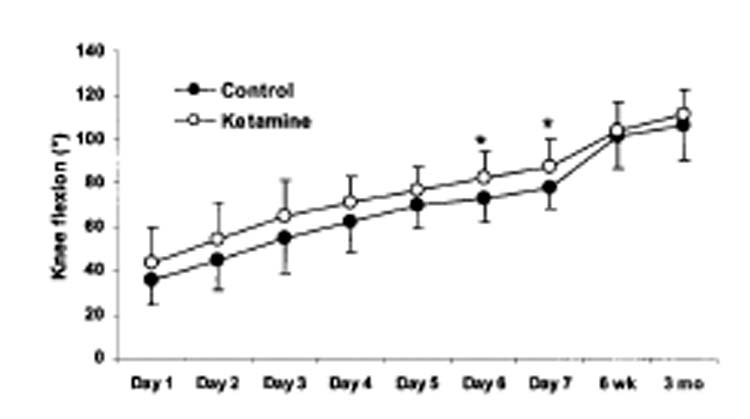
Maximal active knee flexion obtained daily during the first week, at 6 weeks, and at 3 months in each group. The maximal amplitude of knee flexion reached over the first 7 days after surgery was significantly greater in the Ketamine group than in the Control group (P < 0.02). No significant differences were noted for active knee flexion values among the two groups at the 6 weeks and 3 months examinations. Data are expressed in degrees as means ± SDs.
Fig. 4.
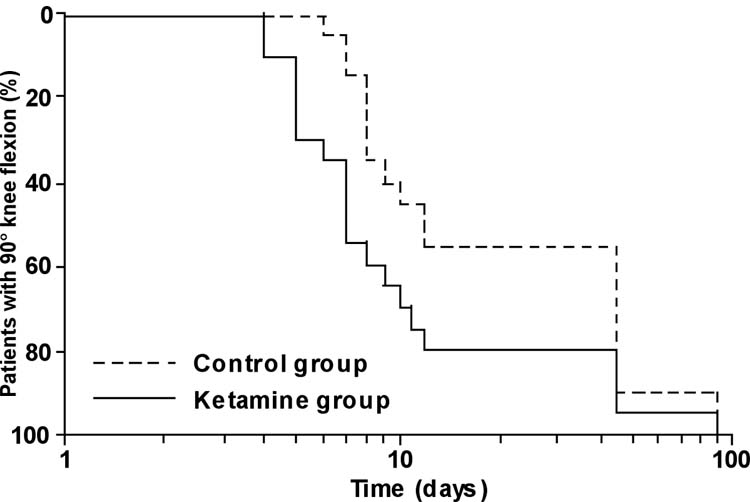
Number of days required to obtain 90° of active knee flexion plotted on semi-logarithmic scale. The log-rank curves representing the two groups studied differed significantly with P < 0.03.
Nausea and vomiting requiring treatment occurred at similar rates in each group. No patient in either group reported sedation, hallucinations, nightmares, or diplopia. Seventy percent of the patients in the Control group and 75% in the Ketamine group were satisfied or very satisfied with their surgeries. The duration of hospital stay was similar in each group with the average for all patients being 11 ± 3 days.
DISCUSSION
Continuous femoral nerve blocks are considered the analgesic technique of choice after open knee surgery (8,9). However, isolated femoral block provides incomplete postoperative analgesia because sensory innervation of the knee is also derived from the obturator, lateral femoral cutaneous, and sciatic nerves. Our primary result is that simply adding an infusion of small-dose ketamine intraoperatively and for 48 postoperative hours reduced morphine requirement by 35% and allowed faster postoperative knee rehabilitation.
There are three possible explanations for this beneficial effect of ketamine. The first is that there is an interaction between ketamine and the femoral block, peripherally. Peripheral ionotropic glutamate receptors, such as NMDA receptors, have been identified on peripheral nerve fibers (13), and their number may increase during inflammation (14). However, although a peripheral analgesic effect of ketamine has been suggested (15), actual evidence for such an effect remains controversial despite local administration (16). The importance of peripheral NMDA receptors could be evaluated with peripherally restricted antagonists, but none is currently available for human use.
