Abstract
Lens regeneration in adult newts is a classic example of faithfully regenerating an entire organ via transdifferentiation1–6. After lentectomy, intriguing regulation allows the pigment epithelial cells (PECs) of the dorsal iris, but not the ventral, to dedifferentiate and then differentiate to form a new lens. This regulation might provide clues to the lack of lens regeneration in higher vertebrates. Six-3 and pax-6 known for their ability to induce ectopic lenses during embryogenesis7,8 and members of the BMP pathway, which are regulators of the dorsal/ventral axis establishment in embryos9 were examined for their role in induction of lens regeneration. Here we show that lens regeneration from the ventral iris is possible by inhibiting the BMP pathway or by transfecting ventral iris cells with six-3 and concomitant treatment with retinoic acid. In intact irises six-3 is expressed higher in the ventral iris. During regeneration, however, only levels in the dorsal iris are significantly increased. Such an increase is seen in ventral irises only when they are induced to transdifferentiate by six-3/RA or BMP inhibitors. Therefore, transcriptional regulation associated with competency for lens regeneration, aims to increase levels over established thresholds and not to merely render a regulatory gene as dorsal-specific. Lack of induction in the axolotl, a salamander incapable of lens regeneration seems to be associated with repression of six-3 expression.
Keywords: lens, regeneration, transdifferentiation, six-3, BMP
In order to determine the role that six-3 and pax-6 play in the induction of transdifferentiation of the ventral iris, ventral iris cells were transfected in the presence or absence or retinoic acid (RA) with the appropriate constructs and examined for induction by utilizing an in vitro transfection/in vivo transplantation system that reproduces the conditions seen in vivo10–12. Retinoids have been shown to affect regeneration and to determine morphogenesis and differentiation of several tissues including the eye and limb13–16. In addition, dorsal or ventral iris explants were treated with soluble BMP-4, BMP-7, chordin and a soluble competitor for BMPR-1A.
Following transfection and implantation of aggregated PECs, scores of eyes were examined (see Supplementary Information). As a rule, untransfected dorsal PEC aggregates transdifferentiate to lens while the ventral ones do not. Under the conditions outlined in Methods short term culturing of cells does not interfere with the potential for lens transdifferentiation. Dorsal aggregates produced a lens in over 83% of the cases (10/12), while the ventral ones, as expected, did not (0/11) (Fig. 1a-c). It has been shown before, through beta galactosidase staining, that the lens is indeed derived from the aggregate12. Dorsal aggregates transfected with the constructs with RA treatment also transdifferentiated to lens (not shown). However, with ventral PECs, only one particular protocol, transfection of PECs with six-3 in the presence of retinoic acid, led to the induction of lens transdifferentiation (Fig 1d-f). This induction occurred at a comparable rate (3/4; 75%) to that seen in the dorsal aggregates. Neither treatment with retinoic acid alone nor transfection of ventral PEC cultures with six-3 alone was able to induce transdifferentiation. In the BMP series we found that inhibition of the pathway by either the BMPR-1A competitor or chordin resulted in the induction of a lens from the ventral explants (3/15 and 1/8 respectively) (Figure 1g-j). The incidence of induction was low (17%), however, we regard this as highly significant in light of the failure of the untreated ventral explants to differentiate to lens (0/27; 0% induction). This is in agreement with the established role of BMPs in maintaining ventral identity during embryogenesis and the fact that inhibition of BMPs binding to receptors results in dorsalization9. Interestingly, treatment of the dorsal iris explants with BMP-7, and to a lesser degree BMP-4, significantly inhibited their ability to transdifferentiate to lens (1/12; 8.3% and 5/12; 41.6% respectively). Such results clearly indicate that BMPs maintain the ventral identity and inhibition of the pathway dorsalizes the ventral iris allowing transdifferentiation.
Figure 1. Lens induction from ventral PECs.
