Abstract
Conditioned responses to cues associated with the administration of drugs of misuse are an impediment to continued abstinence for drug-free addicted individuals. In order to study the neuroanatomical and cellular response of the brain to cues associated with nicotine administration, we conditioned Sprague–Dawley rats to receive an ascending dose regimen of nicotine over 14 days in two distinct non-home cage environments and assessed expression of the early response gene arc in corticolimbic areas in response to the nicotine-associated context. All of the rats received the same dose regimen of nicotine. Three days after the last training day, the rats were exposed to the test environment. The rats that had previously received nicotine exhibited increased motor activity compared with the rats that had received saline in the test environment. After 45 min in the test environment, brains were taken for Northern blotting and in situ hybridization analysis, which revealed an increase in levels of activity-regulated, dendritically localized mRNA for arc in a variety of brain regions (medial and lateral prefrontal cortices, cingulate cortex, primary sensory cortex, sensorimotor cortex, ventral striatum and amygdala). Plasma corticosterone levels were not different between the groups, suggesting that exposure to nicotine cues is insufficient to activate the hypothalamo-pituitary-adrenal axis. Given that Arc plays a direct role in neuronal plasticity and memory consolidation, its induction by nicotine-associated cues in brain regions critical for cognitive and emotional processing suggests that rats may be learning that these cues are no longer necessarily predictive of nicotine administration. Further work will be needed in order to assess the role of arc expression in the extinction of conditioned responses to drug-paired cues.
Keywords: addiction, immediate-early gene, prefrontal cortex, relapse, Sprague—Dawley
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ISH, in situ hybridization
Introduction
Continued abstinence from drugs of misuse is a major challenge for addicted individuals. Although current therapeutic interventions for addiction are effective on a par with treatments for other chronic medical conditions (Leshner, 1999), advances in understanding the neurobiological factors leading to relapse are needed for significant gains in positive outcomes to occur. Three precipitants of relapse (and reinstatement of drug-seeking behavior in animal models) include administration of the drug itself (‘priming’), acute stress and the presentation of drug-associated cues (Shalev et al., 2002; Shaham et al., 2003).
Environmental cues associated with drug administration are capable of eliciting a variety of physiological and psychological conditioned responses when animals are re-exposed to them. Interactions between the environment and internal states generated by nicotine underlie associative learning in corticolimbic circuits conferring reinforcing and motivational properties to drug-associated cues (Robbins & Everitt, 2002). For example, administration of drugs of misuse in a novel environment potentiates the expression of immediate-early genes necessary for neuronal plasticity in cortical and limbic brain regions (Uslaner et al., 2001, 2003; Klebaur et al., 2002; Ostrander et al., 2003; Ferguson et al., 2004). Subsequent administration of drug within the same environment results in increases in motor responses to the same dose of the drug, a phenomenon known as behavioral sensitization (Badiani et al., 1995; Anagnostaras & Robinson, 1996; Anagnostaras et al., 2002). Thus, neuronal plasticity resulting from repeated pairings of drugs and cues underlies alterations in behavior such as sensitization, conditioned locomotor hyperactivity, conditioned place preference and reinstatement of drug-seeking behavior in response to drug-cues, including those with nicotine (Caggiula et al., 2001, 2002a,Caggiula et al., b; Robbins & Everitt, 2002).
The neuroanatomical substrates underlying conditioned responses to drug-cues in humans have been identified using functional neuroimaging. Regional brain metabolism is increased in the anterior cingulate and orbitofrontal cortices and anterior temporal lobe by presentation of visual and tactile cues associated with smoking (Brody et al., 2002). These authors showed that metabolic activity in bilateral orbitofrontal and dorsolateral prefrontal cortices and anterior insula and the right sensorimotor cortex were positively correlated with self-reports of cue-induced cigarette craving. Fos immunohistochemistry reveals a similar neuroanatomical activation profile in the rat brain after rats are exposed to a context associated with passive nicotine administration (Schroeder et al., 2001).
It is unclear whether neural activation within corticolimbic circuits in response to drug cues results in alterations in the expression of genes known to play a role in synaptic plasticity. Arc, unlike activity-regulated transcription factors such as Fos, has direct effects on neuronal processes governing postsynaptic signaling efficacy (Shepherd et al., 2004) and is necessary for long-term memory formation (Guzowski et al., 2000). The expression of arc mRNA can therefore be used not only as a marker for alterations in neuronal activity (Lyford et al., 1995; Guzowski et al., 1999) but also as a marker for neuronal plasticity. In order to characterize further the neuroanatomical and cellular response to nicotine cues, we used an animal model of conditioned responses to contextual cues associated with prior administration of nicotine combined with Northern blotting and in situ hybridization (ISH) analysis for arc in a variety of corticolimbic areas.
Materials and methods
Subjects
Twenty-four male Sprague–Dawley rats (Harlan, Madison, WI, USA) weighing between 250 and 300 g at the beginning of training were used in this study. Pairs of rats were housed in clear plastic cages in an animal colony. Food and water were available at all times except during training and testing. Lighting in the animal colony was on a 12-h light/dark cycle with lights on at 07:00–19:00 h. Rats were handled daily for 3 days prior to the beginning of training. All efforts were made to minimize the number of animals used. All animal care was in strict accordance with our Institutional Animal Care and Use Committee guidelines.
