Abstract
Chronic inflammation in atherosclerosis is responsible for plaque instability through alterations in extracellular matrix. Previously, we demonstrated that major histocompatibility class II (MHC II) transactivator (CIITA) in a complex with regulatory factor for X box 5 (RFX5) is a crucial protein mediating interferon (IFN)-γ–induced repression of collagen type I gene transcription in fibroblasts. This article demonstrates that, in smooth muscle cells (SMCs), IFN-γ dramatically increases the expression of CIITA isoforms III and IV, with no increase in expression of CIITA isoform I. Expression of CIITA III and IV correlates with decreased collagen type I and increased MHC II gene expression. Exogenous expression of CIITA I, III, and IV, in transiently transfected SMCs, represses collagen type I promoters (COL1A1 and COL1A2) and activates MHC II promoter. Levels of CIITA and RFX5 increase in the nucleus of cells treated with IFN-γ. Moreover, simvastatin lowers the IFN-γ–induced expression of RFX5 and MHC II in addition to repressing collagen expression. However, simvastatin does not block the IFN-γ–induced expression of CIITA III and IV, suggesting a CIITA-independent mechanism. This first demonstration that RFX5 and CIITA isoforms are expressed in SMCs after IFN-γ stimulation suggest that CIITA could be a key factor in plaque stability in atherosclerosis.
Keywords: collagen, CIITA isoform, RFX, MHC II, SMCs
Atherosclerosis is a complex inflammatory and fibroproliferative process in which the immune system1-3 and the extracellular matrix environment4 play an important role in the pathogenesis of disease. Initially, atherosclerotic lesions consist of fatty streaks that can develop into mature lesions containing macrophages, T lymphocytes, smooth muscle cells (SMCs), a necrotic core, and a fibrous cap containing extracellular matrix components. Clinical complications of atherosclerosis arise when plaques rupture. Instability of the atherosclerotic plaques is a result of an unbalanced synthesis and degradation of extracellular matrix components including collagens.5
Collagen type I, composed of 2 proteins, α1(I) and α2(I), transcribed by 2 separate genes, COL1A1 and COL1A2, is the most abundant fibrillar protein secreted by SMCs in atherosclerotic lesions6 and by SMCs in culture.7 A lower content of collagen has been observed in atherosclerotic lesions that are prone to rupture.8 However, the mechanism by which the collagen content decreases in plaques has not been fully elucidated.
Chronic inflammation in atherosclerosis is accompanied by secretion of cytokines, a major 1 being the interferon (IFN)-γ, which is secreted by T lymphocytes and macrophages.9,10 IFN-γ decreases collagen gene expression and activates major histocompatibility class II (MHC II) gene transcription through similar transcription regulatory proteins, RFX5 and CIITA, in fibroblast cells.11-13 However, little is known about the expression and the mechanism of regulation of collagen and MHC II genes by IFN-γ in SMCs.
MHC II molecules are surface glycoproteins that play a crucial role in adaptive immunity by presenting antigens to reactivate CD4+ T cells. In inflammatory situations, such as in atherosclerosis, MHC II genes can be induced by IFN-γ in nonprofessional antigen-presenting cells (APCs), such as fibroblasts, SMCs, and endothelial cells. Whereas the expression of MHC II genes has been detected in SMCs in human atherosclerotic lesions,14-16 the regulatory mechanism of MHC II expression in aortic SMCs has not been investigated.
MHC II gene expression is transcriptionally regulated by class II transcriptional activator (CIITA). CIITA does not bind DNA but is recruited to the MHC II promoter by RFX5.17,18 CIITA acts as a scaffold for the assembly of various other transcription factors to differentially regulate a number of other genes such as collagen,11,12 cathepsin E,19 interleukin (IL)-4, IL-10,19,20 and the semaphoring receptor plexin-A1 gene.21
The expression of CIITA is cell specific and tightly controlled by 4 independent promoters in human (pI, pII, pIII, and pIV) and 3 promoters in mouse (pI, pIII, and pIV). CIITA type I isoform is specifically and constitutively expressed in dendritic cells. By contrast, CIITA type III is mainly expressed by B lymphocytes and can be induced by IFN-γ in fibroblasts, endothelial cells, and B lymphocytes.22 Yet, CIITA type IV is the major inducible isoform expressed by a variety of cell types stimulated by IFN-γ.23 CIITA promoters direct transcription of distinct CIITA isoforms with different first exons. Although SMCs produce MHC II in response to IFN-γ, CIITA expression is not understood.
Finally, ongoing clinical trials have shown that statins are of great benefit in prevention of cardiovascular diseases.24,25 Statins are inhibitors of the 3-hydroxyl-3-methylglutaryl co-enzyme A (HMG-CoA) reductase and function by lowering cholesterol.26 Recently, the therapeutic effect of statins has been extended to other inflammatory diseases in which CIITA is the target at least in certain cell types.24,27
This article shows for the first time that CIITA is expressed in SMCs. Our novel findings demonstrate that 2 major CIITA isoforms, III and IV, are induced by IFN-γ in this cell type. Moreover, we demonstrate that IFN-γ represses collagen synthesis and increases expression of MHC II in SMCs through a common coregulator, CIITA. Simvastatin does not block the induction of CIITA in SMCs. However, simvastatin lowers collagen gene expression in the presence of IFN-γ through a separate mechanism independent of CIITA. Therefore, during an inflammatory process, increased expression of CIITA by IFN-γ enhances the inflammatory response by increasing the number of MHC II molecules expressed by SMCs and compromises the maintenance of the fibrous plaque by decreasing collagen synthesis.