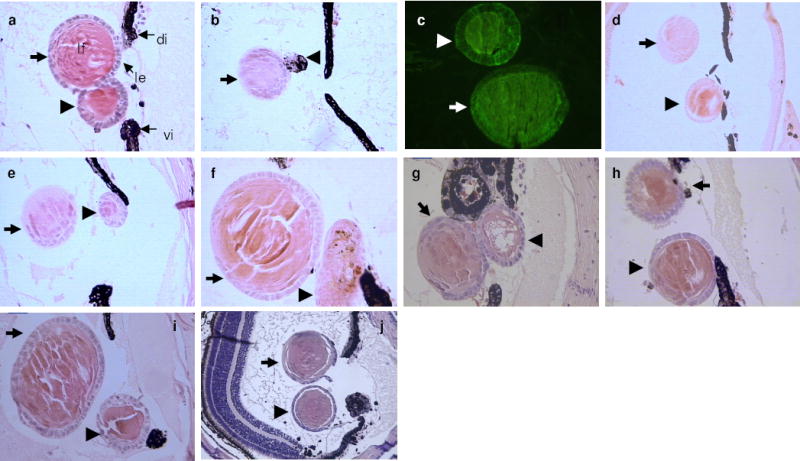
a-f, Lens induction by transplantation of PEC aggregates examined 30 days later. Thick arrows indicate the host regenerated lens and arrowheads the PEC aggregate or the induced lens from the PEC aggregate. a, A control untransfected dorsal PEC aggregate, which has transdifferentiated to lens. le: lens epithelium, lf: lens fibers, di: dorsal iris, vi: ventral iris. b, A control untransfected ventral PEC aggregate (arrowhead), which has remained pigmented and failed to transdifferentiate to lens. c, Detection of crystallin synthesis in both a host lens and an induced lens with a lens fiber-specific antibody to β-crystallin23. d-f, Induced lenses from ventral PEC aggregates transfected with six-3 and treated with RA. g-j, Induced lenses from ventral iris explants treated with BMPR-1A inhibitor (g-i) and with chordin (j).
In order to further probe the mechanism of induction, we decided to undertake a detailed gene expression profiling of six-3 and BMPR-1A during lens regeneration and during the experimental treatments that lead to the induction of lens regeneration from the ventral iris. Pax-6 expression was also assessed because of its known association with six-3. We selected to work with samples of iris isolated 2, 4 and 8 days post lentectomy. During this time, dedifferentiation events that lead to regeneration from the dorsal iris have been initiated. Moreover, at later stages the vesicle starts expressing crystallins and differentiating to lens. Since these genes are also expressed in the differentiating lens their induction-related expression might be ‘contaminated.’ Several interesting points emerged from the expression patterns, which somewhat were very surprising and call for a revision of our view of the mechanism of lens regeneration. First, both dorsal and ventral iris showed expression of all three genes. When the data were analyzed to compare between the dorsal and ventral iris we found that the three genes were expressed higher in the intact ventral iris. This pattern was maintained by day 8 but with a lesser relative fold change (Fig. 2a). When the data, however, were analyzed in a different way to compare expression in the 2, 4 and 8-day dorsal iris with the intact dorsal iris and the 2, 4 and 8-day ventral iris with the intact ventral iris, to correlate expression with the process of regeneration, an interesting pattern emerged: The levels of six-3 were elevated in the dorsal iris only and seem comparable at this time to the ventral ones. BMPR-1A and pax-6 were also slightly up-regulated (Fig. 2b-d). Up-regulation of six-3 in the dorsal iris started at day 4 (Fig. 2c), while for pax-6 and BMPR-1A at day 8 (Fig. 2d). In other words, increase of six-3 levels seems to be important during the dedifferentiation process in the dorsal iris. Since regeneration occurs only from the dorsal iris and since the ventral iris also expresses these genes, our data suggest that gene regulation associated with the competency for lens regeneration aims to increase levels over a particular threshold and not simply rendering a regulatory gene as dorsal-specific. Such a pattern for six-3 is clearly shown when the expression of the different time points is also presented in comparison to intact dorsal iris in one cluster (Fig. 2e).