A second possibility is an interaction between ketamine and morphine. In animals, the concomitant administration of ketamine and morphine results in a synergistic analgesic effect (17). The mechanisms of action for these two classes of analgesic agents are different. Opioids produce their antinociceptive effects acting presynaptically on C-fiber terminals to inhibit neurotransmitter release whereas NMDA receptor antagonists act postsynaptically to reduce the hyperexcitability of spinal nociceptive neurons (18). Furthermore, animal studies indicate that activation of NMDA receptors by opiates mediates tolerance to opioids (19) and that tolerance is thus attenuated by NMDA receptor antagonists including ketamine (20). In our study, the reduction of morphine requirements in the Ketamine group may thus have resulted from attenuation of acute tolerance to opioids.
A third explaination for the morphine-sparing effect of ketamine is that ketamine has a central antihyperalgesic effect. It is widely accepted that tissue injury often results in a prolonged sensitization of the central nociceptive system. Sensitization is at least in part mediated by activation of NMDA receptors in the dorsal horn (2) and ketamine is a non-competitive inhibitor of NMDA receptors (3). The antihyperalgesic effects of ketamine in humans were established using intradermal injection of capsaicin and burn injury (21,22). In contrast to opioids, ketamine reduces secondary hyperalgesia even when given after induction of central sensitization. In rats, NMDA receptors are recruited by inflammation and NMDA receptor antagonists are more effective when the inflammatory reaction is intense (23). Total knee arthroplasty elicits a substantial inflammatory response during the first two or three postoperative days (24), possibly recruiting NMDA receptors. It is therefore likely that the beneficial effect of ketamine we observed results at least in part because the drug prevents development of neuronal hyperexcitability.
The most important information obtained from our study is that continuous intravenous ketamine allowed an improvement in active knee flexion during the first week after surgery and a shorter recovery to 90° knee flexion. Our results are consistent with previous studies that indicate a beneficial effect of ketamine during mobilization after orthopedic surgery (6,7). Ketamine did not improve visual analogue pain scores; but this is unsurprising and simply indicates that patients in both groups used PCA correctly to obtain adequate and comparable analgesia. Similarly, the Ketamine group did not have less pain on movement during physical therapy sessions. However, the maximal degree of active knee flexion tolerated by the patient was greater in the Ketamine group indicating that the drug was an effective adjunct.
That the analgesic effect of ketamine extended well beyond the administration period is consistent with previous reports (5-7,25). For example, Stubhaug et al. (5) found that ketamine still reduced the area of punctuate mechanical hyperalgesia surrounding nephrectomy incision four days after administration. With preemptive administration of ketamine, postoperative morphine consumption decreased in the 48-h period after orthopedic (6) and abdominal surgery (25). Finally, a single intraoperative injection of 0.15 mg/kg ketamine improved postoperative functional outcome for 2 days after outpatient knee arthroscopy (7). Long-lasting postoperative analgesia might be explained as a consequence of the effects of ketamine on central sensitization. However, complementary investigations are necessary to clarify the underlying mechanism(s) of this prolonged analgesic effect of ketamine.
Despite the shorter delay to obtain 90° of active knee flexion in the Ketamine patients, the duration of hospital stay was comparable in the two groups The primary reason is that in our system discharge timing depends largely on rehabilitation center availability rather than surgical recovery per se. Similarly, no differences were observed between the two groups in the active knee flexion at six weeks and three months. But this simply indicates that, as expected, most patients had reached functional recuperation at six weeks (9).
In summary, adding an intravenous infusion of small-dose ketamine to a continuous femoral block for 48 hours after surgery decreased morphine consumption by 35% and improved early rehabilitation without increasing the incidence of adverse effects. The most likely explanation for our findings is that ketamine provided analgesia by preventing or reducing the development of central sensitization elicited by nociceptive inputs (tissue damages) and opioids (acute tolerance).
Footnotes
Received from the Departments of Anesthesia and INSERM E 332, Hôpital Ambroise Pare, Assistance Publique - Hôpitaux de Paris, 92100 Boulogne, France; Hôpital Raymond Poincaré, Assistance Publique Hôpitaux de Paris, 92428 Garches, France; and the Outcomes Research™ Institute and Departments of Anesthesiology and Pharmacology, University of Louisville, Louisville, KY.
Supported by NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). None of the authors has an personal financial interest in this research.
Implications: Adding an intravenous infusion of small-dose ketamine to a continuous femoral block for 48 hours after surgery decreased morphine consumption by 35% and improved early rehabilitation without increasing the incidence of adverse effects. Ketamine may improve analgesia by preventing or reducing the development of central sensitization elicited by nociceptive inputs (tissue damages) and opioids (acute tolerance).