Materials and Methods
An expanded Materials and Methods section can be found in the online data supplement available at http://circres.ahajournals.org.
Human aortic SMCs (Cambrex Bio Science, Walkersville, Md) were grown according to the recommendations of the manufacturer.
Expression of mRNA was measured by real-time PCR using SYBR or TaqMan master mix (PE Applied Biosystems). Protein expression was detected by Western blot and immunocytochemistry. Promoter activity was measured by transfection assays.
Results
Human Aortic SMCs Express Two CIITA Isoforms That Are Highly Inducible by IFN-γ
CIITA is under the control of different promoters schematically represented in Figure 1A. To examine the expression of different CIITA isoforms and their respective induction by IFN-γ, cultured human aortic SMCs were either treated or untreated with recombinant IFN-γ (100 U/mL). Total RNA was extracted from cells, converted to cDNA, and measured by real-time PCR using specific primers for CIITA isoforms I, III, and IV (supplemental Table I).
Figure 1.
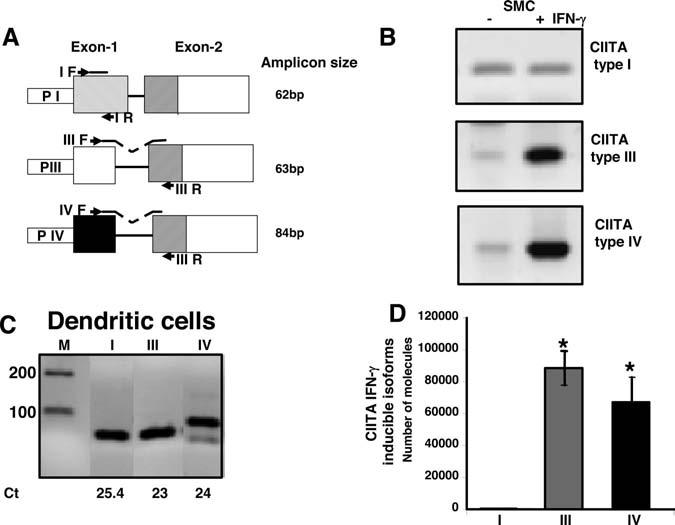
Detection of CIITA type I, III, and IV isoforms by real-time quantitative PCR in human aortic SMCs. A, Schematic representation of primer design. The first and second exons of CIITA types are depicted. The first exon encodes for the 5′ untranslated region (UTR) and the 5′ end of each isoform. Transcription of each individual isoform is directed by 3 different promoters (PI, PIII, PIV). Position of the forward specific primer is shown in the first exon (I F, III F, IV F). For CIITA type III and IV, the TaqMan probes were designed complementary to the first and second exon borders. Reverse primer was common (III) and complementary to the second exon. For CIITA type I, the TaqMan probe and the reverse primer were within the first exon. The size of the amplicons predicted from the cDNA sequence is shown. B, Aortic smooth muscle cell cDNA was amplified with CIITA isoform specific primers by real-time PCR and SYBR green detection. The expression of CIITA type I, III, and IV was measured by SYBR green cycle threshold (Ct) and the products of PCR were also resolved by 1.5% agarose gel electrophoresis. C, RNA from dendritic cells, isolated from human peripheral blood, was extracted and converted to cDNA. Dendritic cell cDNA was amplified with CIITA-specific primers, as in B, and served as a positive control for the expression of all 3 CIITA isoforms. The products of PCR were detected by 1.5% agarose gel. D, Aortic SMCs were treated for 24 hours with or without recombinant IFN-γ (100 U/mL). RNA from 3 wells of cultured cells was extracted, converted to cDNA, and subjected to real-time PCR with TaqMan probes/primers specific for CIITA isoforms. Specific CIITA plasmids were used to construct standard curves and for conversion of Ct values to copy numbers. Results are presented as absolute values and numbers of molecules that are expressed after IFN-γ stimulation. Results are normalized to 18 S ribosomal RNA and error bars represent mean of quadruplicates±SD. The data (n=4) were evaluated for significance by ANOVA followed by Sheffe's post hoc analysis using SPSS software. CIITA type III and CIITA type IV expression was significantly higher than CIITA type I expression. This difference was statistically significant (*P<0.01).
The expression of CIITA was first analyzed by real-time PCR and detected by SYBR green and agarose gel analysis (Figure 1B). The 3 isoforms of CIITA were expressed at very low levels in unstimulated (−IFN-γ) SMCs. After 24 hours of IFN-γ treatment (+IFN-γ), the mRNA expression level of CIITA type III and IV was significantly increased. In contrast, mRNA expression of CIITA type I was not affected by IFN-γ, and the level of expression remained as low as that of untreated SMCs.