Nicotine conditioning procedure
All rats received a daily nicotine injection using an escalating dose regimen (n = 24, 0.40 mg/kg days 1–5, 0.50 mg/kg days 6 and 7, 0.63 mg/kg days 8 and 9, 0.80 mg/kg days 10–14, s.c., dissolved in saline, pH 7.2, Sigma, St Louis, MO, USA) in one of two distinct, non-home cage contexts for 14 days (Fig. 1A). An escalating dose regimen was used in order to minimize any effect of stress from higher-dose nicotine treatments on conditioning. Subjects were randomly assigned to receive injections of nicotine or normal saline (s.c., 1 mL/kg) in context 1 and the alternative treatment in context 2. Although training and housing were carried out in similar polycarbonate cages (19 ×10 × 8 in, San Diego Instruments, San Diego, CA, USA), context 1 and context 2 were distinct from the home cage environment and from each other in a variety of ways. Cages in context 1 had wire mesh floors over aspen chips providing distinct visual, somatosensory and olfactory cues. Cages in context 2 had plain plastic floors and were scented with vanilla extract. Home cages had ground corn cob bedding and food and water present at all times. Daily, rats were placed in context 1 for 90 min following an injection of nicotine or saline. A photobeam activity system (San Diego Instruments) kept track of total horizontal, ambulatory and rearing activity counts (as described in Schroeder et al., 2001) during the daily training sessions. Data were collected on an attached PC in 10-min intervals for 90 min. After 90 min, all rats were returned to the home cages for 30–45 min. The rats were then placed in context 2 after receiving the alternative injections (i.e. rats that received nicotine in context 1 received saline in context 2 and vice versa). After 90 min in context 2, all rats were returned to their home cages until the next day of training. Training was always conducted between 12:00 and 17:00 h. All rats received the same number of injections and the same nicotine dose regimen but in two distinct environments (see Fig. 1A).
Fig. 1.
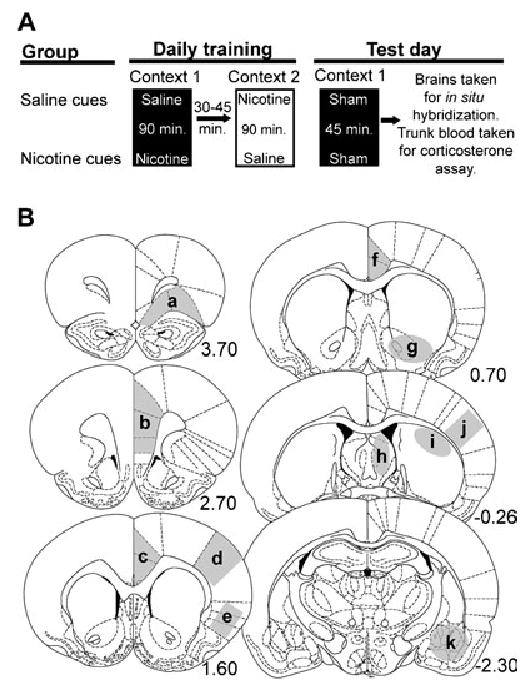
Schematic diagrams of the experimental procedure and brain areas analysed. (A) Rats were trained to receive an ascending dose regimen of nicotine in one of two distinct, non-home cage environments. Rats were placed in context 1 after an injection of nicotine or saline while motor activity was measured. After 90 min in context 1, rats were returned to their home cages for 30–45 min. Rats were then taken to context 2 where they received the counterbalanced injection (rats that received an injection of saline in context 1 received an injection of nicotine and vice versa). After 90 min in context 2, rats were returned to their home cages where they remained until the next training day. The conditioning (training) phase of the experiment lasted 14 days. Three days after the last day of training rats were exposed to context 1 without any injections where they remained for 45 min while motor activity was measured. (B) Brain regions analysed for expression of arc mRNA: (a) lateral prefrontal cortex, (b) medial prefrontal cortex, (c) anterior cingulate cortex, (d) primary sensory cortex, (e) agranular insular cortex, (f) posterior cingulate cortex, (g) ventral striatum, (h) septum, (i) posterior dorsal striatum, (j) sensorimotor cortex, (k) amygdala. Numbers represent distance, in millimeters, from bregma (Paxinos & Watson, 1998).
The ‘saline cues’ control group in these experiments addresses some caveats revealed in our previous studies. The use of this control group, which received the same nicotine dose regimen as the test group but received nicotine in a distinctly different environment from the test cages and the home cages, ensured that any behavioral conditioned responses observed on the test day, and associated changes in gene expression, were due to the environment alone and not treatment history or an interaction of prior treatment with general arousal.
Three days following the final training day, rats were reintroduced into context 1, where they were exposed to either nicotine or saline cues depending on which group they were assigned to during training. Following a mock injection, the rats remained in context 1 where motor activity was measured for 45 min; they were then anesthetized with halothane and decapitated for collection of trunk blood and for brain dissection. Trunk blood was used to assay levels of plasma corticosterone in order to assess whether a stress response is part of the conditioned response. The brain was used for Northern blot analysis and ISH analysis.
Tissue collection
After decapitation, approximately 10 mL of trunk blood was collected in 15-mL conical tubes containing 100 μL of 0.1 m EDTA (Sigma). Tubes were kept on ice until centrifugation at 1700 g at 4 °C for 15 min. Plasma was then stored at −80 °C until assayed. A rat corticosterone EIA kit (ICN Diagnostics, Costa Mesa, CA, USA) was used to quantify plasma corticosterone levels. Intra-assay variability was < 6%.
Heads were cooled for 30 s in −70 °C isopentane. Brains were rapidly dissected from cooled heads and frozen in −40 °C isopentane. Frozen brains were kept at −80 °C until they were used. Two of the brains (n = 1 per group) were sectioned at 20 μm and thaw mounted onto Superfrost slides (Fischer Scientific), warmed at 57 °C for 1 min, and stored at −80 °C for ISH. Twelve of the brains (n = 6 per group) were sectioned for ISH (20 μm) and for lateral prefrontal cortex dissection (see below). Ten of the brains (n = 5 per group) were sectioned (500 μm) and the lateral prefrontal cortex (corresponding to the ventral orbital and lateral orbital cortices, Fig. 1B) was dissected away from the rest of the tissue and stored at −80 °C for subsequent RNA isolation and Northern blot analysis. To analyse the expression of arc, ten (RNA from one sample in each group was not analysed owing to degradation of the samples) animals from the saline- and nicotine-paired groups were analysed via Northern blot analysis and seven animals per group were analysed via ISH analysis.
arc Northern blot
RNA from lateral prefrontal cortex was isolated using the Nucleo-Spin® RNA II Kit (Macherey-Nagel, Palo Alto, CA, USA) using the manufacturer’s suggested protocol. Briefly, tissue punches from brain were homogenized in GITC solution using a tissue tearror™ (Biospec Products, Inc., Bartlesville, OK, USA). Nucleic acids where ethanol precipitated, resuspended in GITC, and then bound to a NucleoSpin™ column (Macherey-Nagel). GITC was removed by centrifugation and samples were DNase treated on the column. DNase treatment was halted with a GITC wash. RNA was then washed twice with 100% ethanol and eluted from the column with RNAse-free water. The concentration of RNA was determined using the RiboGreen method (Molecular Probes, Eugene, OR, USA) and RNA quality was assessed using agarose electrophoresis.
