Abstract
Several previous studies have demonstrated sex differences in cardiovascular autonomic control in healthy young women, but little is known about the regulation of blood pressure in hypertensive elderly women, who have the greatest risk of adverse cardiovascular events. Therefore, we examined sex differences in physiological responses to upright tilt in 21 healthy (13 men and 8 women), 22 controlled hypertensive (10 men and 12 women), and 18 uncontrolled hypertensive (9 men and 9 women) elderly men and women. Of these, 19 normotensives, 18 controlled hypertensives, and 14 uncontrolled hypertensives completed 6 months of observation or pharmacological therapy for uncontrolled hypertension. All of the subjects underwent continuous monitoring of cardiac (RR) interval (ECG), finger arterial pressure (photoplethysmography), and stroke volume (transthoracic impedance) and periodic measurements of forearm blood flow (venous occlusion plethysmography) while resting supine and during a graded head-up tilt. Blood pressure and RR-interval power spectra were computed. Baroreflex gain was estimated by the cross-spectral and sequence methods. In contrast to other groups, elderly hypertensive women increased systemic vascular resistance during tilt. This response was associated with greater low-frequency systolic pressure variability, a presumed marker of sympathetic vascular control. After 6 months of successful antihypertensive therapy, women showed attenuation of the systemic vascular resistance response and a reduction in low-frequency systolic blood pressure variability to levels similar to men and normotensive controls. These results highlight the beneficial effects of antihypertensive therapy on the systemic vasculature, particularly for elderly women in whom enhanced vasoreactivity may contribute to excessive cardiovascular morbidity and mortality.
Keywords: gender, baroreflex, aging, angiotensin converting enzyme, blood pressure, sympathetic nervous system
Although premenopausal women have a lower incidence of cardiovascular disease than men, this difference narrows after menopause and is reversed in later life.1 In fact, cardiovascular disease is the leading cause of death among women in industrialized countries of the world.2,3 Data from the Framingham Study show a higher prevalence of hypertension in elderly women than men.2 For any given level of blood pressure (BP), white women have been shown to have a higher age-adjusted relative risk of coronary artery disease death than white men.4 Therefore, it is important to understand the physiological mechanisms underlying hypertension and its consequences in older women and how these respond to antihypertensive therapy.
Over the past decade, several research studies have demonstrated sex differences in cardiovascular autonomic control, but their relationship to the development of hypertension and adverse cardiovascular events in elderly women has not been well studied. These differences include lower resting muscle sympathetic nerve5 and sympathoadrenal activity,6 a greater parasympathetic influence on heart rate,7,8 and reduced cardiovagal baroreflex gain6,9 in women, all of which would seem to protect against the development of hypertension. In fact, we and others have found that women are more likely to have orthostatic hypotension compared with men.7,10 Because most previous studies have examined normal young subjects, little is known about the cardiovascular responses of hypertensive elderly women to orthostatic stress. Moreover, sex-specific responses to antihypertensive treatment are poorly understood. Therefore, we examined sex differences in physiological responses to upright tilt in healthy, controlled hypertensive and uncontrolled hypertensive elderly men and women during a baseline period and then after 6 months of observation or pharmacological BP control.
Methods
Design
The study was a prospective 3-group comparison of the effect of pharmacological BP reduction versus observation over a 6-month period on cardiovascular responses to a graded head-up tilt. This was part of a larger study of the effects of hypertension and its treatment on the cerebral circulation, which was published previously.11 Elderly subjects were assigned to 3 groups on the basis of the average of 3 BP measures during 3 screening visits, ≈1 week apart: “normotensives” (BP <140/90 mm Hg), on no BP lowering medications; “controlled hypertensives,” well-controlled hypertension (BP <140/90 mm Hg) on chronic BP-lowering medications; and “uncontrolled hypertensives,” with systolic BP >160 mm Hg with or without BP-lowering medications.
Subjects
Subjects ≥65 years of age were recruited from the Harvard Cooperative Program on Aging subject registry, through newspaper advertisements, and from elderly housing sites and physician practices. All of the potential subjects underwent a screening physical examination, laboratory profile, ECG, echocardiogram, and color Duplex carotid ultrasound. They were excluded from the study if they had clinical evidence of heart failure (by history, medication profile, x-ray report, wall motion abnormalities on echocardiogram, or physical evidence of rales, JVD, or a cardiac gallop), hemodynamically significant valvular heart disease (by echocardiogram), cerebrovascular disease or multiinfarct dementia (by history and neurological examination), Alzheimer’s disease, carotid disease (>50% stenosis by carotid ultrasound), angina, myocardial infarction, wall motion abnormalities by echocardiogram, chronic lung disease, history of smoking within the past 10 years, diabetes, Parkinson’s disease, autonomic failure, a pacemaker, severe hypertension [systolic BP (SBP) >200 and/or diastolic BP (DBP) >110 or on >2 drugs for BP control], peripheral vascular disease (claudication and absent peripheral pulses), or if they were receiving hormone replacement therapy or β-blockers.
