Abstract
The bone marrow provides inflammatory cells and endothelial progenitor cells to healing cutaneous wounds. To further explore the bone marrow contribution to skin and healing wounds, we used a chimeric mouse model in which the bone marrow from enhanced green fluorescent protein (EGFP) transgenic mice is transplanted into normal C57BL mice. We found that normal skin is a target organ for bone marrow–derived cells from both the hematopoietic and the mesenchymal stem cell pool. We present evidence that the bone marrow contribution to normal skin and the healing cutaneous wound is substantially greater than the previously recognized CD45+ sub-population, where 15%–20% of the spindle-shaped dermal fibroblasts were bone marrow–derived (EGFP+). Furthermore, the bone marrow–derived cells were able to contract a collagen matrix and transcribe both collagen types I and III, whereas the skin-resident cells transcribed only collagen type I. Whereas endothelial progenitor cells were found early during the wound repair process, bone marrow–derived endothelial cells were not seen after epithelialization was complete. Our data show that wound healing involves local cutaneous cells for reconstituting the epidermis but distant bone marrow–derived cells and the adjacent uninjured dermal mesenchymal cells for reconstituting the dermal fibroblast population.
Keywords: Wound healing, Bone marrow, Stem cells, Mesenchymal stem cells Hematopoietic stem cells, Collagen, Response to injury
Introduction
Normal skin contains bone marrow–derived cells that are involved in host defense and inflammatory processes, including wound healing. After tissue injury, hematopoietic and multipotent progenitor cells are mobilized from the bone marrow into the pool of circulating cells. These cells migrate to the site of injury, where they regulate the proliferation and migration of epithelial cells and dermal mesenchymal cells (MCs) during the early inflammatory phase [1]. As the inflammatory response subsides, the wound is remodeled to form a stratified epithelia layered over a collagen-rich matrix containing several MC types, including fibroblasts interspersed with capillaries and nerves. Although the bone marrow contribution of inflammatory cells in the acute response to injury is well established, the long-term fate and role of bone marrow–derived cells in a healed cutaneous wound remain unclear.
The bone marrow stroma contains precursor cells that are capable of differentiating along hematopoietic cell (HC) and MC lineages [2]. Hematopoietic stem cells can reconstitute the entire circulating population of HCs. Mesenchymal stem cells can differentiate to form osteocytes, chondrocytes, adipocytes, and bone marrow stromal fibroblasts. Both stem cell types retain a high degree of plasticity and are capable of contributing regenerative progenitor cells to hematopoietic and nonhematopoietic tissues, including the skin [3–5]. During cutaneous wound healing, bone marrow–derived cells differentiate into fibrocytes, a subset of CD45+ antigen-presenting fibroblasts [6–8]. Bone marrow–derived cells also differentiate into CD34+ endothelial progenitor cells, which have been shown to form vascular channels in ischemic tissues [9–12] and during the first week of wound healing [13]. It is unclear whether these endothelial progenitor cells remain in the healed wound, because vascular regression occurs during the later phases of wound remodeling.
Most HCs, with the exception of mature red blood cells and their immediate progenitors, express the cell-surface antigen CD45. In addition, a subset of the HCs express CD34, a cell-surface antigen expressed on the surface of hematopoietic progenitor cells. In contrast, mesenchymal stem cells do not express CD34 or CD45. Previous studies on wound healing have relied on cell-surface marker expression, usually CD45 or CD34, to identify cells of bone marrow origin. Given the plasticity of bone marrow–derived cells, reliance on cell-surface marker expression may under-represent the total contribution of the bone marrow to cutaneous wound healing. To circumvent this problem, we used a chimeric mouse model in which the bone marrow from enhanced green fluorescent protein (EGFP) transgenic mice is transplanted into normal C57BL mice. Using this mouse model, we investigated the total contribution of the bone marrow–derived cells to cutaneous wound healing. We show that a significant percentage of cells in the healed dermis are bone marrow–derived spindle-shaped cells, morphologically resembling fibroblasts, and that less than one third of these bone marrow–derived fibroblasts express CD45. Endothelial progenitor cells can be found early during the proliferative phase of wound repair, but the remodeled wound does not maintain the bone marrow–derived endothelial cells after epithelialization is complete. Finally, both the hematopoietic and MC populations provide long-term reconstitution of the healed dermis and produce collagen types I and III, whereas the wound-resident cells produce only collagen type I. Therefore, we propose that in addition to inflammation and blood vessel formation early in the wound repair process, the bone marrow may be a rich source of cells that re-establish the healed cutaneous dermis and are the source for collagen type III production in the skin.
Materials and Methods
Chimeric Mice Preparation and Animal Wound Model
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health [NIH] publication No. 86–23) and approved by the Animal Care Committee of the University of Washington. For the generation of chimeric mice, bone marrow was collected from the tibia and femur of EGFP+/− [C57BL/6-TgN(ACTbEGFP)10sb] donor mice (Jackson Laboratories, Bar Harbor, ME) that had been treated with a single intraperitoneal dose of 5-fluorouracil (5-FU; 150 mg/kg; Sigma, St. Louis) 24 hours before harvesting donor bone marrow. A single-cell suspension was created, and the cells were prepared for immediate transplantation. Recipient adult C57BL mice (stock number 000664; Jackson Laboratories) were immunodepleted using irradiation (n = 96) with 1.0 Gy in two fractionated doses from a dual opposed Co60 source. Approximately 105 EGFP+ cells were prepared for transplantation by resuspension in phosphate buffered saline (PBS) and injected into recipient mice via the tail vein. Two, 4, and 10 weeks after transplantation, peripheral blood from chimeric mice was analyzed for recovery of the total leukocyte counts. For experiments not requiring chimeric mice, C57BL/6 mice were used. To determine whether radiation impacted the wound-healing model, a set of 10 mice was prepared for transplantation using chemotherapy rather than radiation (busulfan, 25 mg/kg injected subcutaneously; Sigma). After 6 days of receiving daily busulfan, mice received 105 EGFP+ bone marrow cells via tail vein injection. Degree of chimerism was assessed by flow cytometry of circulating nucleated cells at 10 weeks.