One microgram of total RNA from each rat was electrophoresed in individual lanes on a 1.2% agarose/formaldehye/1×MOPS gel. The gel was transferred to GeneScreen™ plus (Perkin Elmer Life Sciences, Inc., Boston, MA, USA) using Stratagene’s PosiBlotter™ (Stratagene, Inc., La Jolla, CA, USA). 32P-labeled cDNA (2 × 106 c.p.m./mL) was hybridized to the membrane in Hybrisol™ I (Intergen, Inc., Norcross, GA, USA). Northern blots were washed and exposed to a phosphorimager screen for 1 day. Screens were scanned on a Storm™ phosphorimager (Molecular Dynamics Inc., Sunnyvale, CA, USA). Quantification was performed using Image-Quant™ software (Molecular Dynamics).
Probes used for Northern blotting were generated from rat prefrontal cortex cDNA using PCR amplification. The following primers were used to generate PCR product for arc: forward primer 5′-CCAGGAAGCTGATGGCTACGAC-3′ (693–714), reverse primer 5′-GTGTCAGCCCCAGCTCAATCAAG-3′ (1471–1494). Numbers in parentheses after the primer sequence represent the base number as defined by the rat arc mRNA sequence (accession number NM_019361) in the NCBI nucleotide database. A 983-bp PCR product for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was generated as described previously (Roseboom et al., 1996) and was used as a loading control. mRNA from one rat in the nicotine- and saline-paired group was precluded from Northern blot quantification and statistical analysis owing to obvious degradation of the samples (data not shown).
In situ hybridization
Sections on slides were fixed in 4% paraformaldehyde in PBS for 2 h at 4 °C. Slides were then washed for 5 min in 2 × SSC three times. Slides were incubated in 0.2 μg/mL Proteinase K (Qiagen, Hilden, GmbH, Germany) in 0.1 m Tris base and 50 mm EDTA, pH 8.1, for 10 min at 37 °C. Slides were washed in 2 × SSC at room temperature for 2 min. Slides were incubated in 0.1 m TEA at room temperature with rapid stirring, and acetic anhydride was added to a final concentration of 0.25% (v/v) with rapid stirring for 10 min. Slides were then washed in 2 × SSC for 5 min. Finally, sections were dehydrated in an ascending ethanol series and air dried for 15 min.
Arc template for in vitro transcription was generated as described above except that either the forward (sense arc probe) or reverse (antisense arc probe) was tagged with a T7 polymerase recognition sequence. The specific primer sequences were: forward primer 5′-CCAGGAAGCTGATGGCTACGAC-3′, reverse primer 5′-GGA TCCTAATACGACTCACTATAGGGAGGGTGTCAGCCCCAGCTC AATCAAG-3′ for antisense template; forward primer 5′-GGATCCTA ATACGACTCACTATAGGGAGGCCAGGAAGCTGATGGCTACG AC-3′, reverse primer 5′-GTGTCAGCCCCAGCTCAATCAAG-3′ for sense template (the underlined bases are the T7 polymerase tag and anchor regions). An in vitro transcription reaction using amplified template was used to generate 35S-labeled arc antisense or sense probe. The in vitro transcription was carried out in 1 × Transcription Optimized Buffer, 10 mm DTT, 1 U/μL RNasin, 0.375 mm ATP, CTP, GTP, 1 U/μL T7 RNA polymerase (all Promega, Madison, WI, USA), 3.5 μCi/μL [α-35S]UTP (PerkinElmer) and 100 ng template DNA and incubated for 2 h at 37 °C. RQ1 RNase free DNase (Promega) was added at a concentration of 0.15 U/mL and incubated for an additional 15 min at 37 °C. The labeled probes were purified using ProbeQuant G-50 Micro columns (Amersham Biosciences, Piscataway, NJ, USA). Radioactivity of labeled probe was determined via scintillation counting and riboprobe was diluted in hybridization solution (3 × SSC, 10% dextran sulphate, 1× Denhardt’s solution, 0.2 mg/mL tRNA, 50 mm NaPO4 buffer, and freshly added DTT to a final concentration of 50 mm) to ~106 c.p.m./100 μL. One hundred microlitres of the hybridization solution at 55 °C with labeled probe was applied to each slide. Slides were then coverslipped and incubated at 55 °C in a hybridization chamber saturated with 75% formamide for 16 h.
After hybridization, coverslips were removed and slides were washed in 2× SSC with 2 mm DTT at room temperature for 10 min, three times. Slides were incubated in 1.5 U/mL RNase A (Qiagen) in RNase buffer (10 mm Tris/HCl and 0.5 m NaCl, pH 8.0) at 37 °C for 1 h followed by washes in 1 × SSC with 1 mm DTT at room temperature for 5 min, 0.5 × SSC with 1 mm DTT at room temperature for 5 min and 0.1 × SSC with 2 mm DTT at 70 °C for 1 h. The sections were then dehydrated in an ascending series of ethanol and then air dried. Sections were exposed to a phosphorimager screen for 4 days. Screens were scanned on a Storm phosphorimager and quantification of signal in particular brain regions (Fig. 1B) was performed using ImageQuant software as with the Northern blot. Values for each hemisphere were averaged together in order to arrive at a single value for each region and each rat. Slides were then covered with NTB2 emulsion (Eastman Kodak Co., Rochester, NY, USA) and exposed for 28 days for analysis of silver grain distribution. After development, slides were counterstained with Nissl stain and dehydrated through a graded series of ethanol and xylene. A coverslip was then applied. Images were taken with a Leica DC 300F digital camera linked to Image Pro-Plus software on a PC through a Leica DMRX microscope.