A total of 61 subjects were studied at baseline, including 21 normotensives (13 men and 8 women), 22 controlled hypertensives (10 men and 12 women), and 18 uncontrolled hypertensives (9 men and 9 women). Of these, 19 normotensives, 18 controlled hypertensives, and 14 uncontrolled hypertensives completed 6 months of observation or pharmacological therapy for uncontrolled hypertension. The primary reasons for withdrawal from the longitudinal study were interim illness, medication changes, newly detected cardiac ectopy, or technical problems during the study. The baseline subject characteristics are shown in Table 1.
TABLE 1.
Supine Subject Characteristics
| Normotensives (1)
|
Controlled Hypertensives (2)
|
Uncontrolled Hypertensives (3)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Group P Value | All | Men | Women | All | Men | Women | All | Men | Women |
| No. | 21 | 13 | 8 | 22 | 10 | 12 | 18 | 9 | 9 | |
| Age, y | ns | 70.2±3.7 | 69.8±3.8 | 71.0±3.8 | 71.7±4.5 | 72.2±4.3 | 71.3±5.5 | 73.6±3.8 | 73.3±4.5 | 74.0±3.2 |
| SBP, mm Hg | ‡§ | 130±4 | 134±5 | 127±6 | 139±4 | 140±6 | 139±5 | 166±4 | 163±6 | 169±6 |
| DBP, mm Hg | ‡ | 64±3 | 69±4 | 58±4 | 70±3 | 72±4 | 68±4 | 78±3 | 77±4 | 78±4 |
| MAP, mm Hg | †‡ | 86±3 | 91±4 | 82±4 | 93±3 | 95±4 | 92±4 | 107±3 | 106±4 | 108±4 |
| HR, bpm | ns | 56±2 | 54±3 | 59±3 | 62±2 | 60±3 | 64±3 | 63±2 | 64±3 | 64±3 |
| SV, mL | ns | 74.0±5 | 82.0±6* | 66.4±8 | 63.0±5 | 75.0±7* | 51.0±7 | 66.0±6 | 78.0±8* | 53.0±8 |
| CI, lpm/m2 | ns | 2.7±0.2 | 2.6±0.2 | 2.8±0.3 | 2.3±0.2 | 2.5±0.3 | 2.0±0.2 | 2.4±0.2 | 2.6±0.3 | 2.2±0.3 |
| TPR | ns | 1506±173 | 1552±215 | 1490±268 | 1913±162 | 1625±239 | 2200±218 | 1943±183 | 1710±255 | 2177±256 |
| FVR | ns | 0.40±0.05 | 0.43±0.06 | 0.36±0.08 | 0.44±0.04 | 0.42±0.07 | 0.46±0.07 | 0.46±0.05 | 0.44±0.07 | 0.49±0.08 |
| BRS | ‡ | 13.21±1.33 | 12.02±1.66 | 14.41±2.06 | 8.87±1.27 | 10.64±1.84 | 7.09±7.76 | 6.93±1.41 | 6.84±1.96 | 7.02±1.96 |
| LF gain | ns | 8.99±1.35 | 9.35±1.59 | 8.63±2.12 | 7.01±1.24 | 8.84±1.76 | 5.18±1.76 | 5.75±1.40 | 7.70±2.01 | 3.80±1.88 |
FVR indicates forearm vascular resistance; TPR, total peripheral resistance; BRS, spontaneous baroreflex slope; MAP, mean arterial pressure; CI, cardiac index; LF, low frequency; ns, not significant (P>0.05 for groups).
Data are mean±SE. Statistics are age-adjusted. FVR and TPR are in mm Hg×min×L−1.
P=0.0007, men vs women;
P<0.05 1 vs 2;
P<0.05 1 vs 3;
P<0.05 2 vs 3.
The study was approved by the Hebrew SeniorLife Institutional Review Board, and all of the subjects provided written informed consent. All of the procedures followed were in accordance with institutional guidelines.