Figure 2. Expression during lens regeneration and induction.
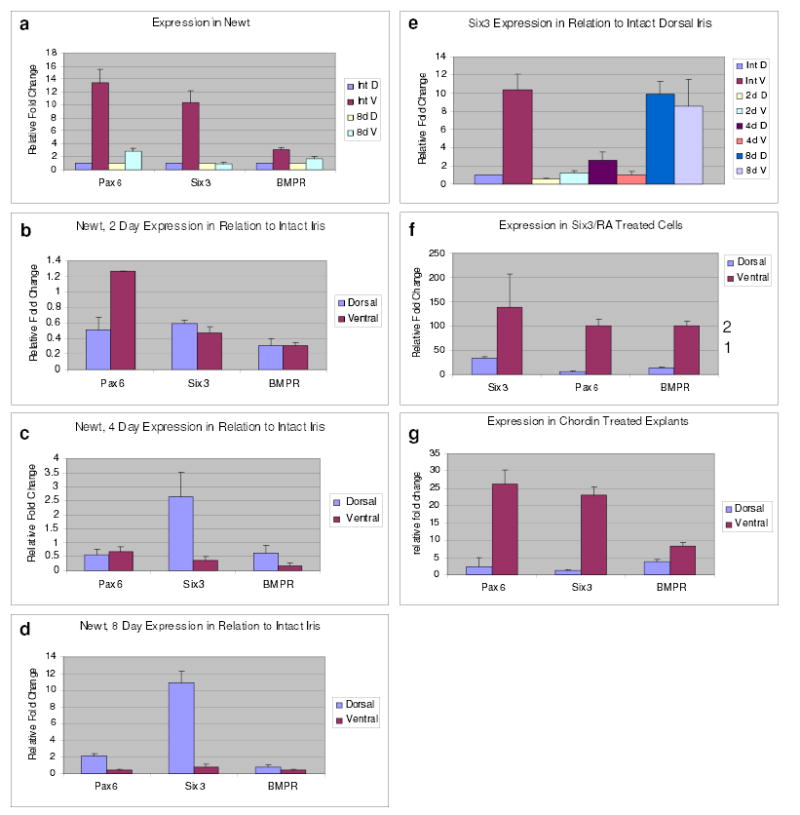
a, Expression of pax-6, six-3 and BMPR-1A in the newt intact and 8-day post-lentectomy dorsal and ventral iris. Comparison between intact dorsal and ventral iris. The values of the dorsal irises have been set to 1 and those of the ventral iris are shown as relative fold changes. b-d, Expression of pax-6, six-3 and BMPR-1A shown by comparing 2, 4 and 8-day irises with intact irises. e, Expression of six-3 at all time points compared with its expression in the intact dorsal iris. f, Expression in six-3/RA treated PECs in relation to the untransfected cells. Note significant increase of exogenous six-3 levels in the ventral PECs. Levels of pax-6 and BMPR-1A are much lower and are represented by the numbers on the right y axis (to accommodate the graph with the very high fold increase for six-3). g, Expression in chordin treated iris explants shown as relative fold change to the untreated explants. Results are means ± s.d.