Data analysis
Behavioral data from the activity cages were analysed using StatView software (SAS Institute, Cary, NC, USA). For the 14-day nicotine treatment, a two-factor, between–within analysis of variance (anova) was performed with treatment as the between-subjects factor and days as the within-subjects factor. For the conditioning (test) day, a two-factor, between–within anova was performed with treatment as the between-subjects factor and time (intervals) as the within-subjects factor. For the arc in situ hybidization data, quantification of phosphorescence was performed using ImageQuant software as described above. The areas analysed were chosen based on the regions previously implicated in our drug-conditioning paradigm (Schroeder et al., 2001). We focused the active search within these specified regions; however, the analysis performed does not preclude the involvement of other brain regions (see Fig. 1B for a depiction of analysed areas). The average pixel intensities for each region were entered into StatView and were analysed first by performing a two-way between–within anova with treatment as the between-subjects factor and brain region as the within-subjects factor. After determining whether a significant group effect or interaction was present, comparisons between individual experimental and control brain regions were performed using a one-way between-subjects anova. Because different coronal levels of the rat brain were run through ISH at different times, all data from ISH were normalized to the control values for each region analysed. Presentation of the data in this manner is meant to avoid inappropriate comparisons of arc mRNA levels between regions.
For qualitative analysis of emulsion autoradiography, images were captured at a magnification of 100× from sensorimotor cortex or 630× from prelimbic and lateral orbital prefrontal cortices. All images were processed using Adobe Photoshop.
Results
During the 14-day treatment period, nicotine had a strong stimulatory effect on locomotor activity (Fig. 2A). Horizontal activity (F1,22 = 16.919, P < 0.0005) and ambulatory activity (F1,22 = 17.008, P < 0.0004) were significantly increased in rats receiving nicotine compared with rats receiving saline injections in context 1 (only horizontal activity is shown in Fig. 2A). The behavioral activation following increasing nicotine dose administration became progressively greater over time (Fig. 2A), reflected by day × time interactions that were significant for horizontal activity (F13,286 = 20.418, P < 0.0001), ambulatory activity (F13,286 = 18.874, P < 0.0001) and rearing (F13,286 = 4.825, P < 0.0001).
Fig. 2.
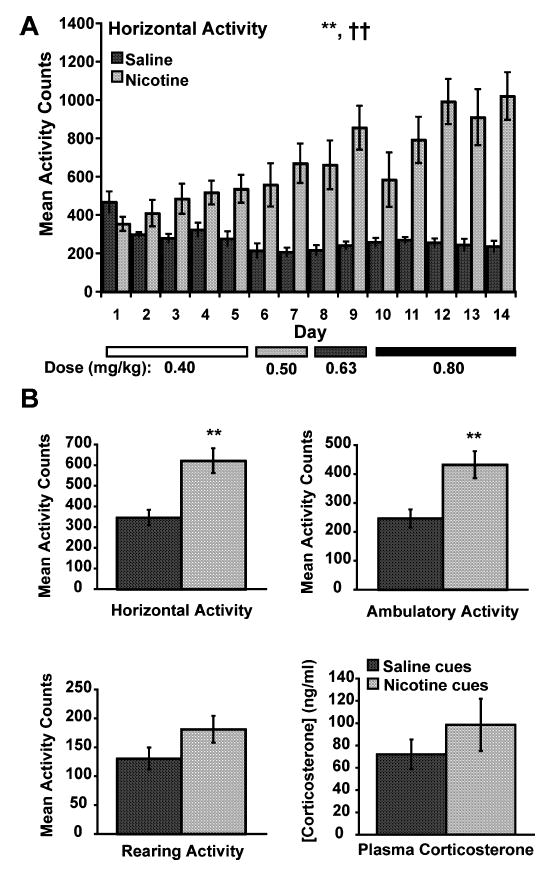
Motor activity data during conditioning and motor activity and corticosterone data during the test day. (A) Mean total horizontal activity (± SEM) during the 14 days of training in context 1 for rats receiving saline or nicotine (n = 12 per group). The bars at the bottom of the graph designate the dose of nicotine the rats received for the overlying days. Rats receiving nicotine in context 1 exhibited greater total horizontal activity than rats receiving saline injections in context 1 (**P < 0.001, see text for statistical details). There was a significant treatment × day interaction in the nicotine group (††P < 0.0001). (B) Conditioned mean total horizontal activity (upper left), mean ambulatory activity (upper right), mean rearing activity (lower left) and mean plasma corticosterone (lower right) on the test day (± SEM). Rats exposed to nicotine cues (n = 12) exhibited significantly higher activity counts for total horizontal activity and ambulatory activity (**P < 0.01, see text for statistical details) compared with rats exposed to saline cues (n = 12). Levels of plasma corticosterone did not differ between rats exposed to saline cues and nicotine cues.
Nicotine treatment also resulted in the development of a conditioned motor response (Fig. 2B). When the rats were re-exposed to the nicotine- or saline-paired environment 3 days later without any drug treatment, rats previously treated with nicotine in context 1 showed significant enhancement of motor activity (horizontal activity F1,22 = 15.223, P < 0.0008; ambulatory activity F1,22 = 10.938, P < 0.0032; rearing F1,22 = 2.849, P < 0.1056). The conditioned motor activation was not accompanied by a significant difference in plasma corticosterone in the two groups of rats (F1,14 = 0.967, P < 0.3421; Fig. 2B, lower right panel).