Medication Management
If uncontrolled hypertensives were taking medications that were ineffective, these were tapered over 2 weeks, while a research nurse, using an automated machine and diary at home, carefully monitored BP. These subjects were drug-free for ≥2 weeks before returning for the baseline protocol. Controlled hypertensives whose BP was well controlled on medications continued the same medication regimen throughout the study. All 3 groups of subjects underwent 2 tilt studies: 1 at baseline and 1 after 6 months of effective antihypertensive therapy or observation (normotensive group).
After the baseline tilt study, uncontrolled hypertensives were started on lisinopril and instructed in medication administration and follow-up. Subjects returned to the laboratory weekly over a 4- to 6-week period for drug titration until their BP reached its target range. Lisinopril was increased in weekly increments from 10 to 40 mg. If this did not achieve a BP <140/90 mm Hg, hydrochlorothiazide 25 to 50 mg was added. If a third medication was needed, amlodipine 5 to 10 mg was used. If lisinopril was not tolerated, an angiotensin receptor blocker was used. The aim of the study was to examine the effect of BP control rather than the effect of any 1 agent. After BP control was achieved, the 6-month treatment period began. These subjects returned monthly for BP checks, safety monitoring, symptom questionnaires, and compliance assessments. All of the subjects were seen at 3 and 6 months for BP monitoring and routine blood tests, including electrolytes, urea nitrogen, and creatinine measurements.
Experimental Protocols
For the baseline study and at the end of the 6-month treatment or observation period, subjects reported to the Hebrew SeniorLife cardiovascular research laboratory at 8:30 am, ≥2 hours after taking their medication and a light breakfast. Both the baseline and follow-up tilt study were conducted at the same time of day, under identical conditions, in a temperature-controlled room.
While lying supine, instrumentation was applied for the following measurements. Heart rate was measured from a 3-lead ECG. Beat-to-beat arterial pressure was determined noninvasively from the middle finger of the nondominant hand, using a photoplethysmographic noninvasive pressure monitor (Finapres), supported by a sling at the level of the right atrium to eliminate hydrostatic pressure effects. This technique provides values that correlate with directly measured radial artery BPs.12 Finapres measurements were initially corroborated by standard measurements of arterial pressure with an oscillometric cuff on the upper arm (Dynamap). Forearm blood flow (FBF) was determined on the other arm by venous occlusion plethysmography using a Hokanson plethysmograph and the procedure of Whitney.13 Cuffs were placed on the upper arm and wrist, and a mercury-in-silastic strain gauge was attached to the forearm. The arm was supported above the level of the right atrium to allow for adequate venous drainage. The wrist cuff was inflated to a pressure of ≈20 mm Hg above arterial pressure to exclude hand blood flow, then a minimum of 1 minute later, the upper arm cuff was inflated rapidly to a venous occlusion pressure of 40 mm Hg. The strain gauge displacement during the venous occlusion represents FBF. Forearm vascular resistance was calculated from the mean arterial pressure divided by FBF.
Transthoracic Impedance
Because of the marked sensitivity of electrical resistance to liquid content, alterations in transthoracic impedance (Zo) can be used to estimate cardiac stroke volume (SV). This noninvasive technique has been used extensively to study the hemodynamic response to orthostatic stress in subjects of all ages. Transthoracic impedance was determined during tilt with an impedance cardiograph (Sorba Medical Systems). Four electrodes were placed on the subject: 2 on the forehead and thigh generating a small current and 2 sensing electrodes at the base of the neck and the midaxillary line at the level of the xiphoid. The SV is computed from its relation to the resistivity of blood (R), the length between the inner electrodes (L), the cardiac ejection time (T), and the change in impedance with each beat of the heart (dZ/dt), according to the expression SV=R×(L2/Zo2)×T×dZ/dt.
Cardiac output (CO) was computed from the product of SV and heart rate and cardiac index by dividing CO by body surface area. Systemic vascular resistance (SVR) was determined from the mean arterial pressure divided by CO.
Carotid Distensibility and Stiffness
These were measured as described previously11,14 using Duplex Doppler ultrasonography to measure beat-to-beat carotid diameter and the Finapres to measure beat-to-beat arterial pressure. Carotid distensibility was calculated as the relative change in carotid diameter during each heartbeat, divided by pulse pressure, according to the formula: [2(Ds−Dd)/Dd]/(SBP−DBP). Stiffness was calculated as: loge(SBP/DBP)×Dd/(Ds−Dd).
