Abstract
Objective To compare oral misoprostol solution with vaginal prostaglandin gel (dinoprostone) for induction of labour at term to determine whether misoprostol is superior.
Design Randomised double blind placebo controlled trial.
Setting Maternity departments in three hospitals in Australia.
Population Pregnant women with a singleton cephalic presentation at ≥ 36+6 weeks' gestation, with an indication for prostaglandin induction of labour.
Interventions 20 μg oral misoprostol solution at ourly intervals and placebo vaginal gel or vaginal dinoprostone gel at six hourly intervals and placebo oral solution.
Main outcome measures Vaginal birth within 24 hours; uterine hyperstimulation with associated changes in fetal heart rate; caesarean section (all); and caesarean section for fetal distress.
Results 741 women were randomised, 365 to the misoprostol group and 376 to the vaginal dinoprostone group. There were no significant differences between the two treatment groups in the primary outcomes: vaginal birth not achieved in 24 hours (misoprostol 168/365 (46.0%) v dinoprostone 155/376 (41.2%); relative risk 1.12, 95% confidence interval 0.95 to 1.32; P = 0.134), caesarean section (83/365 (22.7%) v 100/376 (26.6%); 0.82, 0.64 to 1.06; P = 0.127), caesarean section for fetal distress (32/365 (8.8%) v 35/376 (9.3%); 0.91, 0.57 to 1.44; P = 0.679), or uterine hyperstimulation with changes in fetal heart rate (3/365 (0.8%) v 6/376 (1.6%); 0.55, 0.14 to 2.21; P = 0.401). Although there were differences in the process of labour induction, there were no significant differences in adverse maternal or neonatal outcomes.
Conclusions This trial shows no evidence that oral misoprostol is superior to vaginal dinoprostone for induction of labour. However, it does not lead to poorer health outcomes for women or their infants, and oral treatment is preferred by women.
Trial registration National Health and Medical Research Council, Perinatal Trials, PT0361.
Introduction
Induction of labour is a common intervention,1 performed for medical, obstetric, or social indications. In 2002 in Australia, nearly 27% of pregnant women had their labour induced.2 Prostaglandins to induce labour are used in about 23% of all confinements.3 Misoprostol is an oral prostaglandin compound, structurally related to prostaglandin E14 and manufactured as a treatment for peptic ulcer disease.5,6 Though unlicensed for this indication, misoprostol is being used increasingly in induction of labour, with vaginal7 and oral8 administration.
We conducted a randomised double blind trial to compare 20 μg oral misoprostol solution with vaginal prostaglandin gel (dinoprostone) for induction of labour at term.
Methods
The study took place at the Women's and Children's Hospital and Lyell McEwin Health Service (South Australia) and the Hervey Bay Hospital (Queensland) between April 2001 and December 2004.
Inclusion and exclusion criteria
If the attending obstetrician decided to induce labour we approached any women with a singleton pregnancy at ≥ 36+6 weeks' gestation. We then obtained written informed consent. We excluded women with a “favourable” cervix (defined as a modified Bishop score of ≥ 7), any contraindication to vaginal birth, previous uterine surgery (including caesarean section), or ruptured membranes.
Randomisation schedule and allocation
The randomisation schedule was generated by using a computer sequence with variable blocks and stratification for parity (0 and 1-4) and collaborating centre. The point of randomisation was when the pack was opened. Treatment packs appeared identical and were sealed to prevent tampering.
On admission to the delivery suite the midwife confirmed the trial entry details and allocated a study number by taking the next sequentially numbered, identically appearing treatment pack appropriate for the woman's parity. We also reconfirmed consent at this time.
Treatment schedules
Misoprostol—Each misoprostol pack contained six labelled bottles with 100 μg of crushed misoprostol and two doses of vaginal placebo (tylose) gel in sterile opaque syringes.
Dinoprostone—Each placebo pack contained six labelled white plastic bottles with 25 mg of crushed vitamin B-6 and two doses of dinoprostone gel in sterile opaque syringes and sealed in sterile opaque packages (2 mg for nulliparous women and 1 mg for multiparous women).
If the tracing on the fetal cardiotocogram was within normal limits the the study preparation was given. The midwife or attending doctor performed a vaginal examination, recorded the initial Bishop score, and administered the gel.