To determine whether changes in gene expression accompanied the conditioned motor response to nicotine-paired cues, we examined expression of the mRNA for the activity-regulated, cytoskeletal-associated protein Arc. Northern blot analysis indicated that arc levels in the ventrolateral orbitofrontal cortex were increased in rats exposed to nicotine cues compared with rats exposed to saline cues (Fig. 3A and B; F1,18 = 6.933, P < 0.0169). There was also a strong positive correlation between conditioned total horizontal activity and the arc mRNA phosphorescence signal (Fig. 3Cy = 0.581 + 0.0011x; R2 = 0.582, t = 5.009, d.f. = 18, P < 0.0001).
Fig. 3.
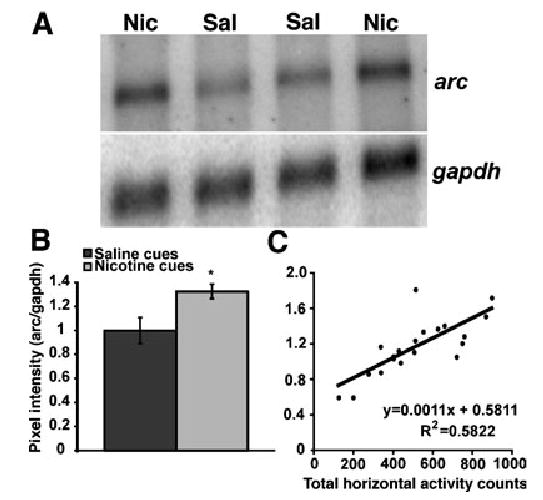
Northern blot analysis of arc mRNA in lateral prefrontal cortex. (A) Representative Northern blot of RNA from the lateral prefrontal cortex of two rats exposed to nicotine cues and two rats exposed to saline cues probed for arc mRNA (upper panel) and normalized to GAPDH mRNA levels (lower panel). Sal = saline cues, Nic = nicotine cues, gapdh = glyceraldehyde-3-phosphate dehydrogenase mRNA. (B) Average pixel intensity of arc signal normalized to gapdh (± SEM) for the saline cues (n = 10) and the nicotine cues (n = 10) groups. mRNA from one rat in the saline cues and in the nicotine cues group each was not included for quantification and statistical analysis due to obvious degradation of the samples (data not shown). A one-way anova indicated an increased level of arc mRNA in the lateral prefrontal cortex of the nicotine cues group compared with the saline cues group (*P < 0.05, see text for statistical details). (C) Total horizontal activity vs. normalized arc mRNA levels in lateral prefrontal cortex. Arc levels in lateral prefrontal cortex are positively correlated with total horizontal activity (R2 = 0.5822, t = 5.009, d.f. = 18, P < 0.0001).
To expand our analysis of arc mRNA expression during the conditioned response, we used ISH histochemistry so that multiple brain regions could be examined for potential changes in arc mRNA levels. Arc expression was measured in selected brain regions (Fig. 1B) 45 min following exposure to the activity cage environment previously associated with nicotine or saline administration using ISH with a 35S-labeled arc antisense riboprobe. Sections from representative animals in the saline- and nicotine-cue groups are shown in Fig. 4A–L. Sections on which ISH with arc sense probe were performed were devoid of signal (data not shown). The overall between–within analysis of the arc data revealed a significant environment effect (F1,12 = 38.717, P < 0.0001), as well as a significant environment × brain region interaction (F10,120 = 2.283, P < 0.0174). Further analysis revealed significant increases in arc mRNA in several cortical, striatal and limbic brain regions (Fig. 4M) including lateral prefrontal cortex (ventral orbital and lateral orbital cortices) (F1,12 = 27.545, P < 0.0002), medial prefrontal cortex (including Cg1, prelimbic and infralimbic cortices) (F1,12 = 19.106, P < 0.0009), anterior cingulate cortex (F1,12 = 11.979, P < 0.0047), primary sensory cortex (F1,12 = 10.721, P < 0.0066), posterior cingulate cortex (F1,12 = 9.301, P < 0.0101), ventral striatum (F1,12 = 7.066, P < 0.0209), sensorimotor cortex (F1,12 = 10.677, P < 0.0067) and amygdala (F1,12 = 12.228, P < 0.0044). Trends for increased arc mRNA signal existed in agranular insular cortex (F1,12 = 3.216, P < 0.0982) and the septum (F1,12 = 3.697, P < 0.0786). There were no significant changes in arc mRNA expression in the posterior dorsal striatum (F1,12 = 1.449, P < 0.2518), indicating that these findings were not simply a result of a generalized increase in arc mRNA expression in the brain. Graphical representation of these data is shown in Fig. 4m.
Fig. 4.
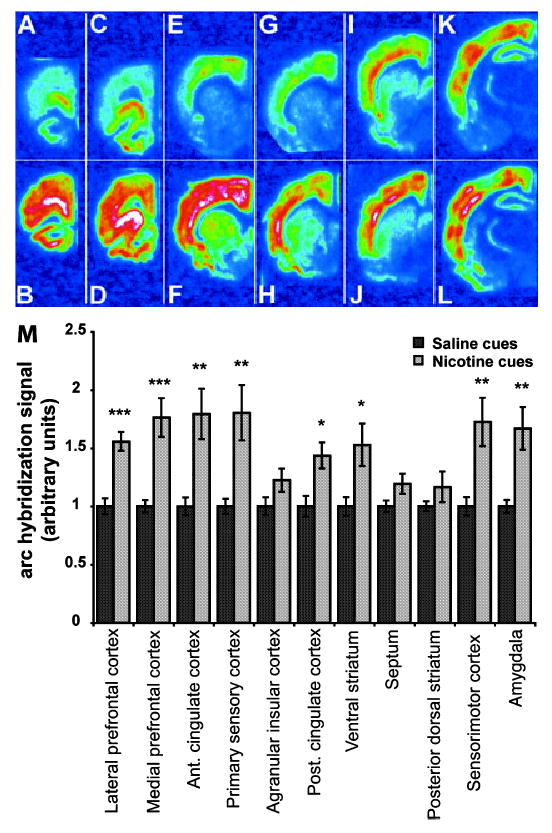
In situ hybridization analysis for the immediate-early gene arc in coronal sections from the brains of rats exposed to saline cues or nicotine cues. (A–L) Representative phosphorescence images of coronal sections hybridized with 35S-labeled arc antisense riboprobe from which pixel intensity measurements were taken. (A, C, E, G, I and K) Brain sections from a rat exposed to saline cues. (B, D, F, H, J and L) Brain sections from a rat exposed to nicotine cues. (A and B) Lateral prefrontal cortex. (C and D) Medial prefrontal cortex. (E and F) Anterior cingulate cortex, primary sensory cortex and agranular insular cortex. (G and H) Posterior cingulate cortex and ventral striatum. (I and J) Septum, posterior dorsal striatum and sensorimotor cortex. (K and L) Amygdala. Note how arc mRNA expression is recruited in upper layers of cortex especially in E compared with F, I compared with J, and K compared with L. (M) Mean arc hybridization signal intensity (± SEM) in the saline-paired (n = 7) and nicotine-paired (n = 7) groups for each area analysed (anova: P < 0.01. *P < 0.05, **P < 0.01, ***P < 0.001, post-hoc one-way anova). Signal intensities from the brain regions of rats exposed to saline cues and nicotine cues were normalized to the average value of the saline cues group for each brain region. Presentation of the data in this manner is meant to prevent inappropriate comparisons of arc expression between regions as ISH was carried out on different areas at different times.
