Abstract
Antimicrobial peptides (AMPs), in addition to their antibacterial properties, are also chemotactic and signalling molecules that connect the innate and adaptive immune responses. The role of AMP [α defensins, LL-37, a cathepsin G-derived peptide (CG117-136), protegrins (PG-1), polymyxin B (PMX) and LLP1] in modulating the respiratory burst response in human and murine macrophages in the presence of bacterial endotoxin [lipopolysaccharide (LPS) or lipooligosaccharide (LOS)] was investigated. AMP were found to neutralize endotoxin induction of nitric oxide and TNFα release in macrophages in a dose-dependent manner. In contrast, macrophages primed overnight with AMP and LOS or LPS significantly enhanced reactive oxygen species (ROS) release compared with cells primed with endotoxin or AMP alone, while no responses were seen in unprimed cells. This enhanced ROS release by macrophages was seen in all cell lines including those obtained from C3H/HeJ (TLR4−/−) mice. Similar effects were also seen when AMP and endotoxin were added directly with zymosan to trigger phagocytosis and the respiratory burst in unprimed RAW 264.7 and C3H/HeJ macrophages. Amplification of ROS release was also demonstrated in a cell-free system of xanthine and xanthine oxidase. Although AMP inhibited cytokine and nitric oxide induction by endotoxin in a TLR4-dependent manner, AMP and endotoxin amplified ROS release in a TLR4-independent manner possibly by exerting a prolonged catalytic effect on the ROS generating enzymes such as the NADPH-oxidase complex.
Introduction
Host antimicrobial peptides (AMPs) are ubiquitous typically cationic molecules involved in innate immune defences (Evans and Harmon, 1995; Bulet et al., 2004; Ganz, 2004). In phagocytes they are constitutively present and stored in the cytoplasmic granules and are released into the developing phagolysosome. In contrast, non-phagocytic cells reacting to stimuli (including endotoxin) can inducibly synthesize AMP. Owing to their non-oxidative killing action, AMP are thought to perform an important role in innate host defence against invading pathogens (Ganz, 1999; Zasloff, 2002). In addition to their antibacterial activities they can act as signalling and/or chemotactic molecules that connect innate and adaptive immune responses (Territo et al., 1989; van Wetering et al., 1999; Aarbiou et al., 2002; Nagaoka et al., 2002; Bowdish et al., 2004; Davidson et al., 2004).
When phagocytes encounter a pathogen, membrane perturbation and phagocytosis can occur, quickly triggering a respiratory burst and subsequent cellular activation. Formation of the phagolysosome and subsequent degranulation results in the rapid release of these molecules into the phagocytic vacuole or secretion to the extracellular fluid (Chaly et al., 2000; Reeves et al., 2002). When secreted to the extracellular fluid, AMP can neutralize endotoxin-induced cellular cytokine and nitric oxide release either by binding directly to lipopolysaccharide (LPS) or by blocking the binding of LPS to LPS-binding protein (LBP) (Hancock and Scott, 2000; Nagaoka et al., 2001). Within phagocytes, AMPs and/or cationic polymers enhance respiratory burst in leukocytes and increase reactive oxygen species (ROS) release (Ginsburg et al., 1985; Ginsburg, 1989; Ammar et al., 1998; Stein et al., 1999). ROS are likely to be important in host defence because of their direct bactericidal activity (Ogino and Awai, 1988; Stohs, 1995) or because they behave as signalling molecules that activate expression of proinflammatory cytokine genes (Baeuerle et al., 1996; Kaul and Forman, 1996).
Endotoxin shed from an invading pathogen can prime host phagocytes for enhanced cellular responses. For example, neutrophils exposed to LPS upregulate NADPH oxidase assembly by inducing flavocytochrome b558 translocation from the specific granules to the plasma membrane resulting in enhanced ROS release (DeLeo et al., 1998). Furthermore, Elssner et al. (2004) reported that LPS-primed monocytes when stimulated with LL-37 show increased IL-1β release resulting from increased caspase-1 activity via the P2X7 receptor.
The aims of this study were to investigate the interaction of AMP with endotoxins of pathogenic bacteria and to determine how these interactions might modulate proinflammatory cytokine and respiratory burst responses. As expected, AMP inhibited the ability of endotoxin to induce the production of TNFα and nitric oxide. In contrast, AMP and endotoxins were synergistic in enhancing ROS release and amplified the respiratory burst in macrophages. Enhancement of ROS release was seen when respiratory burst was triggered with either porbol myristate acetate (PMA) or zymosan and in macrophages primed with endotoxin and AMP or in unprimed macrophages stimulated directly with endotoxin and AMP.
Results
Antimicrobial peptide inhibits TNFα and nitric oxide release from endotoxin-stimulated macrophages
To determine the impact of AMP on the capacity of endotoxin to induce TNFα and nitric oxide release from macrophages, structurally diverse AMP (HNP-1, LL-37, CG117-136, LLP1, PG-1 and PMX) were used (Table 1). A synthetic negatively charged variant of LL-37 in which the positively charged amino acids arginine and lysine were replaced with aspartic acid and glutamic acid, respectively, was used as control to determine the role of peptide cationicity in AMP biologic activity. THP-1 cells or U937 cells were used for TNFα release and RAW 264.7 macrophages for nitric oxide induction overnight. AMP were not toxic to eukaryotic cells as indicated by results from cellular proliferation and viability assays (see Experimental Procedures). When THP-1 cells seeded at 0.75 × 106 cells ml−1 and incubated for 5 days with increasing doses (2, 5, 10 or 20 μg 10−6 cells) of LL-37, CG117-136, LLP1 or PMX, cellular viability was greater than 95% at all AMP concentrations used. Cellular proliferation was not significantly different between THP-1 cells incubated with 2 μg 10−6 cells and 20 μg 10−6 cells of AMPs (1.54 × 106 cells ml−1 and 1.53 × 106 cells ml−1, respectively) which indicated that AMP were not toxic to THP-1 cells.
Table 1.
Cationic peptides used in this study.
| Cationic peptide | Source | Structure | Notes | Reference |
|---|---|---|---|---|
| Defensins α | Neutrophils, monocytes, mast cells | β-Sheet | HNP-1 Neutrophil-derived >95% pure | Ganz (1994) |
| Cathelicidin-derived peptide LL-37 | Neutrophils, monocytes, epithelial, NK, B and T cells | α-Helical | CAP18 cleaved by elastase to release C-terminal antimicrobial domain LL-37. Synthetic peptide | Zanetti (2004) |
| CG117-136 | Neutrophils, phagocytes | α-Helical | Synthetic peptide consisting of residues 117-136 of cathepsin G | Shafer et al. (1996) |
| Protegrins PG-1 | Porcine leukocytes | β-Sheet | Broad-spectrum antimicrobial peptide. Synthetic peptide | Yasin et al. (2004) |
| Lentivirus lytic peptide LLP1 | Lentivirus envelope proteins | Helical | Lytic peptide engineered to have all positive charges on one side of the helix. Synthetic peptide | Tencza et al. (1999) |
| Polymysin B sulphate (PMX) | Bacterial derived cyclic peptide | Cyclic | PMX binds LPS electrostatically | Warren (1982) |
LL-37, LLP1, PMX, CG117-136, PG-1 and HNP-1 neutralized the effect of the Neisseria meningitidis endotoxin (lipooligosaccharide – LOS) as shown by a significantly decreased (P ≤ 0.005) TNFα release from THP-1 cells (Fig. 1A) and nitric oxide release from RAW 264.7 cells (Fig. 1B). PG-1, LLP1, LL-37 and PMX neutralized endotoxin more efficiently than CG117-136 or HNP-1 (Table 2). The cationic AMP were also shown to neutralize the effect of endotoxin in a dose-dependent manner (data not shown). LL-37 at concentrations of 1, 2 and 4 μg ml−1 decreased TNFα induction from THP-1 cells stimulated with 1 pmole ml−1 of N. meningitidis LOS by 36%, 53% and 74% respectively. A dose-dependent reduction of 22%, 38% and 61% of nitric oxide induction from RAW 264.7 cells induced with N. meningitidis LOS in the presence of increasing doses (1, 2 and 4 μg ml−1, respectively) of LL-37 was also found. Similar results were seen with the other cationic AMPs used in this study (data not shown). In contrast, a negatively charged LL-37 control peptide failed to neutralize endotoxin and did not inhibit TNFα or nitric oxide release (Fig. 1A and B); this negatively charged LL-37 peptide variant failed to kill Neisseria gonorrhoeae strain FA19 compared with the normal cationic LL-37 peptide (minimum inhibitory concentration values were > 200 μg ml−1 for the anionic LL-37 variant and 3.12 μg ml−1 for LL-37). Thus, the cationic charge of AMP may neutralize the ability of N. meningitidis LOS to activate macrophages. Although the levels of TNFα and nitrite induced were different, similar reductions were seen in confirmatory experiments with U937 cells and Salmonella typhimurium, Salmonella minnesota and Escherichia coli 55:B5 LPS and AMP (data not shown).