Graded Tilt
After a 10-minute supine rest to reach equilibrium, subjects underwent 3 successive 10-minute tilts at angles of 20°, 40°, and 60°. During each 10-minute segment, continuous RR interval, continuous BP, average SV, and FBF were measured. All of the data were displayed and digitized in real time at 250 Hz using commercially available data acquisition software (Windaq, Dataq Instruments) on a personal computer (NEC Pentium 90 MHZ). Beat-to-beat heart rate and systolic and diastolic pressures were determined from the R wave of the ECG and the maximum and minimum of the arterial pressure waveform.
Analyses
Frequency Analysis
We used frequency analyses of continuous RR interval and BP data to examine low- and high-frequency components of the RR interval and BP variability. The amplitude of low-frequency BP oscillations (0.04 to 0.15 Hz) can be used as a measure of baroreflex-mediated sympathetic control of the vasculature, and the amplitude of high-frequency RR interval oscillations (0.15 to 0.40 Hz) can be used to assess vagal control of the heart (respiratory sinus arrhythmia).15
All of the data segments were visually inspected and edited for artifact and ectopy, and only stationary data were used for this analysis. A power spectrum analysis technique based on the Welch algorithm of averaging periodograms was used. The RR interval and systolic pressure time series were interpolated at 5 Hz to obtain equidistant time intervals and then divided into 3 equal overlapping segments. Each segment was detrended, Hanning filtered, and fast-Fourier transformed to its frequency representation squared. The periodograms were then averaged to produce the spectrum estimate.
Baroreflex Gain: Cross-Spectral Method
The beat-to-beat relation between low-frequency BP and RR interval oscillations can be used as a measure of cardiovagal baroreflex gain. This can be quantified using coherence analyses and computing the transfer function between the signals. Coherence between low-frequency BP and the RR interval was calculated from the cross-spectra and autospectra of stationary data segments using the following formula: Coherence=(cross-spectra)2/(input signal autospectrum)×(output signal autospectrum). The signals were considered coherent over the frequencies at which coherence values exceed 0.5. The transfer function was determined by dividing the cross-spectrum by the input autospectrum. The gain of the transfer function, a measure of baroreflex sensitivity, was calculated for each subject over the low-frequency range (0.04 to 0.15 Hz) where coherence exceeded 0.5.
Baroreflex Gain: Sequence Method
Baroreflex gain was computed by this method for each tilt angle from 0° to 60° using 5 minutes of continuous ECG and BP data. Three or more consecutive heartbeats in which systolic BP and the following RR interval either increased or decreased were identified, and the linear regression for each sequence was determined. The mean of regression slopes was then determined for each subject and used as an index of baroreflex gain.16,17
Statistical Analyses
The baseline supine subject characteristics were compared between groups and between men and women within groups, using a general linear model procedure on SAS software. We examined the relation between group or sex and each of the parameters while controlling for age differences. For spectral measures that were not normally distributed, log transformations were performed before statistical comparisons were made. We also examined the response to 6 months of observation or antihypertensive treatment, using a 2-way repeated-measures ANOVA (PROC MIXED) with group or sex as 1 factor and time as the repeated measure. We used a similar analysis to examine the group and sex-related responses to tilt, with each tilt angle as the repeated measure. Differences were considered statistically significant at P<0.05.
Results
Baseline Characteristics and Responses to Tilt
As shown in Table 1, subjects with uncontrolled hypertension had higher systolic BP than the other 2 groups and higher diastolic BP and mean BP than the normotensive group. Baroreflex sensitivity was lower in the uncontrolled hypertensive compared with the normotensive group. Within all of the groups, women had lower SV than men.
Table 2 shows the SBP and RR interval power spectra results for the 3 groups of subjects. High-frequency systolic BP power was higher, whereas high-frequency RR interval power was lower in the uncontrolled hypertensive compared with the normotensive group. The ratio of low/high frequency RR interval power was greater in men compared with women for all of the groups combined. Otherwise, there were no sex-related baseline differences in the study variables.
TABLE 2.