Emulsion autoradiography of forebrain sections revealed that arc was expressed primarily in cells that contained large, pale stained nuclei (arrows in Fig. 5A–D). A dramatic increase in arc, as evidenced by enhanced silver grain accumulation, was apparent in the prelimbic region of medial prefrontal cortex (Fig. 5A and B) and the ventral orbital region of the lateral prefrontal cortex (Fig. 5C and D) following exposure to nicotine cues. These labeling patterns are consistent with the expression of arc in neurons. Autoradiographic analysis of sensorimotor cortex further revealed silver grain accumulation over cells in layers II–IVand enhanced expression in layers V–VI in animals exposed to nicotine cues (Fig. 5F). Control arc expression was confined primarily to layers V–VI in animals exposed to saline cues (Fig. 5E).
Fig. 5.
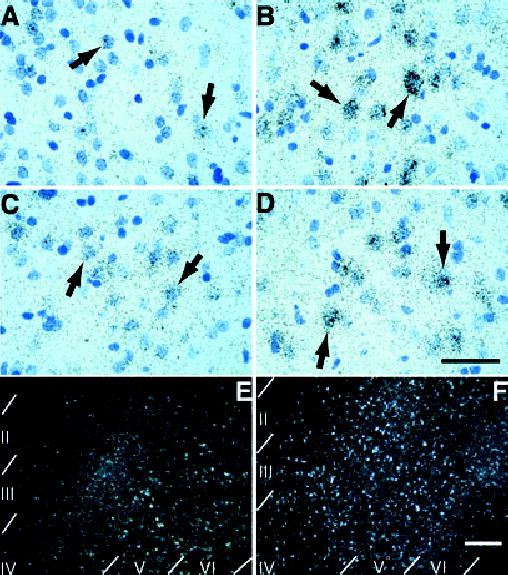
Emulsion autoradiography of forebrain cortical regions hybridized to arc antisense probe. (A and B) Silver grains in layer V/VI of the prelimbic regions of the prefrontal cortex accumulate preferentially over cells with lightly stained, large nuclei (arrows in A and B) in a rat exposed to nicotine cues (B) compared with saline cues (A). This silver grain pattern is consistent with neuronal localization of induced arc (scale bar, 50 μm for A–D). (C and D) Silver grains in layers V/VI of the lateral orbital prefrontal cortex also accumulate preferentially over cells with lightly stained, large nuclei (arrows in C and D) in a rat exposed to nicotine cues (D) compared with saline cues (C). This silver grain pattern is again consistent with neuronal localization of induced arc. (E and F) Lower power magnification, dark-field photomicrographs of sensorimotor cortex (corresponding to Fig. 4I and J). Recruitment of cells expressing arc occurs preferentially in layers II–IV in the sensorimotor cortex of rats exposed to nicotine cues (F) compared with rats exposed to saline cues (E). This laminar pattern of expression was evident in most cortical areas analysed (scale bar, 200 μm for E and F).
Discussion
This paper describes an elevation in arc mRNA expression in specific regions of rat forebrain following the reintroduction of rats into an environment previously paired with nicotine administration. It is the first report that illustrates a nicotine-paired, context-elicited increase in the expression of mRNA encoding a protein (Arc) that is directly involved in modifying postsynaptic signaling efficacy. Changes in the expression levels of arc occurred in neuronal populations within specific corticolimbic regions involved in memory, cognitive functions and reward processing, and imply that pronounced signaling and biochemical changes within these regions occur during exposure to nicotine cues.
The importance of Arc in synaptic plasticity is well documented. Arc mRNA levels are increased by neuronal activity in an NMDA receptor-activation-dependent manner in the hippocampus (Steward & Worley, 2001b). Arc message is trafficked to activated dendrites (Steward & Worley, 2001a) where it is presumably translated by temporally and spatially defined intracellular signals (Steward & Worley, 2002). Arc protein interacts with elements of the cytoskeleton including microtubules and microtubule-associated protein-2 (Fujimoto et al., 2004) and F-actin (Lyford et al., 1995), suggesting that it plays a role in the organization of the cytoskeleton–plasma membrane interface. More recently, Arc has been shown to interact with PSD-95 and CaMKII (Okuno et al., 2004) and the endocytic machinery responsible for trafficking AMPA receptors (Shepherd et al., 2004). These observations support a role for Arc in directly modifying postsynaptic properties responsible for membrane excitation. This role stands in contrast to other immediate-early genes, such as c-Fos (Morrow et al., 1999), that exert their indirect effects on synaptic plasticity and memory by regulating the transcription of other genes.