Fig. 1.
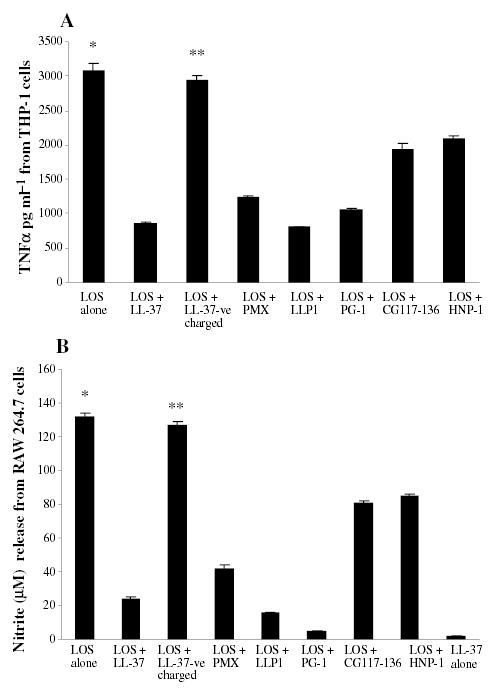
Inhibition by AMP of TNFα and nitric oxide release by endotoxins.
A. THP-1 cells (106 cell ml−1) were stimulated with 0.56 pmole ml−1 (~1 ng ml−1) of N. meningitidis LOS in presence or absence of 2 μg ml−1 of AMPs, LL-37, LLP1, PMX, CG117-136, PG-1 or HNP-1 overnight. TNFα release was measured by ELISA. Each bar represents the mean of quadruplicate measurements of TNFα from a representative experiment and error bars represent the ± SD from the mean. B. RAW 264.7 macrophages (106 cell ml−1) were stimulated with 0.56 pmole ml−1 meningococcal LOS in presence or absence of AMP (see above) with nitric oxide release measured by the Greiss method. LL-37-ve is a negatively charged peptide and was used as control. P-values calculated for AMP + LOS compared with (Student t-test) to LOS alone* or to LOS + LL-37-ve charge** and were < 0.001. The experiment is representative of at least three other experiments.
Table 2.
TNFα, nitric oxide and ROS release with cationic peptides and endotoxin.
| ROS release fold increasea |
||||
|---|---|---|---|---|
| TNFα release ng ml−1 (% of inhibition) vs. LOS alone | Nitric oxide release μM (% of inhibition) vs. LOS alone | Macrophages | Cell-free system | |
| LOS alone | 3.06 (0%) | 132 (0%) | 3.5 | 2.2 |
| LOS + LL-37 | 0.85 (73%) | 23.7 (82%) | 9.6 | 6.2 |
| LOS + LL-37-veb | 2.94 (4%) | 126.7 (4%) | 3.5 | 2.2 |
| LOS + PMX | 1.24 (60%) | 41.9 (68.3%) | 8.0 | 6.6 |
| LOS + LLP1 | 0.81 (74%) | 15.7 (88%) | 6.0 | 5.5 |
| LOS + PG-1 | 1.06 (66%) | 4.89 (96.3%) | 5.2 | 5.1 |
| LOS + CG | 1.9 (38%) | 80.6 (39%) | 11 | 7.2 |
| LOS + HNP-1 | 2.0 (35%) | 85.2 (36%) | 7.3 | 6.4 |
| AMP alonec | ND | ND | 1 | 2 |
ROS fold increase above the baseline ROS release from macrophages triggered for oxidative burst in the absence of LOS and AMP, or the baseline ROS release from the cell free system of xanthine-xanthine oxidase in the absence of LOS or AMP.
LL-37-ve is the negatively charged variant of LL-37 used as control.
AMP alone refers to any AMP listed above.
ND, not detected.
Antimicrobial peptide and endotoxin synergistically amplify respiratory burst
Although AMP-endotoxin interactions inhibited TNFα and nitric oxide release, the molecules together enhanced the respiratory burst response mounted by macrophages. In this respect, we observed increased ROS release as a result of the presence of endotoxin and AMP in human monocytic cell lines. Thus, when THP-1 cells were primed with N. meningitidis LOS (5.6 pmole ml−1) and LL-37 or CG117-136 they released significantly more ROS during the oxidative burst than THP-1 cells primed with N. men-ingitidis LOS or AMP alone (Fig. 2A). Amplification of ROS release by AMP and endotoxin was dose-dependent [i.e. cells primed with 5.6 pmole ml−1 LOS and 5 μg 10−6 cells of AMP released more ROS compared with cells primed with 2 or 1 μg 1−06 AMP (data not shown)]. The synergistic effect of endotoxin and AMP in amplifying ROS release was seen whether the respiratory burst was triggered with a soluble PMA stimulus or by phagocytosis of opsonized zymosan. In contrast, the negatively charged LL-37 control peptide and endotoxin failed to amplify ROS release (Fig. 2A). Confirmatory results were obtained when U937 and MM6 monocytes primed with N. meningitidis LOS (5.6 pmole ml−1) and 2 μg ml−1 of polymyxin B or other AMPs (data not shown).
Fig. 2.
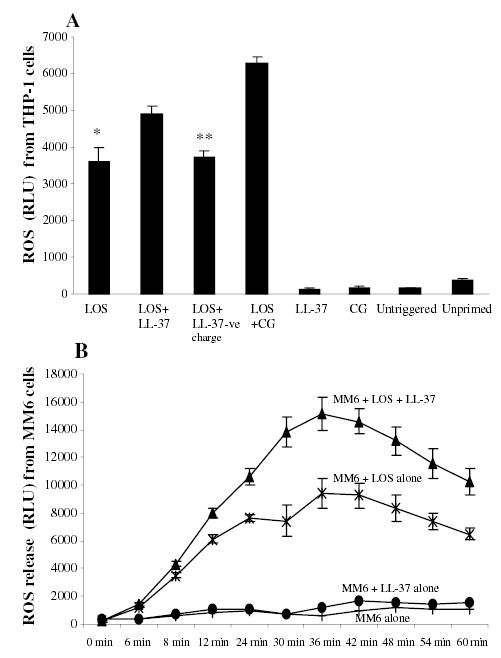
AMP and endotoxin enhance respiratory burst response in macrophages.
A. THP-1 cells primed overnight with N. meningitidis LOS (5.6 pmole ml−1) in presence or absence of LL-37 or CG117-136 (CG) at 2 μg 10−6 cells final concentration. LL-37-ve is a negatively charged peptide used as control. The respiratory burst was triggered with 50 μl of PMA (1 μM) and ROS release was detected with chemiluminescence probe lucigenin (25 μl ml−1 of cells from 1.0 mM stock solution). B. MonoMac6 (MM6) cells 2 × 106 ml−1 were primed with 5.6 pmole ml−1 of N. meningitidis LOS in presence or absence of LL-37 (2 μg 10−6 cells). Other controls were cells primed with LL-37 only and unprimed cells incubated in the same conditions but without LOS. Each bar represents the mean ± SD of quadruplicate readings at the peak of respiratory burst and each experiment is representative of three other experiments. P-values calculated for AMP + LOS compared with (Student t-test) to LOS alone* or to LOS + LL-37-ve charge** and were < 0.001.