For demonstration of engraftment without direct venous injection of bone marrow cells, bone grafts were taken from EGFP transgenic mice, cut into fragments 5-mm long with sterile bone cutters. These morselized femurs were then placed into a subcutaneous anterior thigh pocket of C57BL mice after immunodepletion by irradiation as detailed above. Flow cytometry for circulating EGFP+ cells was done at 3 weeks.
Hematopoietic Cell and Mesenchymal Cell Chimeric Mice Preparation
For the generation of HC and MC chimeras, the bone marrow was collected from the tibia and femur of EGFP+/− donor mice that had been treated with a single intraperitoneal dose (150 mg/kg) of 5-FU 24 hours before harvesting donor bone marrow. A single-cell suspension was created, and the cells were cultured for 72 hours before separation into mesenchymal and hematopoietic fractions before transplantation, as previously described [14]. Recipient adult C57BL mice were partially immunodepleted using a 3-day busulfan (n = 20) regimen (daily subcutaneous injection of 25 mg/kg). Approximately 105 EGFP+ HCs or MCs were prepared for transplantation by resuspension in PBS and injected into recipient mice via the tail vein. Two, 4, and 10 weeks after transplantation, peripheral blood from chimeric mice was analyzed for recovery of the total leukocyte counts and degree of chimerism by flow cytometry.
Wound-Healing Model
Chimeric mice were anesthetized through intraperitoneal injection of ketamine and xylazine mixture (15 and 1 mg/kg, respectively; Phoenix Pharmaceuticals Inc., St. Joseph, MO). The dorsal hair was removed, and the skin was prepared for generation of a standardized 1.5-cm2 full-thickness wound including the panniculus carnosus muscle on the mid back [15]. The wound was covered with a transparent semiocclusive dressing (Tegaderm, 3M; St. Paul, MN) to prevent desiccation. On days 3, 7, 15, 21, 30, and 40, mice were euthanized and the entire wound including the adjacent 2-mm skin margins was excised. The wound was bisected along the cranial-caudal axis. Half of the wound was fixed in 2% paraformaldehyde and then embedded in OCT (Tissue-Tek, Sakura, Torrance, CA) for histological analysis; the other half was processed for flow cytometry. At the time of euthanasia, blood was taken via direct cardiac puncture to determine the proportion of EGFP+ engraftment.
Flow Cytometry
Excised murine skin and wounds were dispersed into single-cell suspension as previously described [16]. Before antibody staining, Fc receptors were blocked with a rat anti-mouse CD16 antibody (Fc BlockTM; Pharmingen, San Diego) to minimize false positive staining. The antibodies used included F4/80 (CALTAG, Burlingame, CA) for identification of monocytes/macrophages, CD34 (Pharmingen) for identification of progenitor cells, CD117 (Pharmingen) for identification of c-kit-expressing cells, GR-1 (Pharmingen) for identification of granulocytes, CD45 (Pharmingen) for leukocytes, and CD31 (Pharmingen) for endothelial cells. The primary antibodies were phycoerythrin (PE) conjugated to avoid overlap with the emission spectra of EGFP. Antibodies were diluted to optimal dilution in PBS + 0.1% sodium azide, added to 106 cells, and incubated overnight on a rocker at 4°C. Cells were washed and resuspended in 1 ml fluorescence-activated cell sorter (FACS) buffer (PBS + 3% fetal bovine serum). The cell suspension was analyzed with a FACS Vantage (Becton, Dickinson, Franklin Lakes, NJ) using excitation at 488 nm and fluorescence detection at 530 nm (EGFP) or 575 nm (PE). Propidium iodide (Sigma-Aldrich Corp., St. Louis, MO) was used to identify and gate out dead cells. All flow cytometry data analysis was done with FlowJo (version 4.1) software.
Collagen Contraction Assays
Dermal fibroblasts from EGFP chimeric mice were obtained from normal skin using enzymatic digestion [16] and sterile sorted into EGFP+ and EGFP− populations. Cells (2 × 105 EGFP+ or EGFP− cells) were cultured in a collagen matrix as previously described [17]. Lattice contraction was determined by measuring the diameter of the circular disk-shaped lattice in perpendicular directions; measurements were taken manually and by digital images, which were analyzed using NIH Image program (http://rsb.info.nih.gov/nih-image/). The mean diameter of the two perpendicular measurements was used to calculate the area of the lattices, which were followed for 7 days.
Reverse Transcription Polymerase Chain Reaction
Excised murine skin from irradiated chimeras was dispersed into a single-cell suspension [16] and sterile sorted into EGFP+ and EGFP− cell populations. RNA extraction, from EGFP+ and EGFP− cells, was performed using RNeasy (Qiagen Corporation, Valencia, CA). Purified RNA was then used for reverse transcription using OneStep reverse transcription-polymerase chain reaction (Qiagen) and primers for collagen type I (5′-tggactcctttcccttcctt-3′ and 3′-gacccggggaaaatatg gta-5′), collagen type III (5′-gaaaccccagcaaaacaaaa-3′ and 3′-actggacataacccaccaac-5′), and β-actin (5′-tgttaccaactgggacgaca-3′ and 5′-ctgggtcatcttttcacggt-3′) as a control to verify RNA integrity. Reactions were run for 15, 20, and 25 cycles and separated on a 2% agarose gel.