Treatment of ventral iris cells with six-3/RA, which resulted in induction of transdifferentiation, showed a similar pattern of up-regulation of six-3, pax-6, and BMPR-1A when compared to the untransfected ventral cells (Fig. 2f). Treatment of the cells with RA alone or transfection of six-3 alone, which failed to induce irises to differentiate to lens, did not show such a pattern (not shown). Similarly, treatment of ventral iris explants with chordin, which also resulted in induction, invoked marked up-regulation of six-3 and pax-6, and to a lesser degree of BMPR-1A, in the treated ventral irises, as compared with the increase in the untreated irises (Fig. 2g). BMPR-1A transcriptional regulation might not be that important for the induction. Interestingly the rate of increase (as relative fold change) in the treated ventral irises is comparable to the increase in the regenerating 8-day dorsal iris. In other words the treated ventral irises that were able to transdifferentiate to lens adopted a gene expression profile (especially for six-3) that is seen only in the dorsal iris during dedifferentiation and regeneration. This in turn indicates that when the ventral irises are coaxed to mimic patterns of regulatory events seen in the dorsal iris they are able to be “dorsalized” and therefore transdifferentiate into lens.
These expression patterns for six-3 pose the following question: Are there subpopulations in the dorsal or ventral iris that might account for these differences? To answer this question we used immunostaining to assess the distribution of six-3 expressing cells. We stained serial sections along the nasal-temporal axis that spanned the whole iris (with distinct dorsal and ventral portions) and we counted the positive cells. Six-3 positive cells were found throughout the examined 8-day dorsal and ventral irises without apparent differences in their distribution (Fig. 3a). These results show that six-3 up-regulation is not attributed to expression in more cells. When the aggregates (or explants) transdifferentiated to lens nearly all cells participated, agruing against six-3 expressing subpopulations as well (Fig. 1). Since expression of six-3 and BMPR-1A in lens regeneration has not been reported before we also examined their expression throughout the process. In Fig. 3b we show expression in dorsal and ventral iris at early stages (before vesicle formation) and in Fig. 3c during later stages (in vesicle or regenerating lens). Ventral iris of the later stages is also positive (not shown). The presence of six-3 in dorsal and ventral iris is consistent with the QPCR data, immunostaining, however, is not quantitative.
Figure 3. Expression of six-3 and BMPR-1A during regeneration.
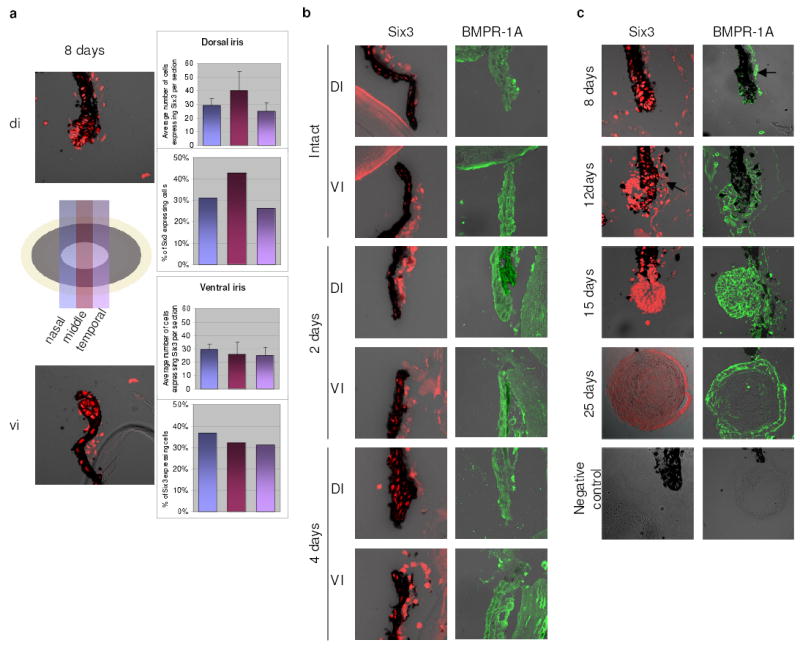
a, Identification of six-3 positive cells in the dorsal and ventral iris 8-day post-lentectomy assessed via immunostaining of serial sections along the nasal-temporal axis (see illustration). No apparent difference in cell numbers or distribution was seen (right panels; results on graphs are means ± s.d.). b, Expression of six-3 and BMPR-1A in both dorsal and ventral iris at early stages (before vesicle formation). Due to heavy pigmentation at these early stages for the BMPR-1A staining sections were bleached. c, Expression of six-3 and BMPR-1A at later stages. At 12-day post-lentectomy a definite lens vesicle has been formed. The lens vesicle and the differentiating lens fibers are positive at 15-day and by 25-day only cells in the lens epithelium express six-3. Same patterns can be seen for the BMPR-1A. Negative controls are shown at the bottom. All images are mergers between the dark field and DIC. The stroma (arrows) always shows unspecific fluorescence.