Because CIITA type I primers detected a small amount of CIITA isoform I in SMCs, cDNA from dendritic cells was used as a positive control. CIITA type I primers amplify a significant amount of dentritic cell mRNA (Figure 1C). CIITA type I expression was 1500 fold higher in dendritic cells than in stimulated or unstimulated SMCs, a factor of 10 difference in cycle threshold (Ct) (dendritic cells Ct 25.4 versus SMCs Ct 35.5 and Ct 36.1). The IFN-γ induced CIITA type III and IV in SMCs was comparable with that of the constitutive expression of CIITA type III and IV in dendritic cells (Figure 1 B and 1C).
For specific detection of CIITA and better quantification of the 3 different isoforms, we used primer/TaqMan probe (supplemental Table I) and plasmid standards. A standard curve was constructed using serial dilution of the plasmid standards to calculate molecule copy numbers. The number of CIITA type I molecules induced by IFN-γ remained very low, in the range of 500 molecules per 50 ng of cDNA assayed (Figure 1D), whereas the numbers of CIITA type III and type IV molecules were greatly increased, reaching 80 000 and 60 000 molecules, after IFN-γ stimulation. The constitutive levels of all CIITA isoforms in untreated cells ranged between 400 and 1000 molecules. Because CIITA is an IFN-γ response gene, the kinetics of its RNA and protein expression in SMCs was examined at different times during IFN-γ treatment. Protein was located equally in nucleus and cytoplasm by 6 hours, as judged by Western and immunochemical analysis (supplemental Figure IA and IB). CIITA isoform III and IV expression increased with similar kinetics (supplemental Figure IC and ID). In some experiments, CIITA protein was detectable at 2 hours and the level was reduced at 24 hours, peaking between 6 and 24 hours, similar to other cells.28,29 RFX5, a DNA-binding protein that recruits CIITA, is constitutively expressed and induced (3-fold) with time by IFN-γ in aortic SMCs (supplemental Figure II). These results show that SMCs produce a significant amount of IFN-γ inducible CIITA and RFX-5 and that CIITA types III and IV are the foremost isoforms present in this cell type.
MHC II Expression Directly Correlates With an Increase in CIITA and RFX Transcription Complex
Because little is understood concerning the mechanism of MHC II expression in cultured aortic SMCs, the expression of HLA-Drα, 1 of the MHC II genes, was investigated in IFN-γ–stimulated SMCs by real-time PCR and Western analysis. HLA-Drα mRNA was absent in untreated cells and expressed at high levels after 24 hours of exposure to IFN-γ (Figure 2). Maximal HLA-Drα expression was achieved after 24 hours of IFN-γ treatment and maintained after 48 hours, although very little mRNA was detected at 6 hours (Figure 2A and 2B). HLA-Drα protein expression reflected that of the mRNA expression as shown in Figure 2C. Thus, expression of HLA-Drα is coordinately expressed with its master activator, CIITA. The results suggest that aortic SMCs express MHC II genes, activated by CIITA under the inflammatory stimulus of IFN-γ.
Figure 2.
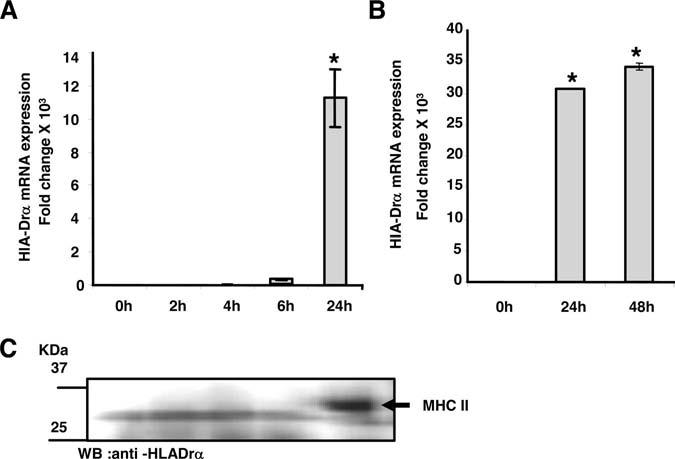
IFN-γ stimulated SMCs express great levels of HLA-Drα. RNA was extracted from human aortic SMCs that were treated with or without 100 U/mL IFN-γ at various times (2, 4, 6, 24, and 48 hours). TaqMan probe and specific primers were used to amplify cDNA for HLA-Dra (A and B) by real-time PCR. Each experiment was repeated at least 3 times and values represented as mean±SD. The data were evaluated for significance by ANOVA followed by Sheffe's post hoc analysis using SPSS software (*P<0.01). C, Cytoplasmic proteins were extracted from human aortic SMCs that were induced with IFN-γ (100 U/mL) at different times (2, 4, 6, and 24 hours). Proteins (20 mg) were separated in 10% SDS gel and electroblotted to a nitrocellulose membrane. The membrane was incubated with HLA-Dra antibody (FL-254; Santa Cruz Biotechnology) that detected a correct size band of 32 kDa after 24 hours of IFN-γ treatment. The chemiluminescence was measured with a Kodak Image station.
Expression of COL1A1 and COL1A2 Inversely Correlates With That of CIITA and RFX5 During IFN-γ Treatment
To study the role of IFN-γ on the expression of both collagen type I genes, COL1A1 and COL1A2, aortic SMCs were stimulated for different times with and without recombinant IFN-γ. Expression of COL1A1 and COL1A2, as measured by real-time PCR, was dramatically repressed after 48 hours of IFN-γ treatment (Figure 3). The steady-state levels began to decrease slowly 2 hours after IFN-γ treatment but remained constant without IFN-γ (data not shown). The results indicate that IFN-γ decreases collagen mRNA expression, which is inversely correlated with CIITA and RFX-5 mRNA expression.