Supine Subject Power Spectral Characteristics
| Normotensives (1)
|
Controlled Hypertensives (2)
|
Uncontrolled Hypertensives (3)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Group P Value | All | Men | Women | All | Men | Women | All | Men | Women |
| N | 21 | 13 | 8 | 22 | 10 | 12 | 18 | 9 | 9 | |
| Age, y | ns | 70.2±3.7 | 69.8±3.8 | 71.0±3.8 | 71.7±4.5 | 72.2±4.3 | 71.3±5.5 | 73.6±3.8 | 73.3±4.5 | 74.0±3.2 |
| LFSBP power | ns | 17.73±5.94 | 16.79±6.14 | 18.67±7.65 | 17.40±4.71 | 15.71±6.83 | 19.09±6.51 | 30.82±5.23 | 18.11±7.27 | 43.53±7.32 |
| HFSBP power | ‡ | 3.23±1.52 | 3.66±1.88 | 2.81±2.35 | 4.04±1.45 | 2.65±2.10 | 5.43±2.00 | 9.13±1.60 | 9.24±2.23 | 9.03±2.25 |
| LFSBP/HFSBP | ns | 6.47±1.41 | 6.33±1.75 | 6.60±2.18 | 6.29±1.35 | 7.51±1.95 | 5.06±1.86 | 6.82±1.49 | 3.68±2.08 | 9.96±2.09 |
| LFRR power | ns | 1268±399 | 1474±496 | 1061±618 | 649±381 | 901±552 | 397±526 | 1067±423 | 1495±587 | 639±592 |
| HFRR power | ‡ | 643±194 | 612±241 | 675±300 | 396±185 | 626±268 | 166±256 | 460±205 | 429±286 | 492±288 |
| LFRR/HFRR | ns | 2.41±0.58 | 3.08±0.73* | 1.75±0.90 | 3.60±0.56 | 4.35±0.81* | 2.84±0.77 | 3.48±0.62 | 4.26±0.86* | 2.71±0.87 |
ns indicates not significant (P>0.05 for groups); LFSBP, SBP low-frequency power (0.04 to 0.15 Hz); HFSBP, SBP high-frequency power (0.15 to 0.5 Hz); LFSBP/ HFSBP, ratio SBP low to high frequency power (0.04 to 0.5 Hz); LFRRP, RR low-frequency power (0.04 to 0.15 Hz); HFRRP, RR high-frequency power (0.15 to 0.5 Hz); LFRRP/HFRRP, ratio RR low to high frequency power (0.04 to 0.5 Hz).
Data are mean±SE. Statistics are age-adjusted.
P=0.03, men vs women for all groups combined;
P<0.05 1 vs 2;
P<0.05 1 vs 3;
P<0.05 2 vs 3.
The cardiovascular responses to graded head-up tilt are shown in Figure 1. BP was well maintained during tilt in all 3 of the groups, but hypertensive women demonstrated a tendency to increase systolic and mean arterial pressure in contrast to men who tended to decrease. This hypertensive response in women was associated with a significantly greater SVR at all tilt angles as compared with normotensive or controlled hypertensive subjects or uncontrolled hypertensive men (Figure 2). Whereas SVR was significantly increased, forearm vascular resistance did not significantly increase. Similarly, there were no significant group or sex differences in the heart rate, SV, or cardiac index responses to tilt.
Figure 1.
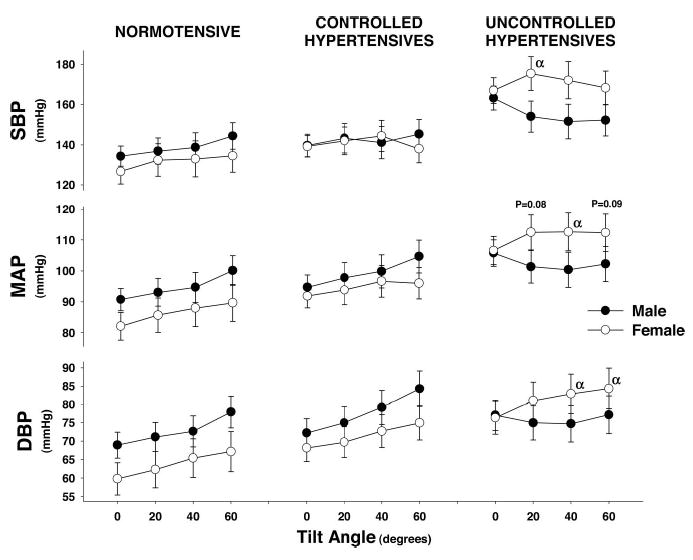
Blood pressure response of all subjects to steady-state tilt during baseline data collections. Symbols represent steady-state 3-minute mean±SE during each tilt angle. α indicates significant difference between men and women at that tilt angle (P<0.05).
Figure 2.