References
- 1.Carr DB, Goudas LC. Acute pain. Lancet. 1999;353:2051–8. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 2.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–16. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 3.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 4.Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–25. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 5.Stubhaug A, Breivik H, Eide PK, et al. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41:1124–32. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 6.Menigaux C, Fletcher D, Dupont X, et al. The benefits of intraoperative small-dose ketamine on postoperative pain after anterior cruciate ligament repair. Anesth Analg. 2000;90:129–35. doi: 10.1097/00000539-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Menigaux C, Guignard B, Fletcher D, et al. Intraoperative small-dose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–12. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Singelyn FJ, Deyaert M, Joris D, et al. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Capdevila X, Barthelet Y, Biboulet P, et al. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 10.McNamee DA, Parks L, Milligan KR. Post-operative analgesia following total knee replacement: an evaluation of the addition of an obturator nerve block to combined femoral and sciatic nerve block. Acta Anaesthesiol Scand. 2002;46:95–9. doi: 10.1034/j.1399-6576.2002.460117.x. [DOI] [PubMed] [Google Scholar]
- 11.Ben-David B, Schmalenberger K, Chelly JE. Analgesia after total knee arthroplasty: is continuous sciatic blockade needed in addition to continuous femoral blockade? Anesth Analg. 2004;98:747–9. doi: 10.1213/01.ane.0000096186.89230.56. [DOI] [PubMed] [Google Scholar]
- 12.Winnie AP, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: the “3-in-1 block”. Anesth Analg. 1973;52:989–96. [PubMed] [Google Scholar]
- 13.Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Carlton SM, Coggeshall RE. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res. 1999;820:63–70. doi: 10.1016/s0006-8993(98)01328-6. [DOI] [PubMed] [Google Scholar]
- 15.Tverskoy M, Oren M, Vaskovich M, et al. Ketamine enhances local anesthetic and analgesic effects of bupivacaine by peripheral mechanism: a study in postoperative patients. Neurosci Lett. 1996;215:5–8. doi: 10.1016/s0304-3940(96)12922-0. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen JL, Galle TS, Kehlet H. Peripheral analgesic effects of ketamine in acute inflammatory pain. Anesthesiology. 1998;89:58–66. doi: 10.1097/00000542-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez P, Saavedra G, Hernandez A, et al. Synergistic antinociceptive effects of ketamine and morphine in the orofacial capsaicin test in the rat. Anesthesiology. 2003;99:969–75. doi: 10.1097/00000542-200310000-00033. [DOI] [PubMed] [Google Scholar]
- 18.Dickenson AH. NMDA receptor antagonists: interactions with opioids. Acta Anaesthesiol Scand. 1997;41:112–15. doi: 10.1111/j.1399-6576.1997.tb04624.x. [DOI] [PubMed] [Google Scholar]
- 19.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 20.Kissin I, Bright CA, Bradley EL., Jr The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combination? Anesth. Analg. 2000;91:1483–88. doi: 10.1097/00000539-200012000-00035. [DOI] [PubMed] [Google Scholar]
- 21.Mikkelsen S, Ilkjaer S, Brennum J, et al. The effect of naloxone on ketamine-induced effects on hyperalgesia and ketamine-induced side effects in humans. Anesthesiology. 1999;90:1539–45. doi: 10.1097/00000542-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Warncke T, Stubhaug A, Jorum E. Preinjury treatment with morphine or ketamine inhibits the development of experimentally induced secondary hyperalgesia in man. Pain. 2000;86:293–303. doi: 10.1016/S0304-3959(00)00260-8. [DOI] [PubMed] [Google Scholar]
- 23.Ren K, Hylden JL, Williams GM, et al. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–44. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- 24.Andres BM, Taub DD, Gurkan I, Wenz JF. Postoperative fever after total knee arthroplasty: the role of cytokines. Clin Orthop. 2003;415:221–31. doi: 10.1097/01.blo.0000093914.26658.55. [DOI] [PubMed] [Google Scholar]
- 25.Fu ES, Miguel R, Scharf JE. Preemptive ketamine decreases postoperative narcotic requirements in patients undergoing abdominal surgery. Anesth Analg. 1997;84:1086–90. doi: 10.1097/00000539-199705000-00024. [DOI] [PubMed] [Google Scholar]