Figure 3.
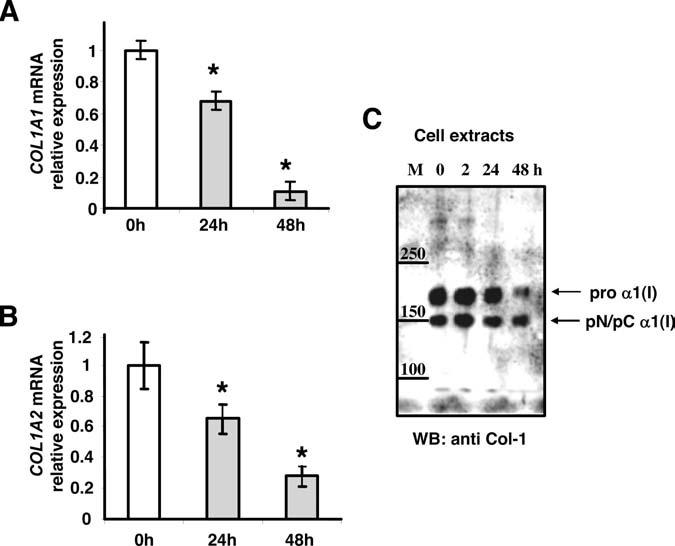
Type I Collagen α1(I) and α2(I) mRNA is repressed at 48 hours. RNA was extracted from human aortic SMCs that were treated with or without 100 U/mL of IFN-γ for 0, 24, and 48 hours. TaqMan probe and specific primers were used to amplify cDNA for COL1A 1 (A) and COL1A 2 (B) by real-time PCR. Each experiment was repeated at least 3 times and values represented mean±SD (n=5). The data were evaluated for significance by ANOVA followed by Sheffe's post hoc analysis using SPSS software (*=P<0.01). C, Cytoplasmic proteins were extracted from human aortic SMCs that were induced with IFN-γ (100 U/mL) at different times (0, 2, 24, and 48 hours). Proteins (20 μg) were separated in 4% Novex Tris–Glycin gel (Invitrogen) and SDS denaturing buffer and electroblotted to a nitrocellulose membrane. The membrane was incubated with anti-collagen type I (Rockland no. 103) at 1:5000 dilution. The antibody has been shown to be specific for α1(I) and not α2(I).50 The chemiluminescence was measured with a Kodak image station.
To test whether inhibition of collagen mRNA by IFN-γ is coupled to protein synthesis, the expression of collagen proteins was measured by Western analysis. Proteins were extracted from IFN-γ untreated and treated SMCs at different times (0, 2, 24, 48 hours). Cytoplasmic proteins were separated by 4% sodium dodecyl sulfate-gel electrophoresis, transferred to nitrocellulose membrane, and detected with antibodies specific for α1(I) (Figure 3C). Protein expression reflected the mRNA expression of COL1A1 with a significant decrease of α1(I) protein expression after 48 hours of IFN-γ treatment (Figure 3C). The reduction in protein expression was greater than 50% for procollagen α1(I) compared with the partial processed form pN/pC α1(I). Thus, IFN-γ reduces not only transcription of collagen but also collagen production in SMCs.
CIITA Types I, III, and IV Repress Collagen Type I and Induce MHC II Promoters
Exogenous expression of CIITA type III enhances transcription of MHC II and represses collagen genes in transient transfected fibroblasts cells.12,30 However, the regulatory properties of all 3 CIITA isoforms in fibroblasts or aortic SMCs have not been tested. SMCs were transfected with COL1A1, COL1A2, or HLA-Drα promoter/luciferase construct, together with expression constructs for either CIITA type I, type III, or type IV. As expected, CIITA significantly (P<0.01) repressed transcription of COL1A1 and COL1A2 promoters, whereas it enhanced transcription of HLA-Drα promoter (Figure 4). CIITA types I, III, and IV had similar effects on all promoters. These findings suggest that the specific domains of the isoforms (the CARD domain of type I and the 24 extra amino acids present in CIITA type III) have no role in the collagen repression and MHC II activation.
Figure 4.