The next question is whether the “newt treatments” are unique to the newt or if they can induce lens transdifferentiation in irises from other vertebrates. To answer this question we used another salamander, the axolotl, which possesses the ability to regenerate limbs or tail, but not the lens. None of the treatments induced transdifferentiation, indicating that the treatments are most likely newt-specific. However, it might be too premature to preclude the participation of six-3 and BMP inhibition in lens regeneration in other species. In the newt the lens is regenerated from the dorsal iris and in the premetamorphic frog from the cornea, two strategies that differ from the embryonic induction of lens development as well. This might argue against absolute conservation of the inductive mechanisms. To receive some insight that could explain the axolotl data we examined gene expression in intact irises, in irises 8 days after lentectomy and in treated irises. In contrast to what was observed in the newt, the expression profiles in the axolotl differ considerably in both intact and 8-day irises (Fig. 4a). The intact and 8-day ventral irises do not show higher levels of expression over the dorsal ones. Moreover, six-3 was severely down regulated in the irises 8-days after lentectomy when compared with the intact irises. Pax-6 and BMPR-1A were slightly upregulated in both dorsal and ventral irises (Fig. 4b). This expression pattern is diametrically opposite to what we observed in the newt and indicates that a negative regulation of six-3 as well as regulation in establishing thresholds might result in inability for lens regeneration in the axolotl. So, why did the treatments fail to induce lens transdifferentiation? We believe because repression of six-3 persists even after the treatments. Indeed, treatment with chordin, which mediated six-3 and pax-6 up-regulation in the induced newt ventral irises failed to do so in the axolotl (compare Fig. 4c with Fig.2g). Several explanations can account for the axolotl results. First, six-3 repression could be regulated more tightly than in the newt possibly by an inhibitor of its transcription and function. Second, the axolotl PECs do not respond to these treatments equally and they may require optimized conditions. Third, the mechanism of induction of lens regeneration in different vertebrates follows unique pathways.
Figure 4. Expression in axolotl.
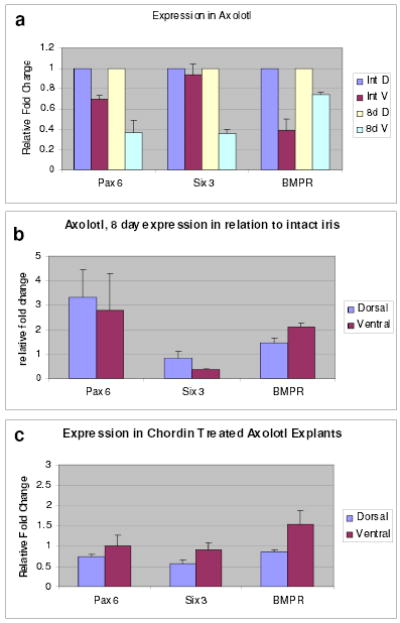
a, Expression of pax-6, six-3 and BMPR-1A in the axolotl intact and 8-day post-lentectomy dorsal and ventral iris. Comparison between intact dorsal and ventral iris. The values of the dorsal irises have been set to 1 and those of the ventral iris are shown as relative changes. b, Expression shown by comparing 8-day irises with intact irises. c, Expression in chordin treated iris explants shown as relative fold change to the untreated explants. Results are means ± s.d.