Arc appears to play a role in classical and operant conditioning. Up-regulation of arc has been observed in a variety of brain regions and the anatomical distribution of its induction depends on the type of stimulus or the type of experience provided to rats. Rats in the early stages of learning how to press a lever for a food reward have higher levels of arc mRNA and protein in brain regions known to be involved in the consolidation of this instrumental behavior compared with rats lever pressing for food but that have already learned how to perform the task (Kelly & Deadwyler, 2002, 2003). Expression of arc in hippocampal neurons depends on the environment in which a rat is placed and it depends on how familiar the rat is with the environment (Guzowski et al., 1999, 2001; Vazdarjanova et al., 2002). Blockade of Arc expression by local infusion of antisense in the hippocampus blocks long-term potentiation and long-term memory, but not acquisition, of a hippocampus-dependent, spatial memory task in rats (Guzowski et al., 2000). Arc is also expressed in response to acute treatment with cocaine (Fosnaugh et al., 1995), acute or chronic treatment with amphetamine (Kodama et al., 1998) and chronic treatments with morphine (Marie-Claire et al., 2004). The environmental context as well as the rate of psychostimulant administration (two factors influencing behavioral sensitization and drug conditioning) modulate the up-regulation of arc (Klebaur et al., 2002; Samaha et al., 2004). The changes in expression of arc in response to drugs of misuse and paired sensory stimuli are thought to underlie some of the changes in behavior associated with repeated drug treatment such as sensitization and tolerance, dependence and withdrawal, and the development of conditioned responses to drug-related stimuli (Ujike et al., 2002). Therefore, the elevation of arc message we observe during the first non-reinforced exposure to the nicotine-paired context may reflect new learning that this context is no longer necessarily predictive of nicotine administration. Arc expression in the mesocorticolimbic system, then, may play a role in the extinction of the conditioned response to the nicotine-paired context.
Remarkable similarities between arc expression patterns and neural activation profiles were found between this study and human neuroimaging studies of cue-elicited drug craving. These similar neural activation profiles extend across cues associated with administration of drugs of misuse from various chemical classes. For example, subregions of the prefrontal cortex in human addicts are activated in response to cigarette (Brody et al., 2002; Due et al., 2002), cocaine (Grant et al., 1996; Maas et al., 1998; Wang et al., 1999; Garavan et al., 2000; Bonson et al., 2002) and alcohol cues (George et al., 2001; Wrase et al., 2002; Tapert et al., 2003; Tapert et al., 2004). Subregions of the cingulate cortex are responsive to cues associated with cigarettes (Brody et al., 2004), cocaine (Maas et al., 1998; Childress et al., 1999; Garavan et al., 2000; Kilts et al., 2001; Wexler et al., 2001), alcohol (Tapert et al., 2003) and opiates (Daglish et al., 2001). The ventral striatum is activated in addicted individuals in response to cigarette (Due et al., 2002) and cocaine cues (Kilts et al., 2001, 2004). The amygdala also exhibits increased activity after presentation of cues associated with cigarettes (Due et al., 2002), cocaine (Grant et al., 1996; Childress et al., 1999; Kilts et al., 2001; Bonson et al., 2002) or alcohol (Schneider et al., 2001). The increases in activity within these structures are often correlated with increases in self-reported drug-craving. We observe a similar correlation between conditioned locomotor hyperactivity and arc expression in the lateral prefrontal cortex. These similarities suggest that the paradigm used here for drug-cue-conditioned responses is an appropriate model for studying cue-elicited drug craving at a cellular and molecular level.
One criticism of the positive correlation between conditioned motor activity and immediate-early gene expression in the prefrontal cortex is that the increased motor activity caused the increase in immediate-early gene expression within the prefrontal cortex. We find this interpretation unlikely because rats exposed to cues associated with highly palatable food have increased Fos expression in prefrontal cortical areas but have no increased motor activity (Schroeder et al., 2001). In addition, rats exposed to cues associated with the anxiogenic drug yohimbine showed no alteration in Fos levels in prefrontal cortical areas yet exhibited a robust conditioned motor activation (Schroeder et al., 2003). We favor the interpretation that the increased neuronal activity resulting in changes in immediate-early gene expression in prefrontal cortical areas (as well as other areas) to drug-associated cues is responsible for the behavioral conditioned response in rats and the psychological conditioned response in humans. This interpretation is further supported by studies showing disruption of appetitive conditioned responses after lesions in prefrontal cortical subregions (Isaac et al., 1989; Pears et al., 2003).
Activity within subregions of the medial and lateral prefrontal cortex (McLaughlin & See, 2003; Fuchs et al., 2004b), nucleus accumbens (Fuchs et al., 2004a) and amygdala (Fuchs & See, 2002; Kantak et al., 2002; McLaughlin & See, 2003) is responsible for cue-induced reinstatement of drug-seeking behavior in animal models of relapse. Cue-induced reinstatement also elicits immediate-early gene expression in these corticolimbic areas (Ciccocioppo et al., 2001). The observations that activity-dependent changes in gene expression in these brain areas parallel activation in human addicts suggest that related processes within corticolimbic circuits are occurring during cue-induced reinstatement as well as during expression of classically conditioned responses to drug cues.
The role of stress in drug-cue-conditioned responses is unclear. Because stress itself can interact with drug-paired cues to elicit or enhance drug-seeking behavior (Liu & Weiss, 2002), it becomes important to understand if stress plays a role in the conditioned response to nicotine-paired cues. We found no appreciable increase in plasma corticosterone in rats exposed to nicotine cues compared with rats exposed to saline cues. Furthermore, plasma corticosterone levels were not correlated with motor activity on the test day or with arc expression in the lateral prefrontal cortex (correlations not shown). Plasma corticosterone levels have been shown to increase in rats during cue-elicited reinstatement of cocaine-seeking behavior (Goeders & Clampitt, 2002) and during exposure to a cocaine-paired context (DeVries et al., 1998). Further work will be needed to determine if the differences in hypothalamic-pituitary-adrenal (HPA) activation in response to nicotine vs. cocaine cues are due to differences in the pharmacological and conditioning properties of nicotine and cocaine or to differences in methodology between studies. Nonetheless, exposure to nicotine cues in this paradigm seems not to activate the HPA axis and stress, per se, seems to not contribute to the behavioral or cellular response to nicotine cues.