The midwife made up the oral solution immediately before administration by mixing the powder with 100 ml of water to produce a solution of 1 μg/ml of misoprostol in the active solution. The woman then took a 20 ml aliquot of solution (20 μg misoprostol solution). This procedure was repeated every two hours (to a maximum of six doses in 12 hours). The vaginal gel was administered every six hours (to a maximum of two doses in 12 hours). All care was according to local hospital guidelines, except for the administration of trial medications as described.
Six weeks after the birth we posted women a questionnaire relating to satisfaction with care. If they did not return the questionnaire within two weeks we contacted them by telephone and they completed the questionnaire over the phone.
Outcome measures
Our primary outcome measures were vaginal birth not achieved in 24 hours (including women who achieved vaginal birth after 24 hours and those women who had a caesarean section), caesarean section (all and for heart rate tracing indicating fetal distress), and uterine hyperstimulation with changes in fetal heart rate.9
We defined uterine hyperstimulation as uterine tachysystole (with five or more contractions in a 10 minute period for two consecutive 10 minute periods) or uterine hypertonus (a uterine contraction lasting for more than two minutes).10 The changes in fetal heart rate that we considered abnormal included persistent decelerations (early, late, or variable decelerations), fetal tachycardia (fetal heart rate > 160 beats per minute), fetal bradycardia (fetal heart rate < 100 beats per minute), or reduced short term variability (< 5 beats per minute).11,12 A single investigator blinded to the treatment allocated reviewed all fetal heart rate tracings from an induced labour to maintain consistency in interpretation.
We measured labour and birth complications, neonatal complications, maternal complications and side effects, and maternal satisfaction with care as secondary outcomes.9 We compared costs with clinical pathways built into the model from the perspective of the healthcare institution (table 1). The midwifery care during induction was costed at a rate of one midwife caring for two women, and care during labour and birth was costed at a rate of 1.25 midwives caring for a single woman, according to hospital standards.
Table 1.
Cost to the hospital associated with induction of labour
| Item | Cost ($A)* |
|---|---|
| 1:2 midwifery care | 13.04/hour |
| 1:1.25 midwifery care | 32.60/hour |
| Misoprostol | 0.35/200 μg |
| Vaginal dinoprostone gel | 40.17/1 mg; 51.64/2 mg |
| Vaginal birth* | 3973.83 |
| Caesarean birth* | 6349.97 |
$A1=£0.43, €0.62.
Including average length of hospital stay.
The midwife caring for the woman completed data forms, which were confirmed and checked before hospital discharge. Data were entered into a database created in Microsoft Access 97.
Data analysis
We analysed data on an intention to treat basis, blind to the allocated treatment, using SAS, version 9 (SAS Institute, Cary, NC). Dichotomous outcomes were compared with χ2 tests or Fisher's exact test, with calculation of relative risks and 95% confidence intervals. We used Student's t test to compare normally distributed continuous data and non-parametric tests (Wilcoxon rank sum) for skewed data. The number of women needed to treat to benefit (NNTB) and to harm (NNTH) with 95% confidence intervals was calculated for significant outcomes. Before the analysis of any outcomes, we considered baseline characteristics and corrected those sufficiently imbalanced (more than 5% difference between treatment groups) using log binomial regression techniques. P < 0.05 was considered significant.
Sample size
Using information from the Cochrane review,8 we calculated that a sample size of 738 women would give 85% power to detect a 50% difference in the number of women who did not achieve a vaginal birth after 24 hours from 20% in the vaginal dinoprostone group to 30% in the misoprostol group (P < 0.05), a 32% difference in caesarean section from 28% in the vaginal dinoprostone group to 19% in the misoprostol group, and a threefold difference in the rate of hyperstimulation with changes in fetal heart rate from 2% in the vaginal dinoprostone group to 6% in the misoprostol group (P < 0.05; power 80%).