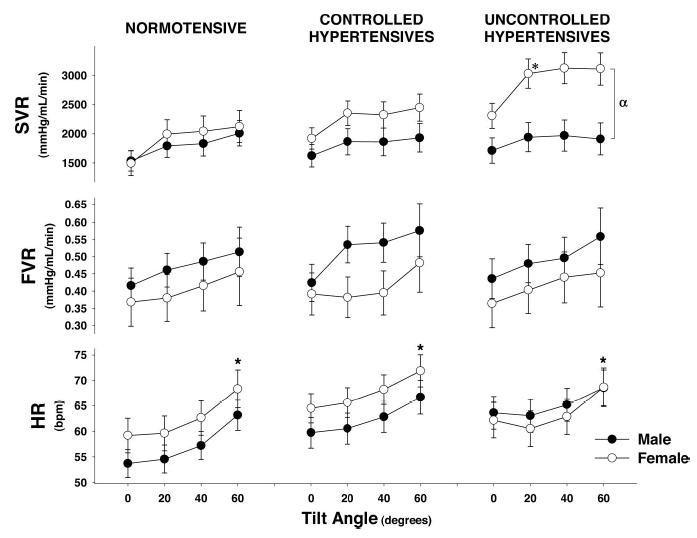
Systemic vascular resistance (SVR), forearm vascular resistance (FVR), and heart rate (HR) response of all subjects to steady-state tilt during baseline data collections. Symbols represent steady-state 3-minute mean±SE during each tilt angle. *Significantly different from supine (0°; P<0.05). *indicates a significant difference in SVR between uncontrolled hypertensive men and women at all levels of tilt (P<0.05).
Response to Antihypertensive Treatment
After 6 months of BP control, the formerly hypertensive women no longer experienced excessive increases in BP or SVR during tilt (Figure 3). Their response was the same as the men in their group and all of the subjects in the other groups. In addition, after 6 months of treatment, both men and women hypertensives demonstrated a significant increase in supine carotid artery distensibility in the supine and 60° upright positions (Figure 4). There was a trend toward supine improvement but no significant change in carotid stiffness.
Figure 3.

Response of uncontrolled hypertensives to 6 months of antihypertensive treatment for SBP, mean arterial pressure (MAP), SVR, and low-frequency SBP power (0.04 to 0.15 Hz). Symbols represent steady-state 3-minute mean±SE during each tilt angle. *Significantly different from supine (0°; P<0.05). α indicates significant difference between men and women at that tilt angle (P<0.05). Uncontrolled hypertensive men and women had significantly different SVR at all levels of tilt during baseline but not after 6 months of anti-hypertensive treatment.
Figure 4.

Response of uncontrolled hypertensives to 6 months of antihypertensive treatment on carotid distensibility and stiffness. *Significantly different from baseline (P<0.05).
Baroreflex Control of BP
There were no sex differences in baroreflex gain within any subject group by both the sequence and cross-spectral (transfer gain) methods. In all of the groups, men and women showed a decrease in baroreflex gain during tilt. Low-frequency SBP power was higher in hypertensive women than men at baseline (P=0.04). This decreased to the same level as men after 6 months of successful antihypertensive therapy (Figure 3).
Discussion
The results of this study demonstrate that elderly hypertensive women have a tendency toward an orthostatic increase in BP during graded head-up tilt that is associated with a significant increase in SVR. This increase in BP and SVR was not seen in hypertensive men. Furthermore, this response is associated with greater low-frequency systolic BP variability, a presumed marker of sympathetic control of the vasculature. Forearm vascular resistance remained unchanged, despite an increase in SVR. After 6 months of successful antihypertensive therapy, BP fell to normal ranges in both men and women and remained stable during tilt. This improvement in BP regulation was associated with attenuation of the SVR response, improved carotid distensibility, and a reduction in low-frequency systolic BP variability.
Our findings are supported by those of Laitinen et al,18 who incidentally observed that older female subjects had a greater increase in mean arterial BP during a 70° head-up tilt than older male subjects. Although the purpose of their study was to determine the effect of age on cardiovascular autonomic responses to posture change in healthy subjects, the average systolic BP of their older subjects was 155 mm Hg. Therefore their results, like ours, may be characteristic of hypertensive elderly women. In a previous study we found greater decreases in systolic BP during head-up tilt in healthy normotensive women of all ages compared with men.7 In the current study, which was limited to elderly subjects, normotensive women had similar responses to men.