It is rather interesting that pax-6 was unable to induce transdifferentiation of the ventral iris, even though it was shown to be up-regulated in the chordin-treated ventral irises. However, in pax-6 transfected cells six-3 was not upregulated (not shown) and this might be the reason why pax-6 was not able to induce transdifferentiation. Based on other results from our laboratories we now believe that pax-6 is rather involved in later events of lens regeneration, such as the proliferation of PECs in both the dorsal and ventral iris and control of crystallin synthesis17. The fact that RA was also necessary for the induction most likely indicates that other factor(s) regulated by RA are involved, a synergism that has been shown in other studies as well16, 18,19. Interestingly, both inhibitors of the BMP pathway and the six-3/pax-6 loop are part of a network identified during induction of eye development20. The up-regulation of six-3 and pax-6 in chordin treated iris explants (Fig. 3f) suggests that the BMP signaling is upstream of the six-3/pax-6 regulatory loop.
Previous work by us and others has shown that other important regulators of lens differentiation, such as FGFs, Sox2, MafB and members of the hedgehog pathway are expressed in both dorsal and ventral iris21,22. This goes against the commonly held belief that regulatory genes involved in lens regeneration should be dorsal-specific. However, the detailed quantitative studies reported here suggest novel regulatory events involved in the induction of lens regeneration. Collectively, our data presented here demonstrate that induction of lens regeneration can be achieved in non-competent adult tissues. This is important because ectopic lens formation has never been shown in adults and opens new avenues in the field of vertebrate lens regeneration.
METHODS
All methods not listed here can be found in Supplementary Information.
Cloning of newt six-3 and BMPR-1A partial cDNAs
BMPR-IA cloning was performed with RNA isolated from newt forelimb blastema (~ 2 weeks post-amputation) using TRI REAGENT® (Molecular Research Center, INC.) according to manufacturer’s instructions. Dorsal PECs were used to clone a partial cDNA for six-3. One microgram of RNA was used to synthesize cDNA using iScript™ cDNA Synthesis Kit (BioRad). For PCR, a portion of the DNA was used along with Taq polymerase, 200 μM dNTPs, and 800 nM primers. Primers used were as follows: BMPR-1A forward 5′-TGCTGYATTGCTGAYYTDGG, reverse 5′-GGRTCATTYGGCACCA; six-3 forward 5′-CACTACCAGGAGGCCGAGAA, reverse 5′- TCCTTGAAGCAGTGCGTCTT. DNA was purified using Qiagen MinElute™ Gel Extraction Kit. The fragment was cloned using the pGEM®-T Easy Vector Systems (Promega) and sequenced.
Immunostaining
Affinity purified polyclonal antibodies were made against six-3 and BMPR-1A peptides. The six-3 antibody was made in rabbit (New England Peptide, Inc.) and the BMPR-1A in chick (Cocalico). Newts were anesthetized and the lens was removed through a slit in the cornea. They were sacrificed at 2, 4, 8, 12, 15 and 25 days post-lentectomy. The eyeballs were enucleated and fixed in 4% formaldehyde for 4 hours, washed in 1X PBS and cryoprotected in 30% sucrose, followed by embedding in OCT (Andwin Scientific, Warner Center, CA), freezing and sectioning at 10μm. Slides with frozen serial sections were washed several times in PBS and 1% saponin (Sigma, St. Louis, MO) and incubated in 10% goat serum/PBS. Occasionally, for reduction of pigmentation, sections were bleached in 0.1% potassium permanganate for 10 minutes, followed by immersion in 0.5% oxalic acid for 5 minutes and rinses in PBS. The samples were incubated at 4°C overnight with primary antibody (anti-newt six3 diluted 1:10 in blocking solution or anti-newt BMPR diluted 1:100 in blocking solution), followed by washes in 0.3% PBST and PBS, and incubation secondary antibody for 2 hours at 37°C: goat anti-rabbit- Alexaflor 546 (Molecular Probes, Invitrogen, Eugene, OR) for six3, and rabbit anti-chicken-FITC (Sigma, St. Louis, MO) for BMPR, diluted 1:200 in 10% goat serum in PBST. The sections were again washed with PBST and PBS, and coverslipped using Vectashield (Vector labs, Burlingame, CA). Pictures were taken using confocal microscopy.