When the respiratory burst was triggered with the soluble stimulus PMA in MM6 monocytes primed overnight with N. meningitidis (5.6 pmole ml−1) LOS and LL-37 (2 μg 10−6 cells), a significant (P < 0.001) increase in ROS release compared with cells primed with LOS alone (Fig. 2B) was observed. No significant difference in ROS release was observed between cells primed with LOS alone or LOS and the negatively charged LL-37 control peptide at all measured time points during the respiratory burst (data not shown). Unprimed MM6 cells or those primed with LL-37 alone did not release significant amounts of ROS (Fig. 2B). Confirmatory results were seen when THP-1 cells and other AMPs were used (Table 2) and when the respiratory burst was triggered by phagocytosis of opsonized zymosan (data not shown). Also when other endotoxins (i.e. LPS from S. minnesota, S. typhimurium and E. coli 55:B5) were used to prime THP-1 cells or MM6 cells enhancement was seen with cationic AMP but not with the anionic LL-37 control peptide. Taken together, these data suggested a synergistic interaction between AMP and endotoxins in priming macrophages for enhanced respiratory burst response in contrast to their inhibitory effect on TNFα and nitric oxide release.
Enhanced ROS release by endotoxin in the presence of AMP does not require TLR4
The priming of macrophages with endotoxin required LBP, CD14 and TLR4 (Zughaier et al., 2004), but the triggering of the respiratory burst was found to be a TLR4-independent event. THP-1 cells blocked with anti-TLR4 or anti-CD14 monoclonal antibodies (5 μg 10−6 cells) prior to priming with N. meningitidis LOS released significantly less ROS when the oxidative burst was triggered with the soluble stimulus PMA (Fig. 3). Furthermore, THP-1 cells primed with endotoxin in serum free conditions (i.e. without soluble CD14 or LBP) released significantly less ROS compared with cells primed in 10% (v/v) fetal bovine serum (FBS). The addition of recombinant LBP (20 ng 10−6 cells) to serum free conditions restored endotoxin priming and ROS release (data not shown). However, when the respiratory burst of functionally TLR4-deficient but NADPH oxidase-sufficient macrophages (C3H/HeJ) was triggered with opsonized zymosan or opsonized LOS-coated polystyrene beads (thus without priming), in presence of the cationic peptide LL-37 (2 μg ml−1) and N. meningitidis LOS, a significant enhancement of ROS release was observed (Fig. 4). TLR4-deficient macrophages primed with endotoxin alone or stimulated with either LOS-coated polystyrene beads alone or LL-37 alone did not show significant enhancement of ROS release. Similar results were seen when 23ScCr (TLR4−/−) murine cells and when other AMP (LLP1, PMX or HNP-1) were used (data not shown). These data suggested that phagocytosis and ROS amplification were TLR4-independent events.
Fig. 3.
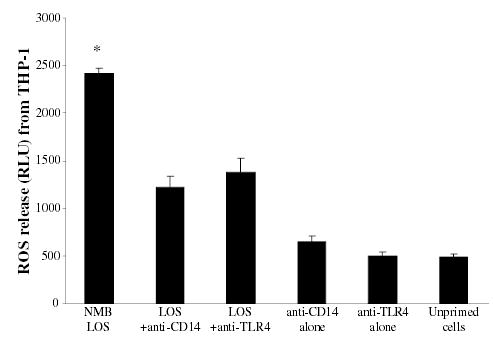
The priming of macrophages with endotoxin for respiratory burst was CD14 and TLR4 mediated. THP-1 cells (2 × 106 cell ml−1) inhibited with 5 μg ml−1 of anti-CD14 or anti-TLR4 monoclonal antibodies prior to priming overnight with N. meningitidis LOS (strain NMB) at 5.6 pmole ml−1 (~10 ng ml−1) of LOS. Unblocked THP-1 cells primed with LOS, unprimed THP-1 cells or cells blocked with anti-CD14 or anti-TLR4 alone were controls. Respiratory burst was triggered with PMA and ROS release was detected with lucigenin. Each bar represents quadruplicate readings at the peak of respiratory burst. *P-values calculated in reference to LOS-primed unblocked THP-1 cells were < 0.001. The experiment is representative of two other experiments.
Fig. 4.
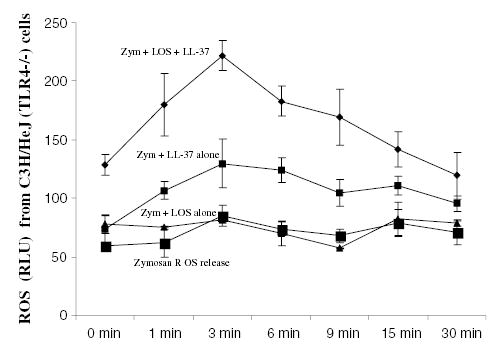
AMP enhancement of endotoxin induced respiratory burst was TLR4-independent. Respiratory burst was triggered in unprimed C3H/HeJ (TLR4-deficient but NADPH oxidase-sufficient) macrophages with 50 μl of opsonized zymosan (500 μg ml−1) in presence or absence of cationic peptide LL-37 (2 μg ml−1) and N. meningitidis LOS (1 pmole ml−1). ROS release was detected with lucigenin and monitored for 30 min. Each point was the mean of quadruplicate wells reading. P-values calculated for LL-37 + LOS compared with (Student t-test) to LOS alone or LL-37 alone were < 0.001. This experiment is representative of three other experiments.
Enhanced ROS release by AMP and endotoxin is exerted through the ROS generating enzymes
The involvement of the ROS generating enzymes in enhanced ROS release by AMP and endotoxin was investigated by the following methods.
Phagocytosis triggered respiratory burst in macrophages
The respiratory burst in unprimed RAW 264.7 macrophages triggered by opsonized zymosan showed that AMP (LLP1, PMX, HNP-1) and N. meningitidis LOS added at the start of the respiratory burst significantly enhanced ROS release compared with macrophages treated with LOS or AMP alone (data not shown). To confirm this observation, the respiratory burst in RAW 264.7 macrophages was triggered by phagocytosis of polystrene beads coated with N. meningitidis LOS and opsonized with 1% (v/v) normal human sera (NHS). ROS release from RAW 264.7 macrophages triggered with LOS-coated polystyrene beads was significantly enhanced, in a dose-dependent manner, in presence of PMX. The peak of ROS release was 1000 ± 100 RLU and 2800 ± 250 RLU in presence of 2 μg or 4 μg of PMX, respectively, which represented an approximately threefold increase over that observed in presence of 2 μg of PMX. Polystyrene beads (uncoated with LOS) added with PMX did not result in significant ROS release (peak of ROS release ~250 ± 30 RLU). These data suggest that the synergistic effect of AMP and endotoxin was a result of a direct effect exerted on the ROS generating enzymes mainly the NADPH oxidase complex.
Xanthine-xanthine oxidase cell free system
In order to further determine whether the enhanced ROS release observed in macrophages with endotoxin and AMP influ-enced the catalytic activity of ROS generating enzymes, the cell free xanthine-xanthine oxidase system was used as a model. N. meningitidis LOS and PMX significantly amplified ROS release compared with the baseline level of xanthine-xanthine oxidase ROS release (Fig. 5). LOS alone or PMX alone added to the reaction were poor inducers of ROS compared with AMP and LOS together. The addition of the ROS scavenger superoxide dismutase (SOD) decreased the synergistic effect of cationic peptides and endotoxin in enhancing ROS release from xanthine-xanthine oxidase system. Similar results were obtained with LLP1, HNP-1, LL-37 (but not the negatively charged variant), CG117-136 (Table 2) and when other endotoxins were used (data not shown). These results further indicated that the endotoxin-AMP complexes enhanced ROS release probably by exerting a catalytic effect on the ROS generating enzymes.