Frozen Tissue Sections and Immunocytochemistry
Expression of EGFP in multiple tissues was evaluated by fluorescence microscopy using a wide-band green filter on fresh frozen 10-μm tissue sections. Immunocytochemistry for anatomical localization of each cell lineage was performed as previously described [18]. Tissues were stained with anti-heavy chain myosin (Biomedical Technologies, Stroughton, MA) and visualized using PE-conjugated secondary antibodies followed by 4′,6-diamidino-2-phenylindole nuclear stain (Boehringer-Mannheim, Indianapolis, IN). Slide images were digitized using IP Lab Spectrum (Scanalytics, Fairfax, VA).
Results
Generation of EGFP Chimeric Mouse
To determine the percentage of bone marrow cells that express EGFP, we harvested bone marrow from EGFP transgenic mice and analyzed the single-cell suspension by flow cytometry, gating out propidium iodide (PI)–positive cells. Compared with C57BL bone marrow cells, we found that 52% of the PI− cells in the bone marrow expressed EGFP above background levels. To track the fate of bone marrow cells in the skin, we created a total of 96 chimeras. There were 13 deaths (mortality rate, 13.5%), all within the first 2 weeks after radiation. All mice surviving past 2 weeks had normal leukocyte counts 10 weeks after radiation (data not shown), with 82%–95% of the circulating cells EGFP+ by flow cytometry (mean, 86.2 ± 2.4%; Fig. 1A). Three of the irradiated chimeras were analyzed at 8 months after transplantation. The average percentage of EGFP-positive cells in circulation remained at 92.4% by flow cytometry. A total of 26 mice survived 1 year after transplantation and maintained similar engraftment, with two random blood samples from this group of mice demonstrating 84% and 91% EGFP+ cells. No animals showed signs of graft versus host disease, immunologic signs of rejection, or infection in their skin or wounds.
Figure 1.
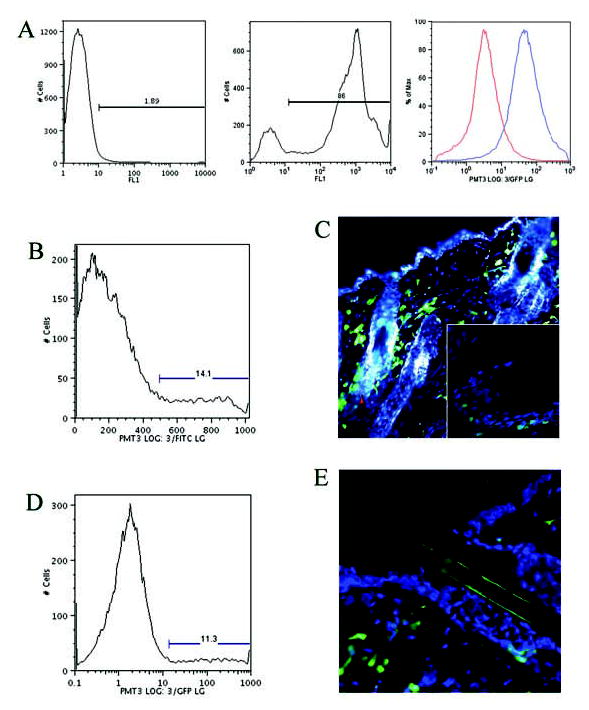
(A): Flow cytometry histogram of circulating cells from a control mouse (left graph) and an EGFP bone marrow–transplanted mouse (middle graph). Note the shift in emission spectra in the FITC channel, with 86% of the circulating cells fluorescing green. To determine degree of autofluorescence, cells were dispersed from normal skin of C57BL mice and EGFP+ mice and analyzed by flow cytometry. The right graph shows the emission characteristics in the FITC channel and the degree of overlap. (B):At 10 weeks, flow cytometry of the normal skin of EGFP bone marrow–transplanted mice shows that 14.1% of the cells in the skin are bone marrow–derived. (C): At 10 weeks, fluorescent microscopy of the normal skin shows large numbers of EGFP-expressing bone marrow cells, especially around the hair follicle. The inset shows integration of bone marrow–derived cells around the base of the hair follicle. (D):At 8 months, flow cytometry of the normal skin confirms a significant number (11.3%) of EGFP+ cells, which persist in the skin at 8 months after transplant. (E): Fluorescent microscopy of the normal skin confirms EGFP+ cells in the dermis at 8 months. Abbreviations: EGFP, enhanced green fluorescent protein; FITC, fluorescein isothiocyanate.
We were concerned that irradiation may interrupt normal wound-healing mechanisms. To determine whether the wound-healing chimeric model was influenced by the amount of radiation given, we created a chimera using chemical immunodepletion with busulfan. The busulfan-treated chimeric mouse model had a 15% reduction in engraftment compared with the irradiated chimeric mouse model, as determined by flow cytometry of the circulating cells at 4 weeks. However, decreased engraftment with busulfan in comparison with radiation is well known [19]. Analysis of the busulfan-treated mice showed no discernible difference in the contribution of EGFP+ cells to normal skin or the percentage of EGFP+ cells in the healing wound compared with the irradiated chimeric mouse model. There was no significant difference between the irradiated and nonirradiated chimeras in the time to achieve wound healing.