Results
Baseline characteristics at trial entry
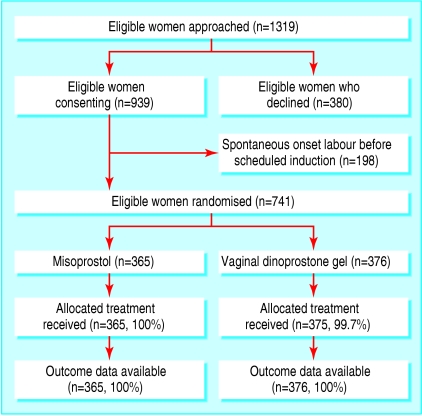
Of 1319 eligible women approached, 939 (71.2%) provided written consent (figure). Of these, 741 (78.8%) were admitted for induction of labour and randomised, 365 women to oral misoprostol, and 376 to vaginal dinoprostone. In total, 740 (99.9%) women received treatment as allocated. We had outcome data for all 741 women up to hospital discharge (figure).
Figure 1.
Flow of women through trial
Baseline characteristics were comparable except for initial Bishop score (table 2). We adjusted for this in the analyses and have presented adjusted results.
Table 2.
Baseline variables at trial entry. Figures are numbers (percentages) of women unless stated otherwise
| Misoprostal (n=365) | Dinoprostone (n=376) | |
|---|---|---|
| Mean (SD) age (years)* | 27.9 (5.6) | 28.0 (5.6) |
| Nulliparous | 213 (58.4) | 221 (58.8) |
| White | 355 (97.3) | 362 (96.3) |
| Public patient | 362 (99.2) | 376 (100.0) |
| Indication for induction of labour: | ||
| After due date | 181 (49.6) | 175 (46.5) |
| Pre-eclampsia | 30 (8.2) | 37 (9.8) |
| Hypertension | 49 (13.4) | 55 (14.6) |
| Intrauterine growth restriction | 24 (6.6) | 32 (8.5) |
| Abnormal glucose tolerance | 29 (8.0) | 46 (12.2) |
| Social | 55 (15.1) | 46 (12.2) |
| Other | 21 (5.8) | 25 (6.6) |
| Initial Bishop score: | ||
| 0-3 | 246 (67.4) | 214 (56.9) |
| 4-6 | 119 (32.6) | 162 (43.1) |
| Mean (SD) gestational age (weeks)* | 40.6 (2.0) | 40.4 (2.1) |
| Mean (SD) height (m)* | 165.1 (5.8) | 165.0 (6.6) |
| Median (IQR) weight at booking (kg) | 72.0 (48.0-96.0) | 75.0 (49.0-101.0) |
| BMI >30 (kg/m2) at booking | 94 (34.0) | 98 (33.1) |
| Smoking at booking | 84 (23.0) | 86 (22.9) |
IQR=interquartile range; BMI=body mass index.
Primary outcomes
There were no significant differences between the two treatment groups for vaginal birth not achieved in 24 hours (misoprostol 46.0% v dinoprostone 41.2%), caesarean section (22.7% v 26.6%), caesarean section for fetal distress (8.8% v 9.3%), or uterine hyperstimulation with changes in fetal heart rate (0.8% v 1.6%) (table 3).
Table 3.
Primary outcomes. Figures are numbers (percentages)
| Outcome | Misoprostol (n=365) | Dinoprostone (n=376) | Relative risk (95% CI)* | P value |
|---|---|---|---|---|
| Vaginal birth not achieved in 24 hours | 168 (46.0) | 155 (41.2) | 1.12 (0.95 to 1.32) | 0.134 |
| Uterine HSS with changes in FHR | 3 (0.8) | 6 (1.6) | 0.55 (0.14 to 2.21) | 0.401 |
| Caesarean section: | ||||
| All | 83 (22.7) | 100 (26.6) | 0.82 (0.64 to 1.06) | 0.127 |
| For fetal distress | 32 (8.8) | 35 (9.3) | 0.91 (0.57 to 1.44) | 0.679 |
HSS=hyperstimulation syndrome; FHR-fetal heart rate.
Adjusted for initial Bishop score at trial entry.
Secondary outcomes
Women in the oral misoprostol group were more likely to have a low Bishop score (< 7) 24 hours after the induction was started, to require vaginal dinoprostone gel, to have infusion of oxytocin, and to have a longer time between induction and birth (table 4). For every 15 women treated with misoprostol, one required further dinoprostone gel (NNTH 15, 95% confidence interval 12 to 4021), and for every 13 women treated with misoprostol, one required infusion of oxytocin (13, 7 to 162).