Fig. 5.
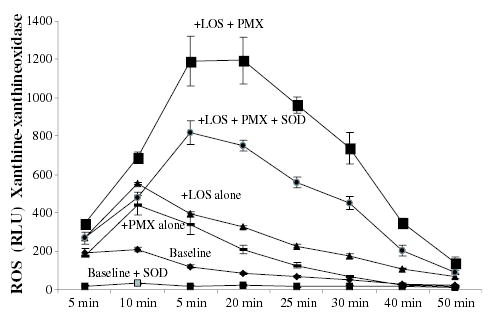
Enhancement by LOS and AMP of ROS release in a cell free system of xanthine-xanthine oxidase. Superoxide anions were generated by the xanthine-xanthine oxidase reaction (the baseline reaction) meningococcal endotoxin (LOS, 1 pmole ml−1) and polymyxin B (PMX) cationic peptide (2 μg ml−1) were added together or alone at the start of the reaction. SOD (300 unit per well) was added into control wells. Chemiluminescence was monitored over 50 min. P-values calculated for PMX + LOS compared with (Student t-test) to LOS alone or PMX alone were < 0.0001. This experiment is representative of four other experiments.
Inhibitable cytochrome c reduction
To confirm enhancement of ROS release by AMP and endotoxin, an inhibitable cytochrome c reduction assay was employed as an alternative approach for the chemiluminescent detection method with lucigenin or luminol (Han et al., 1998). High concentrations of lucigenin have been proposed to effect ROS generation (Heiser et al., 1998; Liochev and Fridovich, 1998). THP-1 cells were primed with 5.6 pmole ml−1 of N. meningitidis LOS overnight in presence or absence of AMP (2 μg ml−1). The respiratory burst was triggered with PMA in presence of 90 μg ml−1 of cytochrome c in all samples and SOD in parallel controls. The results showed that THP-1 cells primed with N. meningitidis LOS and AMP LL-37, LLP1, PMX, CG117-136, PG-1 or HNP-1 released significantly more ROS as reflected by approximately twofold change in cytochrome c reduction OD measured at 550 nm (Fig. 6). In contrast, the negatively charged LL-37 control peptide again failed to enhance ROS release (Fig. 6). The data confirmed a synergistic interaction between endotoxin and cationic peptides in amplifying ROS release from macrophages.
Fig. 6.
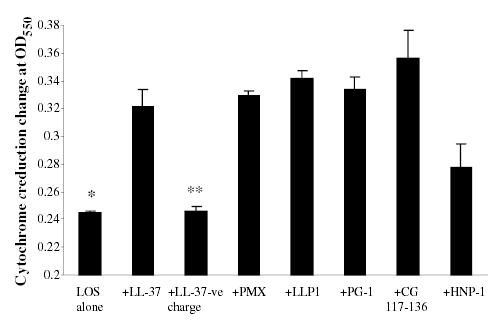
Cytochrome c reduction by ROS was enhanced by AMP and endotoxin. THP-1 cells primed with 5.6 pmole ml−1 of N. meningitidis LOS in presence of 2 μg ml−1 of cationic peptides LL-37, LLP1, PMX, CG117-136, PG-1 and HNP-1 overnight. Respiratory burst was triggered with 100 ng ml−1 PMA in presence of 90 μg ml−1 of freshly dissolved cytochrome c with or without 250 units of SOD. LL-37-ve is negatively charged peptide used as control. Cytochrome c reduction by the generated ROS was recorded as the absorbance of reduced cytochrome c and the change in OD at 550 nm. Each bar represents the mean of triplicate readings after 10 min of incubation. This experiment is representative of four different experiments. P-values calculated for AMP + LOS compared with (Student t-test) to LOS alone* or to LOS + LL-37-ve charge** and were < 0.001.
Discussion
Through their ability to kill invading pathogens, AMP are thought to be important components of the innate host defence against infections (Ganz, 1999; Zasloff, 2002). In addition, several lines of evidence have shown that AMP are signalling and chemotactic molecules that connect the innate and adaptive immune responses (Baeuerle et al., 1996; Kaul and Forman, 1996; Tang et al., 2002; Bowdish et al., 2004; Nemeth et al., 2004). For example, α defensins enhance phagocytosis by macrophages, induce degranulation of mast cells and induce bronchial epithelial cells to release IL-8 that is chemotactic and leads to neutrophil influx (van Wetering et al., 1997; Tomita and Nagase, 2001). Thus, α defensins promote the recruitment and accumulation of neutrophils to the site of inflammation. In addition, β defensin-2 acts directly on immature DC as an endogenous ligand for TLR4, inducing upregulation of costimulatory molecules and dendritic cell maturation (Biragyn et al., 2002); and cathelicidins are multifunctional cationic peptides that play an important role in regulating host defences (Zanetti, 2004). In particular, the sole human cathelicidin LL-37 was reported to induce activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes and bronchial epithelial cells but not in B or T lymphocytes (Bowdish et al., 2004).
The biologic consequences of AMP and bacterial endotoxin interaction on macrophage cytokine and respiratory burst responses were investigated using highly purified endotoxins and synthetic AMP. These highly purified endotoxins were protein, phospholipid, DNA and peptidoglycan free, and activated macrophages via TLR4 (Zughaier et al., 2004). AMP neutralized in a dose-dependent manner the well-recognized TLR4-dependent induction of TNFα or nitric oxide by endotoxin. However, cationic AMP and endotoxin were found to synergistically enhance the respiratory burst and ROS release by macrophages. This synergistic effect was TLR4-independent and was seen in macrophages primed with endotoxin and AMP as well as in unprimed cells when endotoxin and AMP were added at the start of the respiratory burst. The data suggest that the synergistic effect of AMP and endotoxin on ROS release is a result of a catalytic effect exerted on the ROS releasing enzymes such as the NADPH oxidases (Fig. 7). In support of this model, Ginsburg et al. (Ginsburg, 1989) demonstrated that poly l-histidines, poly l -arginine, poly l -lysine (all cationic peptides) are potent stimulators of superoxide generation in human blood leukocytes. Ginsburg et al. (1993) also showed the synergistic effect among hydrogen peroxide, cationic substances, membrane-damaging agents and proteinases in killing of endothelial cells and the release of arachidonic acid. Furthermore, stimulation of ROS free radicals formation by millimolar amounts of aminoglycoside antibiotics has been demonstrated in neutrophils as well as cell-free assays detected by lucigenin or luminol chemiluminescence (Sha and Schacht, 1999). Moreover, AMP such as the frog skin AMP dermaseptin, is reported to stimulate microbicidal activity of neutrophils by enhancing the production of ROS and exocytosis (the release of myeloperoxidase) (Ammar et al., 1998).
Fig. 7.
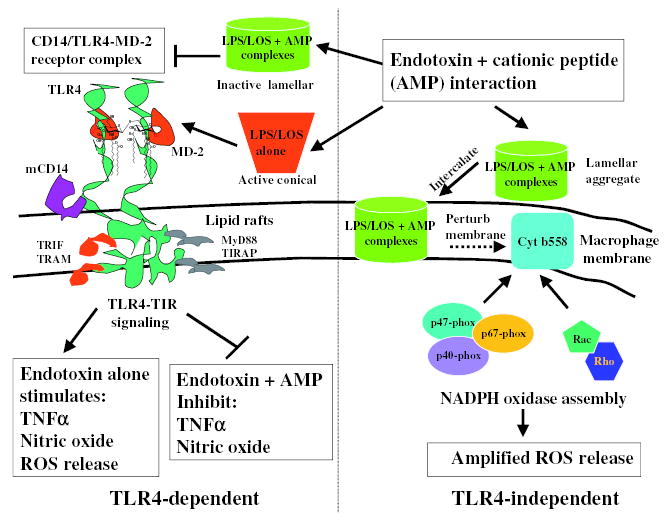
Schematic presentation of a biological model for endotoxin and cationic antimicrobial peptide interactions. When endotoxin interacts with cationic AMP, lamellar (cylinder shaped) aggregate structures are formed that fail to trigger signalling via TLR4 and thus inhibit TNFα and nitric oxide release. But, endotoxin and AMP aggregates intercalate into the macrophage phospholipid membrane, cause membrane perturbation and facilitate NADPH oxidase assembly thus enhancing ROS release in a TLR4-independent manner. The conical supramolecular aggregate structures of active endotoxin stimulate macrophages via the TLR4 receptor complex resulting in TNFα and nitric oxide release and prime macrophages for ROS release.
