Abstract
Bacteria communicate with chemical signal molecules called autoinducers. This process, called quorum-sensing, allows bacteria to count the members in the community and to synchronously alter gene expression of the population. Quorum-sensing-controlled processes are often crucial for successful bacterial-host relationships; both symbiotic and pathogenic. Most quorum-sensing autoinducers promote intra-species communication, but one autoinducer, called AI-2, is produced and detected by a wide variety of bacteria and is proposed to allow inter-species communication1,2. We show here that some species of bacteria can manipulate AI-2 signalling and interfere with other species’ ability to correctly assess and respond to changes in cell population density. AI-2-signalling and interference with it could have important ramifications for eukaryotes in maintaining normal microflora and in protection from pathogenic bacteria.
The bacterial signal molecule called Autoinducer-2 (AI-2) is a product of the LuxS enzyme which is broadly conserved throughout the bacterial world. LuxS enzymes synthesize 4,5-dihydroxy 2,3-pentanedione (DPD) which undergoes spontaneous rearrangements3. Importantly, DPD derivatives interconvert and exist in equilibrium. Different bacteria recognize distinct DPD derivatives, and this family of molecules is generically called AI-24. The interconverting nature of these molecules presumably allows bacteria to respond to their own AI-2 and also to AI-2 produced by other bacterial species, giving rise to the idea that AI-2 represents a universal language fostering inter-species bacterial communication.
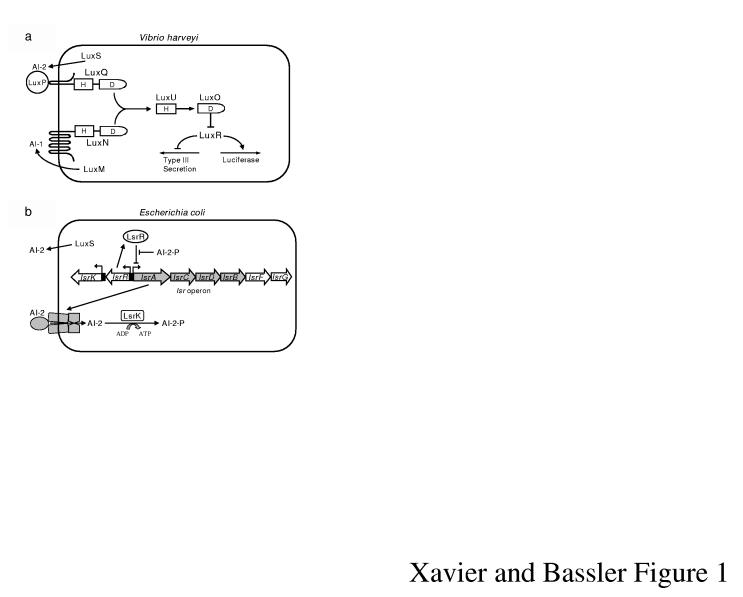
We characterized the quorum-sensing signal production and detection apparatuses in Vibrio harveyi, Vibrio cholerae, Escherichia coli, and Salmonella typhimurium5-8. In V. harveyi, two autoinducers, AI-1 and AI-2, are detected by LuxN and LuxPQ, respectively, and control expression of genes including those for bioluminescence and Type III secretion (TTS) of virulence factors (Fig. 1a)5,9. A related quorum-sensing network exists in V. cholerae and controls expression of virulence genes including hapA encoding the haemagglutinin (H/A) protease6,10.
Figure 1.
AI-2 Signalling Systems. a, V. harveyi quorum sensing. The autoinducers AI-1 and AI-2 are detected by LuxN and LuxPQ, respectively. Information is transduced by phosphorylation. b, The E. coli Lsr transporter imports AI-2. See text for details.