Bone Marrow Contribution to Normal Skin
We examined the uninjured skin of EGFP chimeric mice (irradiated chimeric mouse model) at 10 weeks, 8 months, and 1.5 years after transplantation. Flow cytometry of the normal skin at 10 weeks showed that, on average, 14% of all cells in the epidermis and dermis combined were EGFP+ (Fig. 1B). EGFP+ bone marrow–derived cells were seen infiltrating normal uninjured dermis, with occasional cells exhibiting dendritic extensions into the uninjured epidermis (Fig. 1C). Most EGFP+ cells seen in the epidermis were closely associated with hair follicles, primarily in the region of the inner and outer root sheath and the dermal papillae and occasionally near the bulge region of the hair follicle (Fig. 1C, inset); EGFP+ cells were rarely seen around sebaceous glands or sweat glands. After 8 months, EGFP+ cells constituted approximately 11% of all skin cells, as determined by flow cytometry (Fig. 1D). Fluorescent microscopy of skin sections from mice at 8 months show persistent EGFP+ cells in the dermis (Fig. 1E). These figures show that there is a steady-state contribution of the bone marrow to the normal skin. Similar distribution of EGFP+ cells was observed in the busulfan-treated chimeric mice (data not shown).
To determine whether the EGFP+ cells in the skin were a byproduct of the transplantation protocol where bone marrow cells are injected directly into the circulation, as opposed to a true physiologic model where bone marrow–derived cells target the skin, engraftment was performed by direct implantation of morselized femurs from EGFP+ transgenic donors into the anterior thighs of immunodepleted hosts, without intravenous injection of bone marrow cells. At 3 weeks after implantation, the circulating blood in the hosts was assayed by flow cytometry, and their normal skin was examined by fluorescent microscopy. Flow cytometry demonstrated successful engraftment, with 15% of the circulating cells being EGFP+. Although overall engraftment rates were less efficient than seen with the direct injection method, the substantial presence of bone marrow–derived cells in the normal skin validated this animal model and, in particular, that the skin is a target organ for bone marrow–derived cells.
Bone Marrow Contribution to Other Organs
Bone marrow–derived cells can infiltrate and populate numerous tissues in other organs. To study the long-term fate of the transplanted EGFP+ cells, necropsies were performed on the irradiated chimeric mice between 8 and 12 months after transplantation. Skin, heart, lung, brain, spleen, small intestine, aorta, and kidney sections were examined for the presence of EGFP+ cells by fluorescent microscopy. All tissues had varying amounts of EGFP+ cells; however, normal skin harbored one of the highest concentrations of EGFP+ cells, indicating that normal skin is a primary target for bone marrow–derived cells (Fig. 2).
Figure 2.
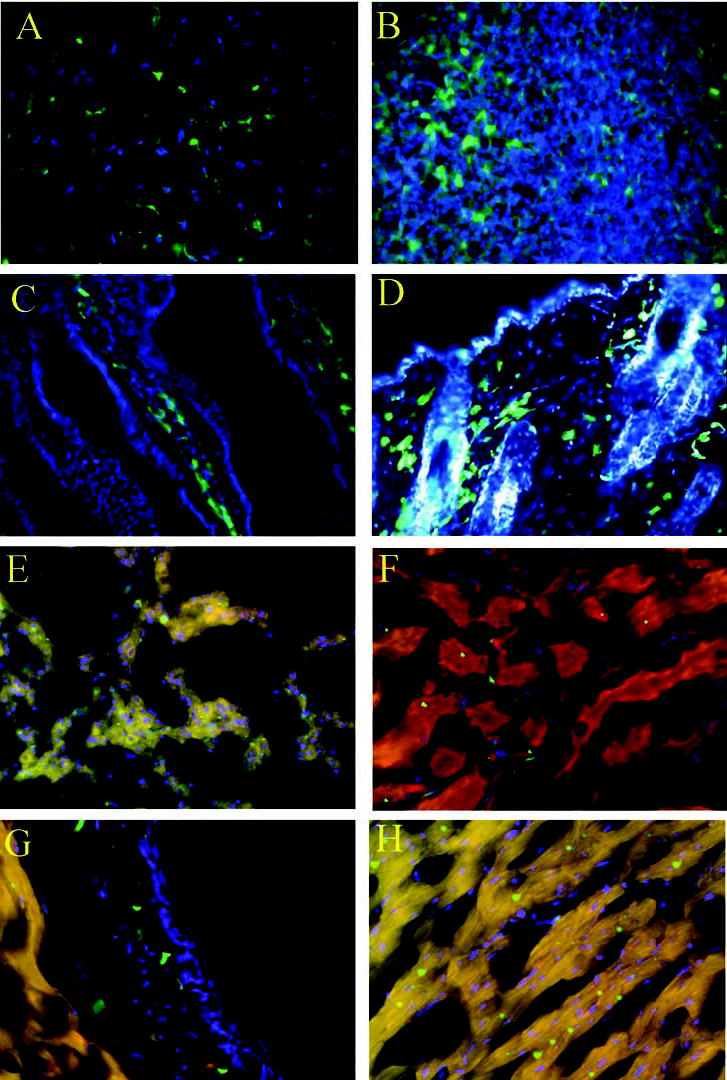
Fluorescent microscopy of various tissues isolated from 8- to 12-month-old chimeras. (A): Brain. (B): Spleen. (C): Small intestine. (D): Normal skin. (E): Lung. (F): Kidney. (G): Cross section of aorta; note the presence of enhanced green fluorescent protein–positive cells in the media. (H): Heart.