We postulated that the difference in orthostatic BP and SVR responses between hypertensive men and women might be because of a sex-related difference in baroreflex gain. In previous studies of young6,9 and middle-aged19 subjects, women had lower baroreflex sensitivity than men, measured by a variety of different methods. Among middle-aged women, baroreflex sensitivity, measured by a cross-spectral method, is lower in hypertensive than normotensive subjects.20 In the current study, both hypertensive men and women had lower baroreflex sensitivity than normotensive subjects, but we were not able to detect any sex differences. This may be because of our relatively small sample size or the insensitivity of the cross-spectral and sequence methods for assessment of baroreflex control of the vasculature.21 Because these methods are better measures of the cardiovagal rather than the vascular-sympathetic baroreflex, it remains possible that sex-related differences in vascular baroreflex gain will explain our findings.
Other possible explanations for the sex-related difference in systemic vascular response to head-up tilt in hypertensive subjects include enhanced sensitivity to circulating vasoconstrictors, such as angiotensin or endothelin, and reduced sensitivity to vasodilators, such as acetylcholine or endothelial nitric oxide. Gandhi et al22 have shown that the renal vasoconstrictor response to angiotensin I and II infusion is greater in young normotensive women compared with men. However, we are unaware of previous studies reporting different vascular responses to angiotensin or other vasoconstrictors in elderly men and women with hypertension.
In support of our findings, 2 previous studies also found greater total peripheral resistance during orthostatic stress in women compared with men.23,24 In our study, there was no difference in forearm vascular resistance in hypertensive women and men, suggesting that increases in resistance took place in other vascular beds (eg, splanchnic, skin, etc). In contrast, Convertino23 reported that both SVR and forearm vascular resistance were increased in women compared with men during lower body negative pressure. However, Convertino23 studied young healthy women in whom peripheral vascular control was likely unaffected by vascular stiffening or other changes associated with hypertension and aging.
Our results are suggestive of greater vascular sympathetic activity in hypertensive women, which is consistent with the observation that aging may cause women to have more sympathetic nerve activity than men and that this may be responsible for the female predominance of hypertension in advanced age.25,26 Although this notion may seem in conflict with the higher ratio of low/high frequency RR interval power found in the men of our current study, it is important to recognize that the lower ratio in women reflects a relatively larger proportion of high-frequency RR interval variability. This finding is consistent with our previous work showing higher cardiac vagal tone in women compared with men.7
After 6 months of antihypertensive therapy with an angiotensin-converting enzyme inhibitor regimen, BP, SVR, and low-frequency SBP power fell in the hypertensive women to the same level as men. Also, carotid distensibility increased. Previous studies in both animals27 and humans28 have shown an inverse relationship between vascular sympathetic activity and arterial distensibility. Therefore, our findings may be because of a reduction in sympathetic tone in the women. It is difficult to explain why men also showed improvements in distensibility without the corresponding changes in vascular resistance and low-frequency SBP power. Unfortunately, we were unable to measure sympathetic nerve activity directly. Another possible explanation for the improvement in distensibility is that treatment with an angiotensin-converting enzyme inhibitor resulted in arterial remodeling.29
Perspectives
A heightened vascular response to sympathetic activation during orthostatic stress may contribute to the excessive cardiovascular morbidity and mortality that has been observed in elderly hypertensive women. The additional after-load placed on the left ventricle may contribute to cardiac ischemia, left ventricular hypertrophy, and, ultimately, cardiac decompensation. Notably, our data demonstrate that 6 months of successful antihypertensive therapy attenuates the vascular response, increases carotid distensibility, and reduces low-frequency BP power, presumably because of reduced sympathetic activity. Because previous work demonstrates that reduced carotid distensibility is associated with increased cardiovascular and all-cause mortality,30 increasing distensibility is likely to be of significant benefit. Our results highlight the beneficial effects of antihypertensive therapy on the systemic vasculature, particularly for elderly women in whom enhanced vasoreactivity may contribute to excessive cardiovascular morbidity and mortality.
Acknowledgments
This work was supported by the Hebrew Rehabilitation Center for Aging/Harvard Research Nursing Home Program Project Grant (P01-AG004390) from the National Institute on Aging, Bethesda, MD. Dr Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
References
- 1.Kannel WB. The Framingham Study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med. 2002;5:27–37. [PubMed] [Google Scholar]
- 2.Franklin SS. Definition and epidemiology of hypertensive cardiovascular disease in women: the size of the problem. J Hypertens. 2002;20(suppl):S3–S5. [PubMed] [Google Scholar]
- 3.Anderson RN. National Vital Statistics Report. In: National Center for Health Statistics, Centers for Disease Control and Prevention Hyattsville, MD: U.S. Department of Health and Human Services; 2002.