The cationic nature of AMP was found to be critical for interaction with endotoxin, both inhibition of cytokine release as well as ROS amplification. Endotoxin is an anionic molecule that binds to cationic peptides by electrostatic and hydrophobic interactions (Kellogg et al., 2001; Andra et al., 2005). The synthetic negatively charged LL-37 control peptide, in which the positively charged amino acids lysine and arginine were replaced with the negatively charged glutamic and aspartic acids, respectively, but retained an identical length and hydrophobicity to the positively charged LL-37, failed to neutralize endotoxin induction of TNFα and nitric oxide and did not amplify ROS release from macrophages. It is likely that the anionic characteristic of the LL-37 variant abrogated its ability to bind endotoxin. In agreement, Dokka et al. reported that ROS mediated pulmonary toxicity in mice induced with the multivalent cationic liposome LipofectAMINE was more pronounced compared with a monovalent cationic liposome DOTAP, whereas neutral and negative charged liposomes did not exhibit lung toxicity (Dokka et al., 2000).
The mechanism by which AMP and endotoxin amplified macrophage respiratory burst is intriguing. Cationic AMP neutralize endotoxin’s TLR4-dependent induction of cellular cytokine and nitric oxide release. This is accomplished either by binding directly to endotoxin or by blocking the binding of endotoxin to LBP thus attenuating or neutralizing endotoxin’s inflammatory effects (Larrick et al., 1995; Scott et al., 2000; Nagaoka et al., 2001). Physico-chemical analysis of endotoxin indicates that lipid A conformation and supramolecular aggregate structure determine TLR4-dependent biological activity, i.e. ‘cubic shaped’ aggregates have biological activity whereas ‘lamellar structure’ aggregates are inactive (Seydel et al., 2003; Mueller et al., 2004) (Fig. 7). Recently, endotoxin-neutralizing protein (ENP) of the horseshoe crab, which is one of the most potent neutralizers of endotoxin, was shown to bind endotoxin from S. minnesota and form inactive lamellar aggregate structures (Andra et al., 2004). The lamellar aggregates of endotoxin and ENP are unable to trigger signal transduction via TLR4 and thus inhibit TNFα, but these aggregates are able to intercalate into phosphilipid liposomes representing cellular membranes.
Although priming of macrophages with endotoxin for respiratory burst or cytokine release was CD14- and TLR4-mediated (Fig. 3), our results suggest that enhanced ROS release by AMP and endotoxin is a TLR4-independent event (Fig. 4). C3H/HeJ and 23ScCr cells are TLR4-deficient, do not respond to endotoxin but are NADPH oxidase-sufficient, thus capable of both phagocytosis and mounting a respiratory burst. Opsonized zymosan alone can trigger phagocytosis and ROS release in these cells and this ROS production was increased in the presence of LOS and AMP. The effect was more pronounced in primed TLR4-sufficient cells that also respond to LPS via TLR4. While cationic peptides could directly enhance phagocytosis and ROS release by opsonization (Iovine et al., 1997), ROS amplification by endotoxin and AMP was also seen in all cell lines when the respiratory burst was triggered with the soluble stimulus PMA, where opsonization would not play a role.
Phagocytosis triggers instant respiratory burst via NADPH oxidase complex activation and results in rapid ROS release. Priming with endotoxin appears to enhance a catalytic effect on the ROS generating enzymes by redistribution of components of the NADPH oxidase complex, shuttling them into the membrane and facilitating the complex assembly (DeLeo et al., 1999) thus promoting the respiratory burst and ROS release (Nisimoto et al., 1995; Reeves et al., 2002). Our data suggest that AMP and endotoxin further enhance these events. Reeves et al. also demonstrated the importance of the interactions of AMP and ROS. Mice deficient in neutrophil-granule cationic proteases (e.g. cathepsin G) but which are NADPH oxidase sufficient fail to kill invading staphylococci or Candida. In wild-type mice killing is mediated through ROS and activation of proteases by K+ flux that lead to cationic peptide release (Reeves et al., 2002). Released cationic peptides could interact with pathogens decorated by anionic molecules such as LPS (Gram-negative bacteria) or lipoteichoic acid (Gram-positive bacteria) to enhance ROS generation and facilitate killing of the invading pathogen.
Other sources of ROS such as mitochondria and enzymes also may contribute to ROS release. As an example, Cytochrome c is a small water-soluble mitochondrial haemoprotein that transfers electrons from cytochrome c reductase to cytochrome c oxidase and ROS reduce cytochrome c (Ames et al., 1995). ROS are also produced as a by-product of xanthine oxidase enzymatic activity (Canas, 1999). The direct catalytic effect of AMP and endotoxin on oxidase enzymes responsible for ROS generation was confirmed in our study using a cell free system of xanthine and xanthine oxidase. The synergistic effect of AMP and endotoxin on amplifying ROS generation from the xanthine- xanthine oxidase system was abrogated by SOD, which suggests that AMP and endotoxin complexes prolonged the catalytic activity of such enzymes by increasing the half life. In support of the enhanced catalytic effect of AMP and endotoxin, unprimed RAW 264.7 cells that phagocytosed opsonized zymosan induced significantly higher ROS release in presence of cationic peptide and endotoxin.
Other studies suggest that AMP and endotoxin enhance respiratory burst responses. The major basic protein (MBP) in eosinophil granules activated neutrophils and amplified ROS release as well as lysosome release, in a dose and Ca+2-dependent manner (Moy et al., 1990). Other compounds, such as glucocorticoids inhibit TNFα and IL-8 release from Chlamydia-primed THP-1 cells while enhancing the magnitude of the respiratory burst (Mouithys-Mickalad et al., 2001; 2004). The plant AMP known as thionins enhance E. coli-stimulated phagocytosis and respiratory burst where the poly cationic structure of intact thionins seemed to be crucial (Stein et al., 1999).
Other biologic effects of the interactions of AMP and endotoxin have been described. Gorter et al. (2003) found that α defensins enhance bacterial adherence to host epithelial cells possibly via binding the lipooligosaccarides of Haemophilus influenzae and N. meningitidis. Furthermore, arginine-rich cationic peptides have been found to increase IL-8 production in LPS-stimulated human whole blood mainly from monocytes and this synergistic effect was CD14-dependent (Bosshart and Heinzelmann, 2002).
Although an important host defence mechanism, excess release of ROS can also lead to cellular injury, host damage and can contribute to the sepsis syndrome (Del Maestro et al., 1980; Simon et al., 1981; Stohs, 1995; Touyz, 2003). For example, pulmonary or systemic infections are associated with significantly increased concentrations of LL-37 and defensin in tracheal aspirates (Schaller-Bals et al., 2002). N. meningitidis is associated with rapid and fatal sepsis. N. meningitidis endotoxin levels in circulation may be very high and are correlated with the severity of meningococcal sepsis (Brandtzaeg et al., 1992). The results of this study suggest that N. meningitidis endotoxin and other endotoxins interact with cationic AMP released upon phagocytosis to amplify ROS release. ROS molecules can further activate the expression of proinflammatory genes such as TNFα, IL-1β and IL-6 (Baeuerle et al., 1996; Kaul and Forman, 1996). In addition, released AMP induce the release of chemotactic chemokines such as IL-8 that recruits neutrophils. Taken together, the synergistic effect of endotoxin and cationic peptides in enhancing ROS release are probably designed to enhance direct killing of the invading microbial pathogen and enhance the innate and adaptive immune response, but if not localized this synergy may contribute to a widespread intravascular inflammatory response and sepsis.