Table 4.
Secondary outcomes. Figures are numbers (percentages) unless stated otherwise
| Outcome | Misoprostol (n=365) | Dinoprostone (n=376) | Relative risk (95% CI)* | P value† |
|---|---|---|---|---|
| Evidence of effect | ||||
| Bishop score <7 after 24 hours | 57 (15.6) | 39 (10.4) | 1.51 (1.03 to 2.20) | 0.031 |
| Further doses of dinoprostone | 70 (19.2) | 47 (12.5) | 1.41 (1.01 to 1.97) | 0.043 |
| Oxytocin infusion | 203 (55.6) | 179 (47.6) | 1.17 (1.01 to 1.34) | 0.034 |
| Median (IQR) induction-birth interval (hours) | 21.2 (8.6-33.8) | 18.4 (6.3-30.5) | — | <0.001‡ |
| Labour and birth complications | ||||
| Uterine HSS-no changes in FHR | 4 (1.1) | 17 (4.5) | 0.23 (0.08 to 0.69) | 0.009 |
| Uterine rupture | 0 | 0 | Not estimable | |
| Need for any analgesia | 351 (96.2) | 347 (92.3) | 1.04 (1.00 to 1.08) | 0.035 |
| Need for epidural | 243 (66.6) | 229 (60.9) | 1.08 (0.97 to 1.21) | 0.149 |
| Meconium stained liquor | 59 (16.2) | 52 (13.8) | 1.14 (0.81 to 1.61) | 0.465 |
| Mean (SD) length of labour (hours) | 7.5 (4.1) | 6.9 (4.0) | 0.073§ | |
| Instrumental vaginal birth | 65 (17.8) | 63 (16.8) | 1.06 (0.77 to 1.46) | 0.712 |
| Blood loss >600 ml | 57 (15.6) | 77 (20.5) | 0.76 (0.55 to 1.04) | 0.081 |
| Blood loss >1000 ml | 17 (4.7) | 20 (5.3) | 0.86 (0.46 to 1.63) | 0.646 |
| Need for blood transfusion | 8 (2.2) | 9 (2.4) | 0.96 (0.37 to 2.47) | 0.927 |
| Neonatal complications | ||||
| Birth weight <2500 g | 15 (4.1) | 11 (2.9) | 1.30 (0.60 to 2.79) | 0.505 |
| Apgar <7 at 5 minutes | 2 (0.6) | 5 (1.3) | 0.42 (0.08 to 2.15) | 0.297 |
| Cord pH <7.18 | 10 (7.6) | 19 (11.6) | 0.62 (0.30 to 1.29) | 0.205 |
| NICU admission | 5 (1.4) | 2 (0.5) | 2.66 (0.52 to 13.75) | 0.242 |
| Neonatal encephalopathy | 0 | 0 | Not estimable | |
| Neonatal death¶ | 0 | 0 | Not estimable | |
| Maternal complications | ||||
| Any side effect | 76 (20.8) | 99 (26.3) | 0.78 (0.60 to 1.01) | 0.063 |
| Nausea | 20 (5.5) | 30 (8.0) | 0.68 (0.39 to 1.19) | 0.175 |
| Vomiting | 4 (1.1) | 10 (2.7) | 0.43 (0.14 to 1.37) | 0.154 |
| Diarrhoea | 5 (1.4) | 9 (2.4) | 0.53 (0.18 to 1.57) | 0.250 |
| Flushing | 6 (1.6) | 4 (1.1) | 1.43 (0.41 to 5.05) | 0.578 |
| Intensive care unit admission | 0 | 0 | Not estimable | |
| Hyperpyrexia | 0 | 0 | Not estimable | |
| Coma | 0 | 0 | Not estimable | |
| Maternal death | 0 | 0 | Not estimable |
IQR=interquartile range; HSS=hyperstimulation syndrome; FHR=fetal heart rate; NICU=neonatal intensive care unit.
Adjusted for initial Bishop score at trial entry.
ϰ2 unless otherwise specified.
Wilcoxon rank sum test.
Student's t test.
Death of liveborn infant within 28 days of birth.