Bone Marrow Contribution to Wound Healing
To determine whether CD45 and other inflammatory cell markers account for the entire bone marrow contribution to wound healing, we examined the inflammatory response to wound healing by flow cytometry in the nonirradiated C57BL control mice (Fig. 3A). The early rise in granulocytes (GR-1+) followed by the gradual rise of monocytes/macrophages (F4/80+) is a typical temporal profile of the inflammatory cell influx seen early during wound healing [16]. In addition, angiogenesis as measured by cells expressing CD31 has been shown to peak between days 10 and 14 in this model of wound healing by immunohistochemistry [18] and to peak on day 14 by flow cytometry (peak, 4.1%; range, 0.9% on day 0 to 4.1% of all cells on day 14). On days 7 and 14, the combination of GR-1 and F4/80 markers accounted for the entire CD45+ cell population. By days 21 and 28, when the wound has completely epithelialized, neither inflammatory cell marker accounts for more than 3% of the CD45+ population of cells in the wound.
Figure 3.
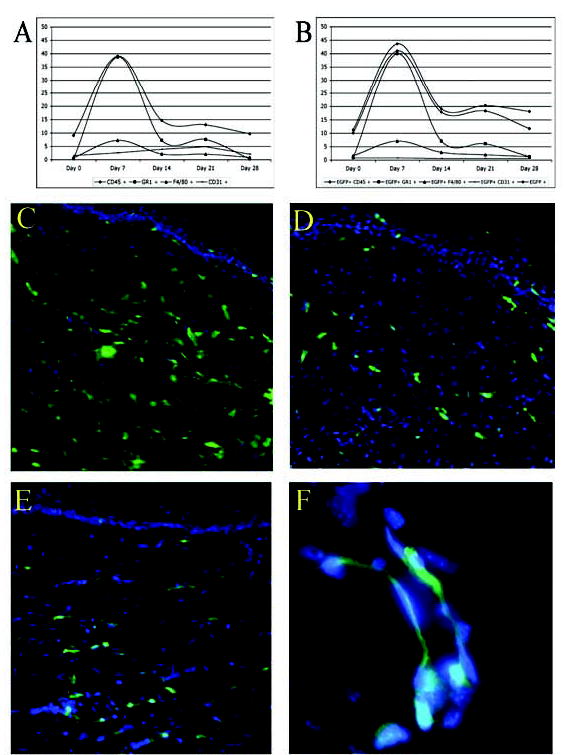
(A): Cumulative graph of flow cytometry data obtained from the control mice, including normal skin (day 0), and during wound healing. The y-axis represents the percentage of cells in the wound that stains with the respective antibodies. (B): Cumulative graph of flow cytometry data obtained from the EGFP-expressing chimeras. The percentages of each cell population in the control and experimental groups are very similar; however, bone marrow–derived cells remain in the healed wound and account for more than 15% of all cells in the healed wound (day 28). On fluorescent microscopy, EGFP+ cells were seen throughout the healing dermis during the early inflammatory phase on days 14 (C) and 21 (D) after wounding. (E): EGFP+ cells persisted in the dermis during the remodeling phase, as seen here on day 42 after wounding. Note the histological resemblance of the EGFP-positive cells to fibroblasts. (F): Microvessel deep in the wound shows EGFP-expressing cells either within the lumen (endothelial cells) or adjacent to the luminal cells (pericytes). Abbreviation: EGFP, enhanced green fluorescent protein.
To determine the cellular contribution of all bone marrow–derived cells to wound healing, we analyzed the wounds of EGFP chimeric mice by two-color flow cytometry on days 7, 14, 21, and 28 and compared them with the results obtained with the control mice (Fig. 3B). All chimeric mice healed their dorsal skin wounds completely between days 14 and 17, identical to the control group. The temporal profile of the inflammatory influx in EGFP chimeric mice was similar to the nonirradiated, nontransplanted controls (Fig. 3A versus Fig. 3B). The EGFP chimeras showed a similar percent of CD45+ cells in normal skin and after wounding; there was no significant difference in the influx and overall percent of GR-1+ or F4/80+ cells between controls and EGFP chimeras during the healing period (p > .05). However, the percentage of EGFP+ cells was far greater than CD45+ cells, and this divergence increased with time from injury, demonstrating that CD45 is not a pan-marker of bone marrow–derived cells in the later stage of wound healing.
EGFP+ cells were seen throughout the healing dermis during the early inflammatory phase (days 14 and 21 after wounding) as well as later in the remodeling phase (day 42; Figs. 3C–E). The bone marrow–derived EGFP+ cells on day 42 (remodeling phase) were spindle-shaped and histologically resembled fibroblasts. The deeper dermal wounds occasionally showed EGFP+ cells intercalating within blood vessels, but this was rare (Fig. 3F). This observation was confirmed by two-color flow cytometry, where less than 0.1% of all cells were CD31+EGFP+ by day 28 (data not shown). Using flow cytometry, we determined that 18% of the cells in the epidermis and dermis of the wound were EGFP+ on day 28 after wounding. To determine the contribution to the dermis alone as well as control for wound contraction, we performed manual counts of high-power histological cross sections across the wound and found the mean percentage of EGFP+ cells in the healed dermis was 37% at day 28 and 19.2% at day 42.