Experimental procedures
Reagents
RPMI 1640 medium, Dulbecco’s Eagle medium, FBS, penicillin/ streptomycin, sodium pyruvate and non-essential amino acids were obtained from Cellgro Mediatech (Herndon, VA). PMA was from GibcoBRL (Grand Island, NY). TNFα, IL-1β and IL-8 ELISA kits and recombinant human LBP were from R&D Systems (Minneapolis, MN). Polystyrene latex beads, zymosan, endotoxin free albumin and lucigenin were from Sigma (St Louis, MO). RAW 264.7 and THP-1 cell lines were obtained from ATCC (Manassas, Virginia). MM6 cell line was kindly provided by Dr Geert-Jan Boon (The Complex Carbohydrate Research Center, University of Georgia, Athens, GA), the U937 cell line was kindly provided by Dr Yusof Abu Kwaik (University of Kentucky School of Medicine, Lexington, KY) and the C3H/HeJ (TLR4−/−) cell line was kindly provided by Dr Bruce Beutler (Scripps Research Institute, La Jolla, CA).
Synthetic cationic peptides LL-37, CG 117–136, PG-1 and negatively charged LL-37 control peptide were synthesized and purified at the Microchemical facility of Emory University as previously described (Shafer et al., 1998). The negatively charged (anionic peptide) LL-37 control peptide was synthesized by replacing the positively charged amino acids lysine and arginine with the negatively charged glutamic and aspartic acids respectively. An engineered analogue of LLP1 (LSA5) was the kind gift of Dr Tim Mietzner (University of Pittsburgh School of Medicine, Philadelphia, PA). Purified polymyxin B and α defensin (HNP-1) were purchased from Sigma Chemical (St Louis, MO).
LOS purification and quantification
Endotoxin from the serogroup B N. meningitidis strain NMB (encapsulated, L2 immunotype) was initially extracted from whole meningococci by the phenol-water method (Kahler et al., 1996). S. typhimurium TV119 Ra mutant, and S. minnesota Re595 mutant (phenol-chloroform-petroleum ether extraction) were initially obtained from Sigma. These endotoxin preparations were further purified and quantified as described (Zughaier et al., 2004). Briefly, residual membrane phospholipids were removed by repeated extraction of the dried LOS/LPS samples with 9:1 ethanol:water. The expected fatty acyl components of 3-OHC12:0, 3-OHC14:0 and C12:0 and the absence of membrane phospholipids was assessed by mass spectroscopy (GC-MS) (Zughaier et al., 2004) (Dr Russell Carlson, Complex Carbohydrate Research Center, University of Georgia, Athens, GA). LOS preparations were examined by silver stained SDS-PAGE and no proteins were visualized. No nucleic acids were detected in the purified LOS samples when measured at ultraviolet wavelengths 260 and 280 nm. No muramic acid was detected by mass spectroscopy, which suggested the absence of peptidoglycan. Purified endotoxins were quantified and standardized based on the number of lipid A molecules per sample (Darvill et al., 1985). LOS or LPS was resuspended in pyrogen free water with 0.5% triethylamine, vortexed for at least 5 min, boiled for 1 h at 65°C, then sonicated for 30 min in a water bath sonicator (L and R, Transisitor/Ultrasonic T-14) to enhance solubility. Endotoxin stock solutions were prepared in pyrogen free water at 10 nmole ml−1 concentration and further diluted with endotoxin free PBS to 1 nmole ml−1 and 100 pmole ml−1 with extensive vortex and sonication prior to each dilution.
Cell cultures
MM6, U937 and THP-1, human macrophage-like cell lines were grown in RPMI 1640 with l-glutamate supplemented with 10% FBS, 50 IU ml−1 of penicillin, 50 μg ml−1 of streptomycin, 1% sodium pyruvate and 1% non-essential amino acids. Culture flasks were incubated at 37°C with humidity under 5% CO2. Murine macrophage cells (RAW 264.7, 23ScCr and C3H/HeJ) were grown in Dulbecco’s Eagle medium supplemented and incubated as mentioned above.
Cellular viability and proliferation assessment
The toxicity of cationic peptides was determined by assessing cellular viability and proliferation using trypan blue exclusion method (Prise et al., 1986). Cells were grown at a starting density of 0.75 million cells ml−1 (final volume 20 ml) in presence of increasing doses (1, 2, 5, 10 and 20 μg 10−6 cells) of cationic peptides PMX, CG117-136, LLP1, PG-1 or LL-37 for 5 days. Cellular aliquots (100 μl) were taken daily and cells were diluted 1:1 with the vital dye trypan blue 0.4% solution in PBS from Cellgro, Mediatech (Herndon, VA) and viable cells were counted. Cellular proliferation was assessed after 5 days of incubation with AMPs where cellular aliquots counted using automated cell counter Sysmex XE 2100 (Roche Diagnostics Quebec, Canada, Laval).
Cytokine induction by LOS
THP-1 human monocytes were differentiated into macrophage-like cells using PMA at a final concentration of 10 ng 10−6 cell and incubated at 37°C for at least 24 h. Freshly differentiated macrophages were washed with PBS, counted and adjusted to 106 cell ml−1, transferred into a 24-well tissue culture plate (1 ml well−1), stimulated with LOS at final concentration of 1, 0.5 or 0.25 pmole ml−1 with or without increasing doses of cationic peptides and incubated overnight at 37°C with 5% CO2. Cell culture supernatants were harvested and saved at −20°C.
Cytokines quantification by ELISA
Human TNFα and IL-1β Duoset kits (R&D Systems, Minneapolis, MN) were used for cytokine quantification according to the manufacturer’s instructions.
Nitric oxide induction in RAW macrophages
Freshly grown RAW 246.7 macrophages adherent to the flask were washed with PBS and incubated with 5 ml of trypsin for 5 min at 37°C. Harvested cells were washed and re-suspended in Dulbecco’s complete media. Approximately 106 macrophages ml−1 were transferred into a 24-well-tissue culture plate, stimulated with 1 pmol ml−1 with or without increasing doses of AMP and incubated overnight. Nitric oxide was quantified using the Griess chemical method (Park et al., 1993).
Cellular respiratory burst (oxidative burst) activity
Freshly grown MM6, U937 or THP-1 cells were adjusted to 2 × 106 ml−1, transferred to a small tissue culture flask, and incubated with 5.6 pmole ml−1 (~10 ng ml−1) LOS or LPS overnight at 37 °C under 5% CO2. Cells were primed with endotoxin in the presence or absence of AMP, which was used at a final concentration of 2 μg 10−6 cells. Unprimed cells were incubated in the same way but without endotoxin. The cells were washed twice with culture medium and resuspended in standard buffer (4.58 mM KH2PO4, 8.03 mM NaHPO4, 0.5 mM MgCl2, 0.45 mM CaCl2, 1% (w/v) glucose, 0.033% (w/v) KCl, 0.76% (w/v) NaCl and 0.1% (w/v) endotoxin-free bovine serum albumin (pH 7.3) at 2 × 106 ml−1. The chemiluminescence probe lucigenin was added to the cell suspension (25 μl ml−1 of cells from 1.0 mM stock solution) and mixed gently. Aliquots (150 μl) of the mixture were transferred into at least quadruplicate wells of a white 96-well plate (FluoroNunc-PolySorp; Nalge Nunc International, Rochester, NY). The respiratory burst was triggered with 50 μl of PMA (1 μM) or with opsonized zymosan (500 μg ml−1). Chemiluminescence was measured in relative light units (a measure of the number of photons generated by the reaction at each time point). Chemiluminescence was measured with luminometer (ML3000, Dynatech Laboratories Chantilly, Virginia) and the plate was read immediately and then at 5 min intervals for the next 90 min (Zughaier et al., 1999).