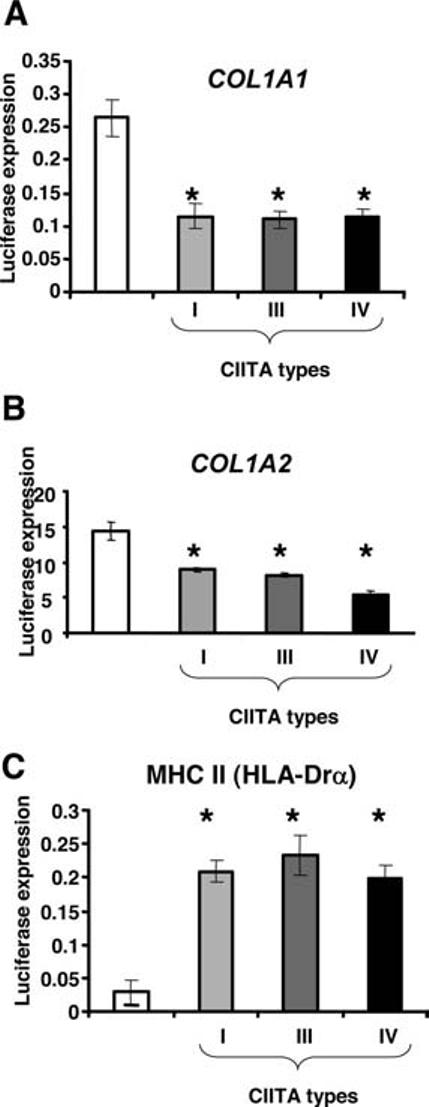
CIITA downregulates collagen type I promoters and activates MHC II promoter in human SMCs. A, COL1A1 promoter construct (−311/+114) (0.5 μg) was cotransfected with GFP construct (0.1 μg) and/or pcDNA3 empty vector or CIITA type I, III, and IV expression vectors (0.5 μg) into human aortic SMCs. B, COL1A2 promoter construct (pGL3-Col-Luc, −357/+55) (0.5 μg) was cotransfected with GFP construct (0.1 μg) and/or pcDNA3 empty vector or CIITA type I, III, and IV expression vectors as in A. C, pGL3-HLA-DRA promoter construct (pGL-300-DRα) was cotransfected with GFP construct and either pcDNA3 empty vector or CIITA type I, III, and IV expression vectors as in A. Luciferase activity, corrected for transfection efficiency with GFP, was normalized by protein concentration and is shown as the mean±SD (n=3). The data were evaluated for significance by ANOVA followed by Sheffe's post hoc analysis using SPSS software (*P<0.01).
Simvastatin Has No Effect on the IFN-γ–Inducible CIITA Isoforms
Because MHC II genes and CIITA type III and IV are highly induced by IFN-γ in SMCs, the role of statins on IFN-γ– induced MHC II and CIITA expression was explored. SMCs were pretreated with 1 μmol/L simvastatin for 18 hours, followed by the addition of IFN-γ (100 U/mL) or a vehicle for an additional 24 hours. Cells appeared healthy and morphologically similar to untreated cells, but they were growth arrested (data not shown).
IFN-γ–induced MHC II mRNA expression was reduced 1.2- to 3-fold by simvastatin, whereas simvastatin alone had no effect on MHC II expression (Figure 5A). Because in endothelial cells, simvastatin treatment abrogated IFN-γ–induced CIITA expression,31 CIITA expression was examined to determine whether decreased MHC II expression was caused by decreased levels of CIITA. Surprisingly, simvastatin did not inhibit the IFN-γ–induced expression of CIITA type III or type IV mRNA in human SMCs. IFN-γ induced CIITA type III and IV (Figure 2C, 2D), but the addition of simvastatin did not lower the level of the induced CIITA mRNAs (Figure 5B). Simvastatin alone had no effect on basal CIITA expression. Results from 6 different experiments demonstrated that simvastatin had no effect on the expression of inducible CIITA mRNA isoforms. In contrast, simvastatin decreased the basal and the IFN-γ–induced expression of RFX5 (Figure 5C). The changes in RFX5 and CIITA protein levels (Figure 5C and 5B) caused by simvastatin treatment were a reflection of the mRNA steady-state levels. Simvastatin alone also decreased the expression of collagen α2(I) and α1(I) mRNA and increased IFN-γ repression (Figure 5D and data not shown), suggesting that the mechanism by which simvastatin works in SMCs is independent of CIITA and IFN-γ pathway. These results suggest that simvastatin may act in a cell type–specific manner.
Figure 5.

CIITA isoforms and MHC II genes are not affected by simvastatin treatment of human SMCs. Aortic human SMCs were pretreated with 1 μmol/L simvastatin or vehicle for 18 hours, followed by 100 U/mL IFN-γ for 24 hours. RNA and proteins were extracted, as described in the expanded Materials and Methods section (supplemental data). TaqMan probes and specific primers were used to amplify cDNA for MHC II (HLA-Drα) (A), CIITA type III and IV (B), RFX5 (C), and COL1A2 (D). Each graph shows results from a representative experiment performed in duplicate repeated 4 times and values represent mean±SD. The data from at least 4 experiments were evaluated for significance by ANOVA followed by Sheffe's post hoc analysis using SPSS software (*P<0.001, **P<0.01, **P<0.05). IFN-γ significantly increased MHC II, RFX5, and CIITA (*P<0.001). Simvastatin decreased COL1A2 (**P<0.01) and RFX5 (***P<0.05). Protein levels were detected by Western blotting and antibodies for RFX5 (C) and CIITA (B).
Discussion
The data presented in this study demonstrate, for the first time, that SMCs express CIITA, a master activator of MHC II that enhances the immune system by activating MHC II expression and reducing the extracellular matrix by repressing collagen expression. Both MHC II and collagen are implicated in the process of atherosclerosis. Our novel findings demonstrate that 2 major CIITA isoforms, III and IV, are induced by IFN-γ in SMCs and all 3 CIITA isoforms regulate collagen and MHC II gene inversely.