Previous studies examining the bone marrow contribution to wound healing have relied on cell marker expression, most commonly CD45, to track bone marrow–derived cells. To determine whether CD45 is a comprehensive marker of bone marrow–derived cells in skin and a healing cutaneous wound, we compared the percentage of cells coexpressing EGFP and CD45. In nonwounded, normal skin of an 8-month-old chimeric mouse, there is a significant population of persistent EGFP+ cells that do not coexpress CD45 (Fig. 4A). In contrast, during the early stages of wound healing, almost all bone marrow–derived cells coexpress EGFP and CD45. However, at later stages of wound healing, approximately 5% of the bone marrow–derived cells are EGFP+CD45−, suggesting that only two of three of all bone marrow–derived cells express CD45 in the healed and remodeling wound (day 28; curves for EGFP and CD45 in Fig. 3B). Day-42 wounds stained with PE-conjugated CD45 antibody show that only a few spindle-shaped EGFP+ bone marrow–derived cells were CD45+ and most were CD45− (Fig. 4B). Manual counts of high-powered histological cross sections of day-42 wounds counterstained with PE-conjugated anti-CD45 showed 17.6% EGFP+ and 10.2% CD45+ but only 2.5% EGFP+/CD45+ cells (Fig. 4D). The divergence in CD45 and EGFP colocalization increased with healing time, becoming statistically significant by day 42 (p < .05) with a difference of 7.4%.
Figure 4.
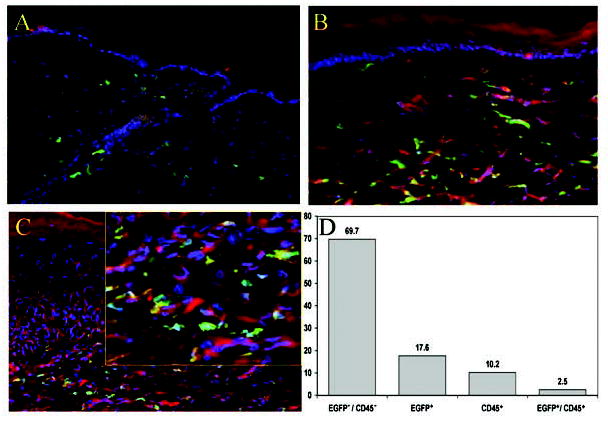
(A): Normal skin 8 months after transplantation shows EGFP+ (green) cells as well as PE-conjugated anti-CD45–labeled cells (red). (B): Day-42 wound stained with PE-conjugated anti-CD45 antibody showing that many of the EGFP+ bone marrow–derived cells within the wound do not coexpress CD45. The EGFP+CD45− cells appear as spindle-shaped cells resembling fibroblasts. (C): Day-42 wound counterstained with anti-heavy chain myosin antibodies. As with the anti-CD45 antibody, most of the EGFP+ cells fail to colocalize anti-myosin and show numerous spindle-shaped EGFP+ bone marrow–derived cells in the dermis that lack heavy chain myosin expression. The yellow framed inset shows a higher-power view of the dermal cells. (D): Manual counts of high-powered histological cross sections of day-42 wounds that are counterstained with PE-conjugated anti-CD45 antibody show that only 2.5% of the dermal cells coexpress EGFP and CD45. More than 7% of the dermal cells are of bone marrow origin (EGFP+) but lack CD45 expression (p < .05). Abbreviations: EGFP, enhanced green fluorescent protein; PE, phycoerythrin.
It has been shown that bone marrow–derived cells can differentiate into myofibroblasts that have contractile properties and express the smooth muscle actin isoform. To determine if the EGFP+ cells had differentiated into myofibroblasts, we stained tissues from day-14, day-21, and day-42 wounds for the smooth muscle form of anti-heavy chain myosin. As with the anti-CD45 antibody, most EGFP+ cells fail to colocalize heavy chain myosin, suggesting that bone marrow–derived cells rarely assume a myofibroblast phenotype in vivo (Fig. 4C).
Hematopoietic Cell and Mesenchymal Cell Contribution to Normal Skin
To determine the respective contribution from the HC or MC lineages, EGFP+ cells derived from the bone marrow of transgenic mice were cultured for separation into MC and HC fractions as previously described [14] and then prepared for transplantation. A total of 10 MC and 10 HC partial chimeras were generated after receiving 3 days of intraperitoneal busulfan. HC chimeras had a circulating population of EGFP+ cells, ranging from 16.6%–82.2% (mean, 44.9 ± 21.9%), whereas MC chimeras had a circulating population of EGFP+ cells ranging from 11.4%–91.4% (mean, 47.7 ± 32.9%). The higher values of circulating EGFP+ cells in the MC chimeras are a consequence of using the plastic-adherent cells from the bone marrow, which contains variable percentage of contaminating HCs. Fluorescent microscopy confirmed the presence of EGFP+ bone marrow cells in the normal uninjured dermis of both chimeric populations (Figs. 5A, 5C). As with the irradiated model, rare EGFP+ cells were seen in the epidermis, associated with hair follicles.
Figure 5.