Real-Time PCR
RNA was isolated from iris tissue and pigmented epithelial cells (PECs) using TRI REAGENT® (Molecular Research Center, INC.) according to manufacturer’s instructions. The following tissues and cells were used: Intact dorsal and ventral iris, 2, 4, 8-day dorsal and ventral iris, isolated cells from dorsal and ventral iris, transfected cells with six-3/RA, six-3 alone, RA alone from the dorsal and ventral iris, explants from dorsal and ventral iris, explants treated with chordin or BMPR-1A from dorsal and ventral iris, axolotl intact and 8-day dorsal and ventral iris and chordin-treated axolotl dorsal and ventral iris. The isolated RNA was used to evaluate expression of six-3, BMPR-1A and pax-6 (along with a suitable reference gene) via Real Time PCR and RT-PCR. RT-PCR was employed to verify that the correct fragment was amplified. Appropriate negative controls were included in all sets. 0.75 micrograms of RNA was used to synthesize cDNA using iScript™ cDNA Synthesis Kit (BioRad). All Real-Time PCRs were performed using the iCycler™ (BioRad). For each Real-Time PCR reaction run in triplicate 2 microliters of cDNA, 800 nM primers, and iQ™ SYBR® Green Supermix (BioRad) were used. Primers were designed from the cloned cDNAs for six-3 and BMPR-1A and from previously published sequence for pax-6. Newt Primers – rpL27: Forward 5′- TACAACCACTTGATGCCA, reverse 5′- CAGTCTTGTATCGTTCCTCA, pax-6: Forward 5′- CTGGGCAGGTATTACGAG, reverse 5′- GTCTCTGATTTCCCAGGC, six-3: Forward 5′- CAAGAAGTTCCCGCTGC, reverse 5′- GGTAGGGGTCCTGTAGGTAC, BMPR-1A: Forward 5′- TGCTGTATTGCTGATTTAGG, reverse 5′- ATAGGTATCAAAGCAGTCCA. Axolotl Primers – RP: Forward 5′- CATCAGATCAAGCAAGCAGTA, reverse 5′- CCAATGCAGCAGTTTAGATG, pax-6: Forward 5′- GAGTGCTCCGCAACCTG, reverse 5′- ATTCGTGTTCTCGCCTCC, six-3: same as newt, BMPR-1A: Forward 5′- CAG TGC TGC ATT GCT GAT, reverse: 5′- GGC TAC TTC CCA AAT AAC C. For each Real-Time PCR the basic program was as follows: Denaturation at 95°C, annealing at 50.6°C, and extension at 72°C (40 cycles). To minimize the background caused by primer-dimer formation, an extra step was added (78°C for 6 seconds) at the end of each cycle. The readings were taken during this step. Data analysis was performed using the Pfaffl method24. The reference genes were rpL27 for the newt and RP for the axolotl. They both encode ribosomal proteins.
Supplementary Material
Acknowledgments
We are grateful to Dr. T. Hayashi for sharing his expertise with the culturing methods for PECs. We thank G. Oliver and D. Englecamp for providing the six-3 and pax-6 expression vectors respectively. We also thank members of our laboratories, Melissa Metheney, Angela Thitoff, Sarah Gainer, Melissa Tsavaris, Matt Tubb and Matt Sander for helping with histology, Dr. V. Mitashov and E. Makarev for providing information on P.Waltl six-3 sequences and Dr. E. Tanaka for providing information on axolotl BMPR-1A sequences. This work was supported by NIH grant EY10540 to P.A.T.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions M.W.G. and M.K.C. contributed equally to this work.