The enhanced expression of arc in layers II–VI in cortical regions involved in the conditioned response to nicotine cues is of interest because principal cells from different layers have differing inputs and outputs. In the parietal cortex, hippocampus and dentate gyrus, arc is expressed in excitatory neurons whereas in the striatum arc is expressed in inhibitory neurons (Vazdarjanova et al., 2004). The pattern of arc expression elicited by nicotine cues suggests a distributed cortical and subcortical neuronal activation possibly resulting in plasticity of both excitatory and inhibitory principal neurons within the circuit.
Conditioned responses to cues associated with drug administration play a role in relapse to drug use. Here we report an animal model that can be used to study classically conditioned responses to cues previously paired with nicotine administration. Expression of at least two immediate-early genes (Fos and arc) has now been shown to increase in a variety of corticolimbic areas in response to nicotine cues. As these genes are known to participate in long-term memory formation (by differing mechanisms), their induction during the first unpaired exposure to conditioned stimuli (i.e. cues associated with nicotine administration) likely signifies that regions involved in the conditioned response may be undergoing plastic changes in order to store information that cues previously paired with nicotine are no longer necessarily predictive of nicotine administration. This hypothesis is especially interesting in terms of addiction and prefrontal cortical function as it relates to decision making, perseverance, reversal learning (Birrell & Brown, 2000; McAlonan & Brown, 2003) and extinction of conditioned responses (Morgan & LeDoux, 1995; Milad & Quirk, 2002; Herry & Mons, 2004). Neuronal activity in the prefrontal cortex thus may not only have a role in the manifestation of conditioned responses to drug-paired cues but also, upon non-reinforced presentation of these cues, a role in the eventual extinction of aspects of the conditioned response to the drug-paired cues. Indeed, in a recent study, extinction training was capable of reversing biochemical neuroadaptations within areas of the mesocorticolimbic system that are a result of chronic drug self-administration, like increased tyrosine hydroxylase immunoreactivity within the nucleus accumbens shell (Self et al., 2004). More work will be necessary to ascertain the role of neuronal activity and immediate-early gene expression in corticolimbic circuits on drug-induced biochemical neuroadaptations and subsequent behavior in response to drug-related cues.
Acknowledgments
This work was supported by grants DA13780 from the National Institute on Drug Abuse and F30 DA016503 to C.A.S. from the National Institute of Drug Abuse.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995;117:443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002a;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002b;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res. 1998;786:39–46. doi: 10.1016/s0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Thomas MJ, Robinson TE. Morphine-induced c-fos mRNA expression in striatofugal circuits: modulation by dose, environmental context, and drug history. Neuropsychopharmacology. 2004;29:1664–1674. doi: 10.1038/sj.npp.1300465. [DOI] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative ‘effector’ immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004a;24:6600–6610. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Tanaka H, Kumamaru E, Okamura K, Miki N. Arc interacts with microtubules/microtubule-associated protein 2 and attenuates microtubule-associated protein 2 immunoreactivity in the dendrites. J Neurosci Res. 2004;76:51–63. doi: 10.1002/jnr.20056. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamopituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci. 1989;103:345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–626. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Ostrander MM, Norton CS, Watson SJ, Akil H, Robinson TE. The ability of amphetamine to evoke arc (Arg 3.1) mRNA expression in the caudate, nucleus accumbens and neocortex is modulated by environmental context. Brain Res. 2002;930:30–36. doi: 10.1016/s0006-8993(01)03400-x. [DOI] [PubMed] [Google Scholar]
- Kodama M, Akiyama K, Ujike H, Shimizu Y, Tanaka Y, Kuroda S. A robust increase in expression of arc gene, an effector immediate early gene, in the rat brain after acute and chronic methamphetamine administration. Brain Res. 1998;796:273–283. doi: 10.1016/s0006-8993(98)00349-7. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Science-based views of drug addiction and its treatment. J Am Med Assoc. 1999;282:1314–1316. doi: 10.1001/jama.282.14.1314. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Courtin C, Roques BP, Noble F. Cytoskeletal genes regulation by chronic morphine treatment in rat striatum. Neuropsychopharmacology. 2004;29:2208–2215. doi: 10.1038/sj.npp.1300513. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Inglis FM, Roth RH. An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. J Neurosci. 1999;19:5666–5673. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Chowdhury S, Worley P, Bito H. Interaction between Arc and CaM Kinase II in dendrites monitored by fluorescence resonance energy transfer. Soc Neurosci Abstracts. 2004:164.16. [Google Scholar]
- Ostrander MM, Badiani A, Day HE, Norton CS, Watson SJ, Akil H, Robinson TE. Environmental context and drug history modulate amphetamine-induced c-fos mRNA expression in the basal ganglia, central extended amygdala, and associated limbic forebrain. Neuroscience. 2003;120:551–571. doi: 10.1016/s0306-4522(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Paxinos, G. & Watson, C. (1998) The Rat Brain in Stereotaxic Coordinates Academic Press, San Diego.
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Coon SL, Baler R, McCune SK, Weller JL, Klein DC. Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137:3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Chowdhury S, Petralia R, Huganir R, Worley P. Arc modulates AMPA receptor trafficking via its interation with the endocytic machinery. Soc Neurosci Abstracts. 2004:971.15. [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci USA. 2001a;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001b;30 :227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–527. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann NY Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Norton CS, Watson SJ, Akil H, Robinson TE. Amphetamine-induced c-fos mRNA expression in the caudate-putamen and subthalamic nucleus: interactions between dose, environment, and neuronal phenotype. J Neurochem. 2003;85:105–114. doi: 10.1046/j.1471-4159.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Worley PF, Guzowski JF, Barnes CA. Behavior induces expression of the plasticity-related immediate-early gene Arc in excitatory and inhibitory CaMKII-positive neurons. Soc Neurosci Abstracts. 2004:329.14. [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]