Preparation of LOS-coated polystyrene beads
Purified LOS from N. meningitidis strain NMB was used to coat latex beads as described (Plested et al., 2001). Briefly, 100 μl of beads were washed three times with 500 μl of borate buffer (0.1 M, pH 8.5) in an Eppendorf tube and then centrifuged for 6 min at 8160 g. The beads were resuspended in 400 μl of bicarbonate buffer (0.1 M, pH 9.68) and coated with 100 μl of LOS at a final concentration of 1 nmole per 100 μl of beads. The beads were vigorously vortexed for 5 min and incubated overnight with shaking at room temperature. The coated beads were resuspended in 0.5 ml blocking solution (1% (w/v) BSA in borate buffer) and incubated for 2 h with shaking at room temperature. Beads were blocked twice and stored in 400 μl of storage buffer (1% BSA, 0.1 sodium azide and 5% glycerol in 0.1 M PBS) and kept at 4°C. Prior to use in phagocytosis experiments, 50 μl of LOS-coated beads in storage buffer were added into 450 μl of PBS, washed three times with PBS, resuspended in 450 μl of final assay buffer and opsonized for 30 min at 37°C with 1% NHS. NHS were pooled from healthy donors that has not been vaccinated or previously infected with Neisseria meningitis.
Phagocytosis of LOS-coated beads measured by enhanced chemiluminescence
Reactive oxygen species release triggered by phagocytosis were quantified by enhanced cellular chemiluminescence method (Zughaier et al., 1999). RAW 264.7 macrophages freshly grown in Dulbecco’s medium or differentiated THP-1 cells grown in RPMI 1640 medium were washed and resuspended in standard buffer (4.58 mM KH2PO4, 8.03 mM NaHPO4, 0.5 mM MgCl2, 0.45 mM CaCl2, 1% glucose, 0.033% KCl, 0.76% NaCl and 0.1% endotoxin free albumin (pH 7.3) at 4 × 106 cell ml−1. Lucigenin was added to the cell suspension (25 μl ml−1 of cells from a 1.0 mM stock solution) and gently mixed. Aliquots (150 μl) of the mixture were transferred into at least quadruplicate wells of a white 96-well plate as mentioned above. The respiratory burst was triggered by phagocytosis of 10 μl from the opsonized LOS-coated beads. Chemiluminescence was measured in relative light units and the plate was read immediately and then at 3 min intervals for 90 min
Xanthine – xanthine oxidase cell free system
To study the effect of AMP and LOS in amplifying respiratory burst in a cell free system, superoxide anions were generated by the xanthine-xanthine oxidase reaction (Aasen et al., 1987) as follows. Lucigenin was diluted in K2HPO4 buffer at a 25 μM final concentration, and 50 μl was dispensed into quadruplicate wells of a white 96-well plate. Fifty microlitres of 10 mM xanthine was added, and the reaction was triggered by the addition of 10 μl of xanthine oxidase (1.0 U). LOS and AMP were added together or alone at different doses at the start of the reaction. Chemiluminescence was monitored for 60 min
Cytochrome c reduction assay
Freshly grown THP-1 (2 × 106 cell ml−1) were primed with N. meningitidis LOS at 5.6 pmole ml−1 (~10 ng ml−1) final concentration with or without AMP (2 μg 10−6 cells) and incubated overnight at 37 °C. Primed cells were washed with PBS and resuspended in standard buffer (4.58 mM KH2PO4, 8.03 mM NaHPO4, 0.5 mM MgCl2, 0.45 mM CaCl2, 1% glucose, 0.033% KCl, 0.76% NaCl and 0.1% endotoxin free albumin (pH 7.3) density of 4 × 106 cells ml−1. Freshly dissolved cytochrome c was used at 90 μg ml−1 final concentration (Nisimoto et al., 1995; Han et al., 1998). Oxidative burst was triggered with 100 ng ml−1 of PMA and the change in OD was monitored for 5 min at 550 nm using a Bio-TEK plate reader (Bio-TEK Instruments, Winooski Vermont). SOD was used at 250 U in parallel controls.
Statistical analysis
Mean values of at least four independent determinations ± SD and P-values (Student t-test) were calculated with Excel software.
Acknowledgments
This work was supported by NIH Grants R01 AI033517 to D.S.S and AI 043316 to W.M.S. W.M.S is the recipient of a Senior Research Career Scientist Award from VA Medical Research Service. We thank Dr Anup Datta and Dr Russell Carlson at the Complex Carbohydrate Center, University of Georgia for purification and quantification of endotoxins, Dr Jan Pohl at the Micro-chemical facility of Emory University for preparing synthetic peptides and Lane Pucko for administrative assistance.
References
- Aarbiou J, Rabe KF, Hiemstra PS. Role of defensins in inflammatory lung disease. Ann Med. 2002;34:96–101. doi: 10.1080/07853890252953482. [DOI] [PubMed] [Google Scholar]
- Aasen TB, Bolann B, Glette J, Ulvik RJ, Schreiner A. Lucigenin-dependent chemiluminescence in mononuclear phagocytes. Role of superoxide anion. Scand J Clin Lab Invest. 1987;47:673–679. [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271:165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- Ammar B, Perianin A, Mor A, Sarfati G, Tissot M, Nicolas P, et al. Dermaseptin, a peptide antibiotic, stimulates microbicidal activities of polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1998;247:870–875. doi: 10.1006/bbrc.1998.8879. [DOI] [PubMed] [Google Scholar]
- Andra J, Garidel P, Majerle A, Jerala R, Ridge R, Paus E, et al. Biophysical characterization of the interaction of Limulus polyphemus endotoxin neutralizing protein with lipopolysaccharide. Eur J Biochem. 2004;271:2037–2046. doi: 10.1111/j.1432-1033.2004.04134.x. [DOI] [PubMed] [Google Scholar]
- Andra J, Lohner K, Blondelle SE, Jerala R, Moriyon I, Koch MH, et al. Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide. Biochem J. 2005;385:135–143. doi: 10.1042/BJ20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle PA, Rupec RA, Pahl HL. Reactive oxygen intermediates as second messengers of a general pathogen response. Pathol Biol (Paris) 1996;44:29–35. [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Bosshart H, Heinzelmann M. Arginine-rich cationic polypeptides amplify lipopolysaccharide-induced monocyte activation. Infect Immun. 2002;70:6904–6910. doi: 10.1128/IAI.70.12.6904-6910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–3765. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Ovsteboo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650–652. doi: 10.1093/infdis/166.3.650. [DOI] [PubMed] [Google Scholar]
- Bulet P, Stocklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- Canas PE. The role of xanthine oxidase and the effects of antioxidants in ischemia reperfusion cell injury. Acta Physiol Pharmacol Ther Latinoam. 1999;49:13–20. [PubMed] [Google Scholar]
- Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- Darvill AG, Albersheim P, McNeil M, Lau JM, York WS, Stevenson TT, et al. Structure and function of plant cell wall polysaccharides. J Cell Scisupplement. 1985;2:203–217. doi: 10.1242/jcs.1985.supplement_2.11. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- Del Maestro R, Thaw HH, Bjork J, Planker M, Arfors KE. Free radicals as mediators of tissue injury. Acta Physiol Scand Supplement. 1980;492:43–57. [PubMed] [Google Scholar]
- DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- Dokka S, Toledo D, Shi X, Castranova V, Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- Evans EW, Harmon BG. A review of antimicrobial peptides: defensins and related cationic peptides. Vet Clin Pathol. 1995;24:109–116. doi: 10.1111/j.1939-165x.1995.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Ganz T. Biosynthesis of defensins and other antimicrobial peptides. Ciba Found Symp. 1994;186:62–71. doi: 10.1002/9780470514658.ch4. Discussion 71–66. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins and host defense. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. Cationic polyelectrolytes: potent opsonic agents which activate the respiratory burst in leukocytes. Free Radic Res Commun. 1989;8:11–26. doi: 10.3109/10715768909087968. [DOI] [PubMed] [Google Scholar]