Author Information The authors declare no competing financial interests.
References
- 1.Colucci VL. Sulla rigenerazione parziale dell’occhio nei Tritoni-Istogenesi e sviluppo. Studio sperimentale. Mem R Acad Sci 1st Bologna Ser. 1891;51:593–629. [Google Scholar]
- 2.Wolff G. Entwicklungsphysiologische Studien. I Die regeneration der urodelenlinse. Wilhelm Roux Arch Entwickl-Mech Org. 1895;1:380–390. [Google Scholar]
- 3.Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi G. Electron microscopic studies on lens regeneration I: Mechanism of depigmentation of the iris. Embryologia. 1963;8:45–62. [Google Scholar]
- 5.Eguchi G. Electron microscopic studies on lens regeneration. II Formation and growth of lens vesicle and differentiation of lens fibers. Embryologia. 1964;8:247–287. [Google Scholar]
- 6.Yamada, T. Control mechanisms in cell-type conversion in newt lens regeneration. Monographs in Dev. Biol.13, Karger, Basel (1977). [PubMed]
- 7.Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene six-3. Mech Dev. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- 8.Altmann CR, Chow RL, Lang RA, Hemmati-Brivanlou A. Lens induction by Pax-6 in Xenopus laevis. Dev Biol. 1997;185:119–123. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- 9.DeRobertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto M, Ito M, Owaribe K. Difference between dorsal and ventral iris in lens producing potency in normal lens regeneration is maintained after dissociation and reaggregation of cells from the adult newt, Cynops pyrrhogaster. Develop Growth Differ. 1998;40:11–18. doi: 10.1046/j.1440-169x.1998.t01-5-00002.x. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Hayashi T, Kuroiwa A, Okamoto M. Lens formation by pigmented epithelial cell reaggregate from dorsal iris implanted into limb blastema in the adult newt. Develop Growth Differ. 1999;41:429–440. doi: 10.1046/j.1440-169x.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, et al. Highly efficient transfection system for functional gene analysis in adult amphibian lens regeneration. Develop Growth Differ. 2001;43:361–370. doi: 10.1046/j.1440-169x.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 13.McCaffery P, Lee M, Wagner MA, Sladek NE, Drager UC. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development. 1992;115:371–382. doi: 10.1242/dev.115.2.371. [DOI] [PubMed] [Google Scholar]
- 14.Tsonis, P.A. Limb Regeneration. Cambridge: Cambridge University Press (1996).
- 15.Tsonis PA, Trombley MT, Rowland T, Chandraratna RAS, Del Rio-Tsonis K. Role of retinoic acid in lens regeneration. Dev Dyn. 2000;219:588–593. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1082>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- 17.Madhavan, M. et al. The role of pax-6 in lens regeneration. Dev. Biol (submitted, 2005). [DOI] [PMC free article] [PubMed]
- 18.Lee SH, Fu KK, Hui JN, Richman JM. Noggin and retinoic acid transform the identity of avian facial prominences. Nature. 2001;414:909–912. doi: 10.1038/414909a. [DOI] [PubMed] [Google Scholar]
- 19.Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of pre-adipocytes. J Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuber ME, et al. Specification of the vertebrate eye by a network of eye field transcription factors. Dev. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, et al. FGF2 triggers iris-derived lens regeneration in newt eye. Mech Dev. 2004;121:519–526. doi: 10.1016/j.mod.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Tsonis PA, et al. A novel role of the hedgehog pathway in lens regeneration. Dev Biol. 2004;267:450–451. doi: 10.1016/j.ydbio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Sawada K, Agata K, Yoshiki A, Eguchi G. A set of anti-crystallin monoclonal antibodies for detecting lens specificities: beta-crystallin as a specific marker for detecting lentoidogenesis in culture of chicken lens epithelial cells. Japn J Ophthalmol. 1993;37:355–368. [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