CIITA activates the expression of MHC II in all types of professional APCs.32,33 Very few studies investigate the role of CIITA in nonprofessional cells. Our earlier studies indicate that CIITA and RFX5 complex play critical roles in IFN-γ–induced repression of collagen expression by fibroblasts.11,12 In this article, aortic SMCs MHC II gene expression was dramatically enhanced by IFN-γ, as measured by both mRNA and protein levels. MHC II gene expression correlated with induced expression of CIITA isoforms III and IV, as well as with that of RFX5. In contrast, collagen α1(I) and α2(I) expression decreased. The kinetics of MHC II, collagen, CIITA, and RFX5 gene expression were in agreement with the role of the CIITA/RFX5 complex as an activator for MHC II34-37 and a repressor for collagen genes.12,30,38 Thus, these findings suggest that CIITA/RFX5 complex exists in SMCs as a common regulator of MHC II and collagen genes.
Because the human CIITA gene is controlled by at least 3 promoters (pI, pIII, and pIV), each of which leads to the production of 3 different isoforms,23 it was of interest to explore the expression and the inducibility of the different CIITA isoforms in SMCs. The major isoforms induced by IFN-γ are III and IV, not I, in agreement with other cell types from human sources.39,40 Recently, we have demonstrated (Xu et al, manuscript submitted for publication) that, in contrast to human, mouse SMCs as well as adventitia and lung fibroblast cells express IFN-γ–inducible CIITA type I, similar to mouse microglial cells.41 Yet, the regulatory mechanism by which IFN-γ induces CIITA type I remains to be elucidated in mice. Interestingly, mouse SMCs do not produce CIITA type III, suggesting that isoforms I and IV can compensate for the missing function of isoform III. The results by us and others42,43 suggest that species-specific regulatory mechanisms might exist for CIITA isoforms, reflecting diversities in the immunological response.
Because translation of CIITA mRNA isoforms begins from exon 2 (isoform IV) or exon 1 (isoform I and III), the resulting isoforms contain a distinct N terminus. CIITA type III protein contains 24 additional N-terminal amino acids that are not in type IV. CIITA type I includes a unique larger domain (94 amino acids) encoded from its distinct first exon, which is homologous to a caspase recruitment domain (CARD). This domain in some cases confers a strong transactivation potential to the CIITA isoform I for the MHC II promoter.19,44 Indeed, it was intriguing that, despite the selectivity in the IFN-γ–induced endogenous expression of CIITA isoforms in SMCs, all 3 CIITA types were capable of repressing collagen and activating MHC II promoters when CIITA was exogenously expressed in transient transfection assays. Therefore, the N-terminal divergences among the 3 isoform are not relevant for either repression of collagen or activation of MHC II promoters. Repression of collagen and activation of MHC II promoters is not isoform specific in this cellular context. The regulatory property of CIITA type III and IV is probably most relevant in vivo in human SMCs, because both isoforms III and IV were endogenously highly expressed after IFN-γ stimulation (Figure 1B and 1D and supplemental Figure I).
Interestingly, CIITA and RFX5 were the first regulatory factors discovered with genetic defects that are responsible for diseases.43,45 Altered expression of CIITA caused by a single nucleotide polymorphism in the human promoter of CIITA type III (168A->G) is associated with increased susceptibility to rheumatoid arthritis, multiple sclerosis, and myocardial infarction.46 Therefore, it is tempting to speculate that alteration of CIITA expression may result in dysregulated expression of MHC II and collagen that may affect the pathogenesis of atherosclerosis by altering the immune response and extracellular matrix deposition. It would be interesting to explore the progression of atherosclerosis with the expression of MHC II and collagen in SMCs from atherosclerotic lesions from patients carrying the above polymorphism.
Statins are largely used for prevention of cardiovascular disease. Their therapeutic effect is not limited only to lowering the cholesterol but also to decreasing the expression of MHC II genes.27,31 CIITA has been demonstrated to be a target of statins,27,31,41 and this effect conferred an antiinflammatory role to the statins used largely also for other inflammatory diseases.47,48 Our results demonstrate that simvastatin did not alter the expression of CIITA III or IV mRNA or protein levels, suggesting that statin inhibition of MHC II is not CIITA dependent in SMCs. The discrepancy in data could be explained by different cell types, SMCs versus human endothelial,27,31,47 macrophages,27 or mouse microglial41 cells. In a recent article, Kuipers et al49 demonstrated that statins do not alter MHC II transcription significantly but rather change cell surface expression of MHC II molecules by disrupting cholesterol-containing microdomains involved in MHC II intracellular transport. In agreement with our data, these authors demonstrate that simvastatin treatment did not reduce the levels of CIITA induced by IFN-γ in HeLa cells. Simvastatin treatment produces a small decrease in IFN-γ–stimulated MHC II levels in SMCs, which was consistently lower but not statistically significant, similar to HeLa cells. Because RFX5 is another component of CIITA/RFX5 complex necessary for MHC II activation, it is possible that RFX5 is a target for the small decrease in MHC II expression by simvastatin. Indeed, our results shows that simvastatin lowered both the constitutive and IFN-γ–induced mRNA and protein expression of RFX5 (Figure 5C).