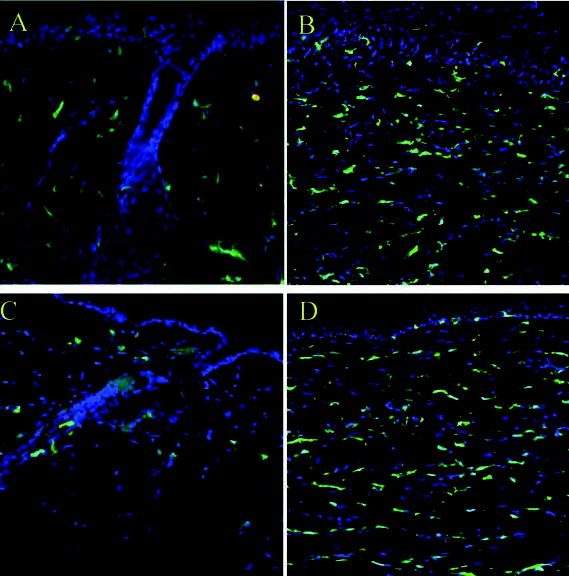
(A): Normal skin of HC chimeric mice shows EGFP+ cells in the dermis confirmed by fluorescent microscopy. (B): Day-42 wound in an HC chimeric mouse; on fluorescent microscopy, EGFP+ cells were seen throughout the healing dermis in the remodeling phase. (C): Normal skin of MC chimeric mice shows EGFP+ cells in the dermis confirmed by fluorescent microscopy. (D): Day-42 wound in an MC chimeric mouse; on fluorescent microscopy, EGFP+ cells were also seen throughout the healing dermis in the remodeling phase. Abbreviations: EGFP, enhanced green fluorescent protein; HC, hematopoietic cell; MC, mesenchymal cell.
Hematopoietic Cell and Mesenchymal Cell Contribution to Wound Healing
We analyzed the wounds of EGFP+ HC and MC chimeric mice by two-color flow cytometry and fluorescent microscopy on days 21 and 42 after wounding to determine the cellular contribution of hematopoietic and mesenchymal bone marrow–derived cells to wound healing. All partial chimeras healed their dorsal skin wounds completely between days 14 and 17. By fluorescent microscopy, EGFP+ cells were seen throughout the healing dermis during the remodeling phase (day 42; Figs. 5B, D). Analysis of healed wounds on day 21 by flow cytometry showed that EGFP+ cells in the HC chimeras ranged from 12.8%–36.1% (mean, 23.6 ± 9.9%), whereas EGFP+ cells in MC chimeras ranged from 13.8–18.7% (mean, 15.9 ± 2%). By day 42, EGFP+ cells in HC chimeras ranged from 8.5%–23.5% (mean, 13.9 ± 6.3%), whereas EGFP+ cells in MC chimeras remained stable at 10.3%–20.7% (mean, 16.2 ± 4.4%). These values were corroborated by manual cell counts and show that during the inflammatory phase of wound healing, there is a direct increase of bone marrow–derived cells that originates from the HC pool, whereas the cells derived from the MC pool maintain a stable population in the skin throughout the wound repair process. The MC chimeras should be regarded as MC-enriched, because murine HCs can remain adherent for 72 hours in culture and thus account for some of the observed difference; however, these data do suggest a potential divergence in the role of the two bone marrow compartments.
Characterization of the EGFP+ Cells
To characterize the function of the EGFP+ cells that populate the healed wound, we began by studying the contraction potential of these cells in vitro. Collagen contraction assays were performed on cells obtained from seven different chimeras. EGFP+ and EGFP− cells were obtained from the skin of chimeric mice and subjected to FACS. EGFP+ cells contracted the collagen lattice to a mean of 31.3% of the initial starting area by day 1, 20.7% by day 2, 16% by day 3, 14.1% by day 4, 12.4% by day 5, and 11.5% by day 6. EGFP− cells contracted the collagen lattice to a mean of 34.1% of the initial starting area by day 1, 23.1% by day 2, 18.2% by day 3, 16.1% by day 4, 14.3% by day 5, and 13.2% by day 6. The control for the collagen lattices (consisting of only collagen and culture media) remained at 96% of the initial starting area throughout the 7-day collection period. Although EGFP+ cells did not show heavy chain myosin staining in situ, comparison of the EGFP+ with the EGFP− cells showed that both cell types were able to contract a collagen gel in vitro.
We also determined expression of collagens type I and III, the two most ubiquitous collagens in the skin, by skin-resident EGFP+ cells. Single-cell suspensions from normal skin of chimeric mice were sterile sorted by FACS into EGFP+ and EGFP− subpopulations, and RNA was extracted to determine collagen transcription. All EGFP+ cells (bone marrow–derived) transcribed both collagen types I and III, whereas EGFP− cells transcribed collagen type I only, not collagen III. All subsets were positive for β-actin mRNA (Fig. 6). These data suggest that, in the skin, collagen type III is produced by the bone marrow–derived cell population, not by the skin-resident dermal fibroblasts. In contrast, both the bone marrow–derived cells and the MCs in the skin produced collagen type I.
Figure 6.
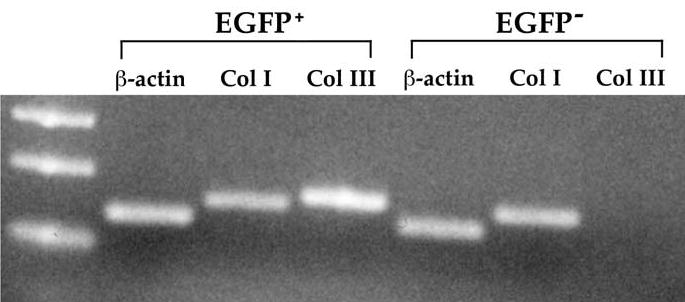
Reverse transcription polymerase chain reaction analysis of cells collected from normal skin via fluorescence-activated cell sorter. Bone marrow–derived EGFP+ cells and skin-resident EGFP– cells were examined for collagen (Col) type I, collagen type III, and β-actin mRNA. Lane 1 represents the DNA molecular standards. In all four chimeras examined, only the EGFP+ and, hence, bone marrow–derived cells in the skin expressed collagen type III. Abbreviation: EGFP, enhanced green fluorescent protein.