- 4.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 5.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 6.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 7.Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC, Lipsitz LA. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension. 1999;33:1195–1200. doi: 10.1161/01.hyp.33.5.1195. [DOI] [PubMed] [Google Scholar]
- 8.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 9.Beske SD, Alvarez GE, Ballard TP, Davy KP. Gender difference in cardiovagal baroreflex gain in humans. J Appl Physiol. 2001;91:2088–2092. doi: 10.1152/jappl.2001.91.5.2088. [DOI] [PubMed] [Google Scholar]
- 10.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- 12.Parati G, Casadei R, Groppelli A, De Rienzo M, Mancia G. Comparison of finger and intra-arterial monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–655. doi: 10.1161/01.hyp.13.6.647. [DOI] [PubMed] [Google Scholar]
- 13.Whitney RJ. The measurement of changes in human limb-volume by means of a mercury-inrubber strain gauge. J Physiol. 1949:5P–6P. [PubMed] [Google Scholar]
- 14.Mukai S, Gagnon M, Iloputaife I, Hamner JW, Lipsitz LA. Effect of systolic blood pressure and carotid stiffness on baroreflex gain in elderly subjects. J Gerontol A Biol Sci Med Sci. 2003;58:626–630. doi: 10.1093/gerona/58.7.m626. [DOI] [PubMed] [Google Scholar]
- 15.Task Force of the European Society of Cardiology and the North Am Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 16.Steptoe A, Sawada Y. Assessment of baroreceptor reflex function during mental stress and relaxation. Psychophysiology. 1989;26:140–147. doi: 10.1111/j.1469-8986.1989.tb03145.x. [DOI] [PubMed] [Google Scholar]
- 17.Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol. 1995;268:H1682–H1687. doi: 10.1152/ajpheart.1995.268.4.H1682. [DOI] [PubMed] [Google Scholar]
- 18.Laitinen T, Niskanen L, Geelen G, Lansimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol. 2004;96:2333–2340. doi: 10.1152/japplphysiol.00444.2003. [DOI] [PubMed] [Google Scholar]
- 19.Huikuri HV, Pikkujamsa SM, Airaksinen KE, Ikaheimo MJ, Rantala AO, Kauma H, Lilja M, Kesaniemi YA. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation. 1996;94:122–125. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 20.Sevre K, Lefrandt JD, Nordby G, Os I, Mulder M, Gans RO, Rostrup M, Smit AJ. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension. 2001;37:1351–1356. doi: 10.1161/01.hyp.37.6.1351. [DOI] [PubMed] [Google Scholar]
- 21.Lipman RD, Salisbury JK, Taylor JA. Spontaneous indices are inconsistent with arterial baroreflex gain. Hypertension. 2003;42:481–487. doi: 10.1161/01.HYP.0000091370.83602.E6. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension. 1998;31:90–96. doi: 10.1161/01.hyp.31.1.90. [DOI] [PubMed] [Google Scholar]
- 23.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998;275:R1909–R1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 24.Gotshall RW, Tsai PF, Frey MA. Gender-based differences in the cardiovascular response to standing. Aviat Space Environ Med. 1991;62:855–859. [PubMed] [Google Scholar]
- 25.Tank J. Does aging cause women to be more sympathetic than men? Hypertension. 2005;45:489–490. doi: 10.1161/01.HYP.0000160319.33841.ff. [DOI] [PubMed] [Google Scholar]
- 26.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 27.Dabire H, Lacolley P, Chaouche-Teyara K, Fournier B, Safar ME. Relationship between arterial distensibility and low-frequency power spectrum of blood pressure in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39:98–106. doi: 10.1097/00005344-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Giannattasio C, Failla M, Lucchina S, Zazzeron C, Scotti V, Capra A, Viscardi L, Bianchi F, Vitale G, Lanzetta M, Mancia G. Arterial stiffening influence of sympathetic nerve activity: evidence from hand transplantation in humans. Hypertension. 2005;45:608–611. doi: 10.1161/01.HYP.0000157368.09939.88. [DOI] [PubMed] [Google Scholar]
- 29.Korshunov VA, Massett MP, Carey RM, Berk BC. Role of Angiotensin-converting enzyme and neutral endopeptidase in flow-dependent remodeling. J Vasc Res. 2004;41:148–156. doi: 10.1159/000077144. [DOI] [PubMed] [Google Scholar]
- 30.London GM, Cohn JN. Prognostic application of arterial stiffness: task forces. Am J Hypertens. 2002;15:754–758. doi: 10.1016/s0895-7061(02)02966-7. [DOI] [PubMed] [Google Scholar]