In conclusion, this publication is the first to demonstrate that CIITA is induced by IFN-γ in SMCs and functions as a common regulator of both collagen and MHC II genes. Moreover, CIITA is not affected by simvastatin treatment. Therefore, during an inflammation process when IFN-γ is produced, increased expression of CIITA will enhance the inflammatory response by increasing the number of MHC II molecules in aortic or vascular SMCs and lower collagen production, which may compromise plaque stability. It would be interesting to explore the role that these molecules play using in vivo models of atherosclerotic lesions. Ongoing studies of CIITA-deficient mouse models will help to elucidate the physiological role of inflammation and extracellular matrix genes in atherosclerosis.
Supplementary Material
Acknowledgments
This work was funded by National Heart, Lung, and Blood Institute grant P01-HL013262-31. We thank Drs Jenny P.-Y. Ting and Cheong-Hee Chan for kindly providing CIITA expression constructs and Dr Barbara M. Schreiber for critical reading of the manuscript.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am J Cardiol. 2000;86:3J–8J. doi: 10.1016/s0002-9149(00)01339-4. [DOI] [PubMed] [Google Scholar]
- 4.Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003;10:267–274. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- 5.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999;34:513–525. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 6.Rekhter MD, Zhang K, Narayanan AS, Phan S, Schork MA, Gordon D. Type I collagen gene expression in human atherosclerosis. Localization to specific plaque regions. Am J Pathol. 1993;143:1634–1648. [PMC free article] [PubMed] [Google Scholar]
- 7.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 8.Rekhter MD, Hicks GW, Brammer DW, Hallak H, Kindt E, Chen J, Rosebury WS, Anderson MK, Kuipers PJ, Ryan MJ. Hypercholesterolemia causes mechanical weakening of rabbit atheroma: local collagen loss as a prerequisite of plaque rupture. Circ Res. 2000;86:101–108. doi: 10.1161/01.res.86.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25(suppl 2):S9–S12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- 10.Harvey EJ, Ramji DP. Interferon-gamma and atherosclerosis: pro- or anti-atherogenic? Cardiovasc Res. 2005;67:11–20. doi: 10.1016/j.cardiores.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Wang L, Buttice G, Sengupta PK, Smith BD. Interferon gamma repression of collagen (COL1A2) transcription is mediated by the RFX5 complex. J Biol Chem. 2003;278:49134–49144. doi: 10.1074/jbc.M309003200. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Wang L, Buttice G, Sengupta PK, Smith BD. Major histocompatibility class II transactivator (CIITA) mediates repression of collagen (COL1A2) transcription by interferon gamma (IFN-gamma) J Biol Chem. 2004;279:41319–41332. doi: 10.1074/jbc.M404174200. [DOI] [PubMed] [Google Scholar]
- 13.Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 14.Jonasson L, Holm J, Skalli O, Gabbiani G, Hansson GK. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985;76:125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stemme S, Fager G, Hansson GK. MHC class II antigen expression in human vascular smooth muscle cells is induced by interferon-gamma and modulated by tumour necrosis factor and lymphotoxin. Immunology. 1990;69:243–249. [PMC free article] [PubMed] [Google Scholar]
- 16.Warner SJ, Friedman GB, Libby P. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis. 1989;9:279–288. doi: 10.1161/01.atv.9.3.279. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XS, Linhoff MW, Li G, Chin KC, Maity SN, Ting JP. Transcriptional scaffold: CIITA interacts with NF-Y, RFX, and CREB to cause stereospecific regulation of the class II major histocompatibility complex promoter. Mol Cell Biol. 2000;20:6051–6061. doi: 10.1128/mcb.20.16.6051-6061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masternak K, Barras E, Zufferey M, Conrad B, Corthals G, Aebersold R, Sanchez JC, Hochstrasser DF, Mach B, Reith W. A gene encoding a novel RFX-associated transactivator is mutated in the majority of MHC class II deficiency patients. Nat Genet. 1998;20:273–277. doi: 10.1038/3081. [DOI] [PubMed] [Google Scholar]
- 19.Yee CS, Yao Y, Li P, Klemsz MJ, Blum JS, Chang CH. Cathepsin E: a novel target for regulation by class II transactivator. J Immunol. 2004;172:5528–5534. doi: 10.4049/jimmunol.172.9.5528. [DOI] [PubMed] [Google Scholar]
- 20.Yee CS, Yao Y, Xu Q, McCarthy B, Sun-Lin D, Tone M, Waldmann H, Chang CH. Enhanced production of IL-10 by dendritic cells deficient in CIITA. J Immunol. 2005;174:1222–1229. doi: 10.4049/jimmunol.174.3.1222. [DOI] [PubMed] [Google Scholar]
- 21.Wong AW, Brickey WJ, Taxman DJ, van Deventer HW, Reed W, Gao JX, Zheng P, Liu Y, Li P, Blum JS, McKinnon KP, Ting JP. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat Immunol. 2003;4:891–898. doi: 10.1038/ni960. [DOI] [PubMed] [Google Scholar]