Discussion
Immediately after tissue injury and formation of the fibrin clot, the bone marrow is known to contribute inflammatory cells, such as granulocytes and monocytes, to initiate the process of wound repair. Most inflammatory cells in the wound are presumed to either undergo apoptosis or migrate back into the circulation once the wound-derived chemotactic signal has subsided. Subpopulations of bone marrow–derived cells integrate into the healed wound as antigen-presenting dendritic cells, often termed skin-associated lymphoid tissue (SALT) and fibrocytes [6, 7, 20]. These cells were defined as bone marrow–derived cells because they expressed the cell-surface marker CD45.
This study shows that the bone marrow is a source of both CD45+ and CD45− cells in uninjured, normal dermis. Our data show that under steady-state conditions, a substantial population of cells in the normal, uninjured skin is EGFP+CD45−, and that during wound healing, the percentage of EGFP+CD45− cells increases after the wound has epithelialized and is undergoing tissue remodeling. These cells represent a population of cells distinct from SALT or fibrocytes, because most lack CD45 expression. The EGFP+CD45− pool of cells did not represent mature reticulocytes and their immediate progenitors, because these cells exclude their nuclei at an early stage; therefore, any residual EGFP protein would be degraded. Because only approximately half of the bone marrow cells in EGFP transgenic mice express EGFP, the degree of bone marrow contribution to skin and healing wounds may in fact be much greater than our results show.
The fact that the EGFP+ cells did not immunostain with muscle cell markers means that these cells were not differentiating into myofibroblasts, although they did appear to have a contractile phenotype when placed in vitro. Our data also show that there is a unique contribution from both hematopoietic and MC lineages during the early phase of wound healing and later remodeling phase. The inflammatory cells derived from the HC pool clearly increased during the early phase of wound repair, as expected during the inflammatory phase. An unexpected finding was that cells derived from the MC pool maintained a stable presence of EGFP+ cells throughout all phases of cutaneous wound repair. These results suggest a potential difference in the role of bone marrow–derived hematopoietic and MCs in skin biology, but because our MC selection method enriches for MC without eliminating contaminating HC, additional experiments are necessary to confirm these findings.
In normal skin, bone marrow–derived cells achieve steady-state presence in the dermis and are responsible for the production of collagen type III in skin. Collagen type I is the predominant collagen in normal human skin and exceeds collagen type III by a ratio of 4:1. During wound healing, this ratio decreases to 2:1 because of an early increase in the deposition of collagen type III [21]. Based on our data, it would appear that the transcription of collagen type III is unique to the bone marrow–derived cells; EGFP+ cells transcribed both collagen types I and III, whereas EGFP− (wound-resident) cells transcribed only collagen type I.
Circulating endothelial progenitor cells have been shown to contribute to the wound-healing neovasculature. Our experiments confirmed that this contribution is transient and decreases as the wound matures and vascular regression occurs. Although we did observe an EGFP+ microvessel in a day-42 wound, this was an unusual finding. Other groups have confirmed our findings and have also observed rare microvessels of bone marrow origin in the mature, healed wound [8]. Although tissue ischemia is a potent stimulus for the recruitment of endothelial progenitor cells, we propose that during the later stages of wound healing, when there is vascular regression, endothelial progenitor cells are not maintained within the healed cutaneous wound.
Summary
In adults, cutaneous wound healing forms stratified epithelia layered over a collagen-rich matrix containing MCs, resulting in scar tissue that is architecturally distinct from surrounding uninjured normal skin. Expanding on this paradigm, we demonstrate that the bone marrow–derived cells provide a population of inflammatory (CD45+) and noninflammatory (CD45−) cell types that are responsible for the production of type III collagen in the skin. The bone marrow–derived cells originate from both the HC pool, which increases during the early inflammatory phase of wound repair, and the MC pool, which maintains a stable representation of cells in the dermis throughout the repair process.
Acknowledgments
This research was supported by NIH RO1 GM-062771.
References
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow–derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 4.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 5.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 6.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 8.Badiavas EV, Abedi M, Butmarc J, et al. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow–derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Takakura N, Watanabe T, Suenobu S, et al. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 12.Crosby JR, Kaminski WE, Schatteman G, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 13.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 14.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang YC, Tsou R, Gibran NS, et al. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery. 2000;127:696–704. doi: 10.1067/msy.2000.105858. [DOI] [PubMed] [Google Scholar]
- 16.Wilson L, Fathke C, Isik F. Tissue dispersion and flow cytometry for the cellular analysis of wound healing. Biotechniques. 2002;32:548–551. doi: 10.2144/02323st07. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich HP, Wyler DJ. Fibroblast contraction of collagen lattices in vitro: inhibition by chronic inflammatory cell mediators. J Cell Physiol. 1983;116:345–351. doi: 10.1002/jcp.1041160312. [DOI] [PubMed] [Google Scholar]
- 18.Jang YC, Arumugam S, Gibran NS, et al. Role of alpha(v) integrins and angiogenesis during wound repair. Wound Repair Regen. 1999;7:375–380. doi: 10.1046/j.1524-475x.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- 19.Mauch P, Down JD, Warhol M, et al. Recipient preparation for bone marrow transplantation, I: efficacy of total-body irradiation and busulfan. Transplantation. 1988;46:205–210. doi: 10.1097/00007890-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Gibran NS, Heimbach DM, Holbrook KA. Immunolocalization of FXIIIa+ dendritic cells in human burn wounds. J Surg Res. 1995;59:378–386. doi: 10.1006/jsre.1995.1179. [DOI] [PubMed] [Google Scholar]
- 21.Peacock EEJ. Wound Repair. Philadelphia: WB Saunders, 1984.