For every 30 women treated with oral misoprostol, there will be one less with uterine hyperstimulation without changes in fetal heart rate (NNTB 30; 18 to 95). The use of analgesia was high in both groups, and there were no significant differences between the two groups for other labour and birth complications (table 4). There were no significant differences in the occurrence of neonatal complications, maternal complications, or side effects (table 4).
Over half of the women (58.5%) expressed a preference for an oral induction agent, and those women in the misoprostol group were more likely to say that they “liked everything” with their labour and birth.
The cost per woman induced with misoprostol was $A4948.81 compared with $A5059.64 for vaginal dinoprostone gel, a difference of $A110.83 (€69.13, £47.25) in favour of misoprostol (range $A15.88 to $A121.87) (table 5).
Table 5.
Costs ($A) for all women
|
Outcome measured
|
Misoprostol group (n=365)
|
Dinoprostone (n=376)
|
||
|---|---|---|---|---|
| No of units | Cost | No of units | Cost | |
| Median length of labour (induction-birth; 1:2 midwifery care) (hours) | 13.7 | 65 207.25 | 11.5 | 56 384.96 |
| Mean length of labour (1:1.25 midwifery care) | 7.5 | 89 242.50 | 6.9 | 84 577.44 |
| Doses of trial medication | 1 668 | 583.80 | 342×2 mg | 17 660.88 |
| 239×1 mg | 9 600.63 | |||
| Further doses of 2 mg dinoprostone gel* | 70 | 3 614.80 | 47 | 2 427.08 |
| Vaginal birth | 282 | 1 120 620.00 | 276 | 1 096 777.00 |
| Caesarean birth | 83 | 527 047.51 | 100 | 634 997.00 |
| Total cost | 1 806 315.80 | 1 902 424.90 | ||
| Total cost per woman | 4 948.81 | 5 059.64 | ||
| Lower threshold | ||||
| Median length of labour (induction-birth; 1:2 midwifery care) (hours) | 5.2 | 24 749.92 | 2.9 | 35 547.04 |
| Mean length of labour (1:1.25 midwifery care) (hours) | 3.4 | 40 456.60 | 3.4 | 16 670.34 |
| Doses of trial medication | 1 668 | 583.80 | 342×2 mg | 17 660.88 |
| 239×1 mg | 9 600.63 | |||
| Further doses of 2 mg dinoprostone gel* | 42 | 2 168.88 | 28 | 1 445.92 |
| Vaginal birth | 297 | 1 180 227.50 | 294 | 1 168 306.00 |
| Caesarean birth | 68 | 431 797.96 | 82 | 520 697.54 |
| Total cost | 1 679 984.60 | 1 736 587.60 | ||
| Total cost per woman | 4 602.70 | 4 618.58 | ||
| Upper threshold | ||||
| Median length of labour (induction-birth; 1:2 midwifery care) (hours) | 22.2 | 105 663.12 | 19.6 | 96 099.58 |
| Mean length of labour (1:1.25 midwifery care) (hours) | 11.6 | 138 028.40 | 10.9 | 133 607.84 |
| Doses of trial medication | 1 668 | 583.80 | 342×2 mg | 17 660.88 |
| 239×1 mg | 9 600.63 | |||
| Further doses of 2 mg dinoprostone gel* | 109 | 5 628.76 | 73 | 3 769.72 |
| Vaginal birth | 262 | 1 041 143.40 | 252 | 1 001 405.10 |
| Caesarean birth | 103 | 654 046.91 | 124 | 787 396.28 |
| Total cost | 1 945 094.30 | 2 049 540.00 | ||
| Total cost per woman | 5 329.03 | 5 450.90 | ||
Refers to further doses administered after completion of the two doses of vaginal gel provided as trial medication in treatment packs.
Discussion
Oral misoprostol was not associated with significant differences in the number of women who achieve vaginal birth within 24 hours after induction, caesarean section, or uterine hyperstimulation with changes in fetal heart rate, compared with vaginal dinoprostone gel.
Oral misoprostol was associated with an increased need for further doses of vaginal dinoprostone gel and infusion of oxytocin, but a significant reduction in uterine hyperstimulation without changes in fetal heart rate. Differences in the process of induction did not lead to poorer health outcomes for women or infants. Women preferred an oral induction agent, and use of misoprostol was associated with a modest cost saving to institutions.