- Ginsburg I, Borinski R, Malamud D, Struckmeier F, Klimetzek V. Chemiluminescence and superoxide generation by leukocytes stimulated by polyelectrolyte-opsonized bacteria. Role of histones, polyarginine, polylysine, polyhistidine, cytochalasins, and inflammatory exudates as modulators of oxygen burst. Inflammation. 1985;9:245–271. doi: 10.1007/BF00916275. [DOI] [PubMed] [Google Scholar]
- Ginsburg I, Mitra RS, Gibbs DF, Varani J, Kohen R. Killing of endothelial cells and release of arachidonic acid. Synergistic effects among hydrogen peroxide, membrane-damaging agents, cationic substances, and proteinases and their modulation by inhibitors. Inflammation. 1993;17:295–319. doi: 10.1007/BF00918992. [DOI] [PubMed] [Google Scholar]
- Gorter AD, Oostrik J, van der Ley P, Hiemstra PS, Dankert J, van Alphen L. Involvement of lipoo-ligosaccharides of Haemophilus influenzae and Neisseria meningitidis in defensin-enhanced bacterial adherence to epithelial cells. Microb Pathog. 2003;34:121–130. doi: 10.1016/s0882-4010(02)00193-6. [DOI] [PubMed] [Google Scholar]
- Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67 (phox) J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser I, Muhr A, Elstner EF. Production of OH-radical-type oxidant by lucigenin. Z Naturforsch [C] 1998;53:9–14. doi: 10.1515/znc-1998-1-204. [DOI] [PubMed] [Google Scholar]
- Iovine NM, Elsbach P, Weiss J. An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains. Proc Natl Acad Sci USA. 1997;94:10973–10978. doi: 10.1073/pnas.94.20.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CM, Carlson RW, Rahman MM, Martin LE, Stephens DS. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul N, Forman HJ. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic Biol Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- Kellogg TA, Lazaron V, Wasiluk KR, Dunn DL. Binding specificity of polymyxin B, BPI, LALF, and anti-deep core/lipid a monoclonal antibody to lipopolysaccharide partial structures. Shock. 2001;15:124–129. doi: 10.1097/00024382-200115020-00008. [DOI] [PubMed] [Google Scholar]
- Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. Lucigenin as mediator of superoxide production: revisited. Free Radic Biol Med. 1998;25:926–928. doi: 10.1016/s0891-5849(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Mouithys-Mickalad A, Deby-Dupont G, Nys M, Lamy M, Deby C. Oxidative processes in human promonocytic cells (THP-1) after differentiation into macrophages by incubation with Chlamydia pneumoniae extracts. Biochem Biophys Res Commun. 2001;287:781–788. doi: 10.1006/bbrc.2001.5643. [DOI] [PubMed] [Google Scholar]
- Mouithys-Mickalad A, Deby-Dupont G, Mathy-Hartert M, Habraken Y, Nys M, Henrotin Y, et al. Effects of glucocorticoids on the respiratory burst of Chlamydia-primed THP-1 cells. Biochem Biophys Res Commun. 2004;318:941–948. doi: 10.1016/j.bbrc.2004.04.120. [DOI] [PubMed] [Google Scholar]
- Moy JN, Gleich GJ, Thomas LL. Noncyto-toxic activation of neutrophils by eosinophil granule major basic protein. Effect on superoxide anion generation and lysosomal enzyme release. J Immunol. 1990;145:2626–2632. [PubMed] [Google Scholar]
- Mueller M, Lindner B, Kusumoto S, Fukase K, Schromm AB, Seydel U. Aggregates are the biologically active units of endotoxin. J Biol Chem. 2004;279:26307–26313. doi: 10.1074/jbc.M401231200. [DOI] [PubMed] [Google Scholar]
- Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14 (+) cells. J Immunol. 2001;167:3329–3338. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, et al. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol. 2002;9:972–982. doi: 10.1128/CDLI.9.5.972-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypo-ferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisimoto Y, Otsuka-Murakami H, Lambeth DJ. Reconstitution of flavin-depleted neutrophil flavocytochrome b558 with 8-mercapto-FAD and characterization of the flavin-reconstituted enzyme. J Biol Chem. 1995;270:16428–16434. doi: 10.1074/jbc.270.27.16428. [DOI] [PubMed] [Google Scholar]
- Ogino T, Awai M. Lipid peroxidation and tissue injury by ferric citrate in paraquat-intoxicated mice. Biochim Biophys Acta. 1988;958:388–395. doi: 10.1016/0005-2760(88)90224-x. [DOI] [PubMed] [Google Scholar]
- Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol. 1993;54:119–124. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- Plested JS, Ferry BL, Coull PA, Makepeace K, Lehmann AK, MacKinnon FG, et al. Functional opsonic activity of human serum antibodies to inner core lipopolysaccharide (galE) of serogroup B meningococci measured by flow cytometry. Infect Immun. 2001;69:3203–3213. doi: 10.1128/IAI.69.5.3203-3213.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise KM, Gaal JC, Pearson CK. Increased protein ADPribosylation in HeLa cells exposed to the anti-cancer drug methotrexate. Biochim Biophys Acta. 1986;887:13–22. doi: 10.1016/0167-4889(86)90116-3. [DOI] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549–553. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- Seydel U, Hawkins L, Schromm AB, Heine H, Scheel O, Koch MH, Brandenburg K. The generalized endotoxic principle. Eur J Immunol. 2003;33:1586–1592. doi: 10.1002/eji.200323649. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res. 1999;128:112–118. doi: 10.1016/s0378-5955(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Shafer WM, Hubalek F, Huang M, Pohl J. Bactericidal activity of a synthetic peptide (CG 117–136) of human lysosomal cathepsin G is dependent on arginine content. Infect Immun. 1996;64:4842–4845. doi: 10.1128/iai.64.11.4842-4845.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RH, Scoggin CH, Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981;256:7181–7186. [PubMed] [Google Scholar]
- Stein GM, Schaller G, Pfuller U, Wagner M, Wagner B, Schietzel M, Bussing A. Characterisation of granulocyte stimulation by thionins from European mistletoe and from wheat. Biochim Biophys Acta. 1999;1426:80–90. doi: 10.1016/s0304-4165(98)00139-1. [DOI] [PubMed] [Google Scholar]
- Stohs SJ. The role of free radicals in toxicity and disease. J Basic Clin Physiol Pharmacol. 1995;6:205–228. doi: 10.1515/jbcpp.1995.6.3-4.205. [DOI] [PubMed] [Google Scholar]
- Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencza SB, Creighton DJ, Yuan T, Vogel HJ, Montelaro RC, Mietzner TA. Lentivirus-derived antimicrobial peptides: increased potency by sequence engineering and dimerization. J Antimicrob Chemother. 1999;44:33–41. doi: 10.1093/jac/44.1.33. [DOI] [PubMed] [Google Scholar]
- Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Nagase T. [Defensins as a mechanism of host defense and innate immunity] Nippon Ronen Igakkai Zasshi. 2001;38:440–443. doi: 10.3143/geriatrics.38.440. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev Cardiovasc Ther. 2003;1:91–106. doi: 10.1586/14779072.1.1.91. [DOI] [PubMed] [Google Scholar]
- Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- Warren JR. Polymyxin B suppresses the endotoxin inhibition of concanavalin a-mediated erythrocyte agglutination. Infect Immun. 1982;35:594–599. doi: 10.1128/iai.35.2.594-599.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 1999;104:1131–1138. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- Yasin B, Pang M, Wagar EA. A cumulative experience examining the effect of natural and synthetic antimicrobial peptides vs. Chlamydia trachomatis. J Pept Res. 2004;64:65–71. doi: 10.1111/j.1399-3011.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zughaier SM, Ryley HC, Jackson SK. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect Immun. 1999;67:908–913. doi: 10.1128/iai.67.2.908-913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zughaier SM, Tzeng YL, Zimmer SM, Datta A, Carlson RW, Stephens DS. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect Immun. 2004;72:371–380. doi: 10.1128/IAI.72.1.371-380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