- 22.Piskurich JF, Gilbert CA, Ashley BD, Zhao M, Chen H, Wu J, Bolick SC, Wright KL. Expression of the MHC class II transactivator (CIITA) type IV promoter in B lymphocytes and regulation by IFN-gamma. Mol Immunol. 2006;43:519–528. doi: 10.1016/j.molimm.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mach F. Statins as immunomodulators. Transpl Immunol. 2002;9:197–200. doi: 10.1016/s0966-3274(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 25.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 27.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri G, Deffrennes V, Prod'homme T, Vedrenne J, Baton F, Cortes C, Fischer A, Bono MR, Lisowska-Grospierre B, Charron D, AlcaideLoridan C. Isoforms of the class II transactivator protein. Int Immunol. 2002;14:839–848. doi: 10.1093/intimm/dxf060. [DOI] [PubMed] [Google Scholar]
- 29.Zika E, Fauquier L, Vandel L, Ting JP. Interplay among coactivator-associated arginine methyltransferase 1, CBP, and CIITA in IFN-{gamma}-inducible MHC-II gene expression. Proc Natl Acad Sci U S A. 2005;102:16321–16326. doi: 10.1073/pnas.0505045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu XS, Ting JP. A 36-amino-acid region of CIITA is an effective inhibitor of CBP: novel mechanism of gamma interferon-mediated suppression of collagen alpha(2)(I) and other promoters. Mol Cell Biol. 2001;21:7078–7088. doi: 10.1128/MCB.21.20.7078-7088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghi MM, Tiglio A, Sadigh K, O'Donnell L, Collinge M, Pardi R, Bender JR. Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation. 2001;71:1262–1268. doi: 10.1097/00007890-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 32.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 33.Ting JP, Baldwin AS. Regulation of MHC gene expression. Curr Opin Immunol. 1993;5:8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- 34.DeSandro AM, Nagarajan UM, Boss JM. Associations and interactions between bare lymphocyte syndrome factors. Mol Cell Biol. 2000;20:6587–6599. doi: 10.1128/mcb.20.17.6587-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villard J, Muhlethaler-Mottet A, Bontron S, Mach B, Reith W. CIITA-induced occupation of MHC class II promoters is independent of the cooperative stabilization of the promoter-bound multi-protein complexes. Int Immunol. 1999;11:461–469. doi: 10.1093/intimm/11.3.461. [DOI] [PubMed] [Google Scholar]
- 36.Kern I, Steimle V, Siegrist CA, Mach B. The two novel MHC class II transactivators RFX5 and CIITA both control expression of HLA-DM genes. Int Immunol. 1995;7:1295–1299. doi: 10.1093/intimm/7.8.1295. [DOI] [PubMed] [Google Scholar]
- 37.Jabrane-Ferrat N, Nekrep N, Tosi G, Esserman LJ, Peterlin BM. Major histocompatibility complex class II transcriptional platform: assembly of nuclear factor Y and regulatory factor X (RFX) on DNA requires RFX5 dimers. Mol Cell Biol. 2002;22:5616–5625. doi: 10.1128/MCB.22.15.5616-5625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta P, Xu Y, Wang L, Widom R, Smith BD. Collagen alpha1(I) gene (COL1A1) is repressed by RFX family. J Biol Chem. 2005;280:21004–21014. doi: 10.1074/jbc.M413191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piskurich JF, Linhoff MW, Wang Y, Ting JP. Two distinct gamma interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor beta. Mol Cell Biol. 1999;19:431–440. doi: 10.1128/mcb.19.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikcevich KM, Piskurich JF, Hellendall RP, Wang Y, Ting JP. Differential selectivity of CIITA promoter activation by IFN-gamma and IRF-1 in astrocytes and macrophages: CIITA promoter activation is not affected by TNF-alpha. J Neuroimmunol. 1999;99:195–204. doi: 10.1016/s0165-5728(99)00117-4. [DOI] [PubMed] [Google Scholar]
- 41.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 42.Waldburger JM, Suter T, Fontana A, Acha-Orbea H, Reith W. Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J Exp Med. 2001;194:393–406. doi: 10.1084/jem.194.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- 44.Nickerson K, Sisk TJ, Inohara N, Yee CS, Kennell J, Cho MC, Yannie PJ, 2nd, Nunez G, Chang CH. Dendritic cell-specific MHC class II transactivator contains a caspase recruitment domain that confers potent trans-activation activity. J Biol Chem. 2001;276:19089–19093. doi: 10.1074/jbc.M101295200. [DOI] [PubMed] [Google Scholar]
- 45.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 46.Swanberg M, Lidman O, Padyukov L, Eriksson P, Akesson E, Jagodic M, Lobell A, Khademi M, Borjesson O, Lindgren CM, Lundman P, Brookes AJ, Kere J, Luthman H, Alfredsson L, Hillert J, Klareskog L, Hamsten A, Piehl F, Olsson T. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet. 2005;37:486–494. doi: 10.1038/ng1544. [DOI] [PubMed] [Google Scholar]
- 47.Kwak B, Mulhaupt F, Veillard N, Pelli G, Mach F. The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced MHC class II expression in human vascular endothelial cells. Swiss Med Wkly. 2001;131:41–46. doi: 10.4414/smw.2001.06144. [DOI] [PubMed] [Google Scholar]
- 48.Mach F. Immunosuppressive effects of statins. Atheroscler Suppl. 2002;3:17–20. doi: 10.1016/s1567-5688(01)00010-1. [DOI] [PubMed] [Google Scholar]
- 49.Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum Immunol. 2005;66:653–665. doi: 10.1016/j.humimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Stefanovic B, Schnabl B, Brenner DA. Inhibition of collagen alpha 1(I) expression by the 5′ stem-loop as a molecular decoy. J Biol Chem. 2002;277:18229–18237. doi: 10.1074/jbc.M108065200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