Strengths of this study
Our trial is the second double blind study comparing oral misoprostol with dinoprostone gel,13 and the first involving low dose oral misoprostol solution. We blinded participants to treatment, as recommended,14-16 which should reduce bias and increase confidence in the validity of our results. Our inclusion criteria represented the spectrum of indications for induction, and with over 70% of the women we approached agreeing to participate, our results have external validity and are applicable to the general obstetric population requiring induction of labour.
Our trial is the largest to date of oral misoprostol and dinoprostone gel13,17-20 and was large enough to detect clinically important differences in caesarean birth and vaginal birth not achieved within 24 hours. We were powered to detect a threefold difference in the less common outcome of uterine hyperstimulation with changes in fetal heart rate. For rare maternal and neonatal complications we were powered to detect only large differences. Given the low frequency of such events it would be necessary to recruit tens of thousands of women and their infants. Nevertheless, our results provide reliable evidence on the use of oral misoprostol for induction of labour at term and contribute to the available information about its safety.
Weaknesses of this study
Our findings of reduced efficacy raise the possibility that our dosing regimen was too low. An incremental increase in dose to 40 μg after four hours in the absence of uterine activity, as described by Hofmeyr et al18 and later Dallenbach et al,19 may be more appropriate, but difficult to achieve in our trial while maintaining blinding.
Unanswered questions and future research
The outcome of vaginal birth not achieved in 24 hours9 covers women who deliver by caesarean or who give birth vaginally after 24 hours. Vaginal birth achieved after 24 hours reflects a longer time from induction to birth and may reflect an inappropriately low dose of misoprostol. An increase in caesarean birth may reflect uterine hyperstimulation or worrying changes in fetal heart rate. For completeness and to ensure clarity of information, future trials should report both components of this composite outcome.
While the use of misoprostol as an induction agent is associated with cost savings, this is unlikely to propel manufacturers towards seeking appropriate product licensing,21 and its use in pregnancy has medicolegal implications for individual practitioners and institutions. Agencies funding health care, however, may be willing to provide indemnity for its use.
While the extent of rare but potentially serious adverse complications such as uterine rupture, maternal or perinatal death, and neonatal acidaemia remain uncertain, regular audit of clinical practice and reporting of such adverse outcomes should be a requirement of clinicians and institutions adopting the use of misoprostol for the induction of labour. Efforts should be directed to ensure the availability of a licensed low dose (20 μg) formulation for use in pregnancy, that is easy to administer orally, while retaining its low cost to enable widespread use, particularly in under-resourced countries.
What is already known on this topic
More than one in four pregnant women have induced labour
Prostaglandins are used to induce labour in more than one in five confinements, and misoprostol, a prostaglandin E1 analogue, is being used increasingly
What this study adds
There was no significant difference between oral misoprostol and vaginal dinoprostone gel in the risk of not achieving vaginal birth in 24 hours, caesarean section, uterine hyperstimulation with changes in fetal heart rate, or adverse health outcomes for the woman and her infant
Women preferred the oral treatment
Contributors: JMD (guarantor) developed the original trial protocol, submitted the protocol to research and ethics committees, obtained funding, developed information sheets and data sheets, coordinated education sessions for midwives and medical staff, prepared treatment packs, recruited women at the Women's and Children's Hospital, collected data, checked all data forms and data entry, analysed and interpreted data, and prepared and revised the manuscript and approved the final version. CAC and JSR developed the original trial protocol, submitted the protocol to research and ethics committees, obtained funding, analysed and interpreted data, and prepared and revised the manuscript and subsequent revisions and approved the final version. Sheree Agett (research midwife) prepared treatment packs, recruited women at the Women's and Children's Hospital and the Lyell McEwin Health Service, and collected data. Kristyn Willson (statistician) gave statistical advice. Neil Hotham (pharmacist) gave advice on the preparation of treatment packs. Gus Dekker coordinated the trial at the Lyell McEwin Health Service. Dirk Ludwig coordinated the trial at the Hervey Bay Hospital.
Funding: During the time this trial was conducted, JMD was the recipient of the Mayne Women's Health Fellowship provided by the research foundation of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists, and a postgraduate medical scholarship provided by the research foundation of the Women's and Children's Hospital. Financial support for a research midwife was obtained through a project grant provided by the Research Foundation of the Women's and Children's Hospital.
Competing interests: None declared.
Ethical approval: Ethical approval was obtained from each institution.
References
- 1.Riskin-Mashiah S, Wilkins I. Cervical ripening. Obstet Gynecol Clin North Am 1999;26: 243-57. [DOI] [PubMed] [Google Scholar]
- 2.Laws PJ, Sullivan EA. Australia's mothers and babies 2002. Sydney: Australian Institute of Health and Welfare (AIHW), National Perinatal Statistics Unit (NPSU). 2004.
- 3.Nassar N, Sullivan EA, Lancaster P, Day P. Australia's mothers and babies 1998. Sydney: AIHW National Perinatal Statistics Unit, 2000.
- 4.Garris RE, Kirkwood CF. Misoprostol: a prostaglandin E1 analogue. Clin Pharm 1989;8: 627-44. [PubMed] [Google Scholar]
- 5.Collins PW, Pappo R, Dajani EZ. Chemistry and synthetic development of misoprostol. Dig Dis Sci 1985;30: 114-7S. [DOI] [PubMed] [Google Scholar]
- 6.Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev 1990;10: 149-72. [DOI] [PubMed] [Google Scholar]
- 7.Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and labour induction in late pregnancy. Cochrane Database Syst Rev 2005;(3): CD000941. [DOI] [PubMed]
- 8.Alfirevic Z. Oral misoprostol for induction of labour. In: Cochrane Database Syst Rev 2005;(2): CD001338. [DOI] [PubMed]
- 9.Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database Syst Rev 2005;(3): CD002074.
- 10.Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. J Reprod Med 1987;32: 91-5. [PubMed] [Google Scholar]
- 11.Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Clinical guidelines: intrapartum fetal surveillance. East Melbourne, Victoria: RANZCOG, 2001.
- 12.Royal College of Obstetricians and Gynaecologists (RCOG). Evidence-based clinical guideline number 8: the use of electronic fetal monitoring. London: RCOG Press, 2001.
- 13.Tessier F, Danserau. A double blind randomized controlled trial comparing oral misoprostol to vaginal prostaglandin E2 for the induction of labor at or near term. Am J Obstet Gynecol 1997;176: S111. [Google Scholar]
- 14.Chalmers I, Hetherington J, Elbourne D, Keirse M, Enkin M. Materials and methods used in synthesizing evidence to evaluate the effects of care during pregnancy and childbirth. In: Chalmers I, Enkin M, Keirse M, eds. Effective care in pregnancy and childbirth. Oxford: Oxford University Press, 1989: 39-65.
- 15.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273: 408-12. [DOI] [PubMed] [Google Scholar]
- 16.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ 1998;317: 1185-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gherman RB, Browning J, O'Boyle A, Goodwin TM. Oral misoprostol vs intravaginal prostaglandin E2 for preinduction cervical ripening. A randomized trial. J Reprod Med 2001;46: 641-6. [PubMed] [Google Scholar]
- 18.Hofmeyr GJ, Alfirevic Z, Matonhodze B, Brocklehurst P, Campbell E, Nikodem VC. Titrated oral misoprostol solution for induction of labour: a multi-centre, randomised trial. Br J Obstet Gynaecol 2001;108: 952-9. [DOI] [PubMed] [Google Scholar]
- 19.Dallenbach P, Boulvain M, Viardot C, Irion O. Oral misoprostol or vaginal dinoprostone for labor induction: a randomized controlled trial. Am J Obstet Gynecol 2003;188: 162-7. [DOI] [PubMed] [Google Scholar]
- 20.Shetty A, Livingstone I, Acharya S, Rice P, Danielian P, Templeton A. A randomised comparison of oral misoprostol and vaginal prostaglandin E2 tablets in labour induction at term. Br J Obstet Gynaecol 2004;111: 436-40. [DOI] [PubMed] [Google Scholar]
- 21.Weeks A, Fiala C, Safar P. Misoprostol and the debate over off-label drug use. Br J Obstet Gynaecol 2005;112: 269-72. [DOI] [PubMed] [Google Scholar]