Some bacteria produce and consume AI-2. For example, E. coli and S. typhimurium release AI-2 in exponential phase, and import AI-2 at the transition into stationary phase. This occurs because one target that is activated by AI-2 is the Lsr (for LuxS Regulated) transporter that imports AI-2 (Fig. 1b)7,8. In the absence of AI-2, LsrR represses the lsr operon. Following AI-2 release, low-level internalization occurs, and intracellular AI-2 is phosphorylated by LsrK8,11. Phospho-AI-2 (AI-2-P) antagonizes LsrR, which leads to de-repression of lsr expression, assembly of the Lsr transporter, and rapid AI-2 internalization. LsrR- strains are avid AI-2 consumers because the lsr operon is de-repressed. By contrast, LsrK- strains never consume significant AI-2 due to their inability to de-repress lsr transcription (Fig. 1b, supplementary Fig. 1S, and 2S)8,11.
In mixed-species consortia, production and consumption of AI-2 by enterics should have reciprocal effects on gene regulation in other bacterial species that communicate with AI-2. When the enterics supply AI-2, a nearby species could use the information to count the enteric cells in the mixed-species community or possibly prematurely activate the expression of quorum-sensing-regulated genes. However, when the enterics remove AI-2, a neighboring species could underestimate cell number and fail to initiate or incorrectly terminate quorum sensing. Thus, consuming AI-2 could allow enterics to interfere with AI-2-mediated communication in other bacteria.
When E. coli is grown in pure culture, AI-2 is required to activate lsr expression (Fig. 2a; compare WT to LuxS-). Induction of lsr expression also occurs when LuxS- E. coli is mixed with WT V. harveyi (third bar) and depends on the AI-2 supplied by V. harveyi because no lsr activation occurs when LuxS- E. coli is combined with LuxS- V. harveyi (fourth bar). Thus, E. coli detects and responds to its own AI-2 and also to that produced by V. harveyi.
Figure 2.
E. coli and V. harveyi communicate with AI-2. a, V. harveyi AI-2 induces the E. coli lsr operon. β-galactosidase activities of an E. coli lsr-lacZ fusion for E. coli mono-cultures and co-cultures with V. harveyi. Abbreviations: WT (AI-2+), LuxS- (AI-2-), β-gal units (Abs420min-1 × 109 ml-1)/( E. coli cfu ml-1). Error bars represent standard deviations. b, E. coli consumes AI-2 and inhibits V. harveyi bioluminescence expression. Light production in V. harveyi WT (black), LuxN- (gray), LuxQ- (white) in co-culture with E. coli WT, LsrR-, LsrK-. V. harveyi bioluminescence is the percentage of WT (140,000 counts per min (cpm) ml-1/( V. harveyi cfu ml-1).
We also examined the effects of E. coli AI-2 production and consumption on V. harveyi using bioluminescence as the readout. When incubated with WT E. coli, WT V. harveyi at high cell density produces only 18% of the light it produces in pure culture (Fig. 2b; left two black bars). Thus, E. coli consumes both its and V. harveyi’s AI-2 causing reduced light output by V. harveyi. Co-culture of WT V. harveyi with LsrR- E. coli (constitutive AI-2 importer) causes a greater reduction in V. harveyi bioluminescence (third black bar). Diminution of light is due exclusively to Lsr-mediated transport of AI-2 into E. coli because no reduction in light production occurs when V. harveyi is co-cultured with the LsrK- E. coli mutant that does not internalize AI-2 (fourth black bar).
In addition to AI-2, V. harveyi produces an autoinducer called AI-1 and responds to it via the LuxN sensor (Fig. 1a)5. V. harveyi LuxN- strains produce less light than WT because LuxN- strains induce bioluminescence only in response to AI-2 whereas WT responds to AI-1 and AI-2 (Fig. 2b; first gray bar). Growth of LuxN- V. harveyi with WT and LsrR- E. coli reduces light output to 2% and <1%, respectively (second and third gray bars). E. coli has a stronger effect on LuxN- V. harveyi than on WT V. harveyi because WT V. harveyi retains its response to AI-1, the levels of which are not altered by E. coli consumption of AI-2. When the LuxN- V. harveyi is co-cultured with the LsrK- E. coli mutant, increased light production occurs in response to the additional AI-2 provided by the internalization-defective E. coli (fourth gray bar). Finally, these AI-2 effects on V. harveyi require the LuxQ sensor (Fig. 1a) because manipulating extracellular AI-2 levels by co-culture with E. coli does not alter light production by the LuxQ- V. harveyi (Fig. 2b; white bars).
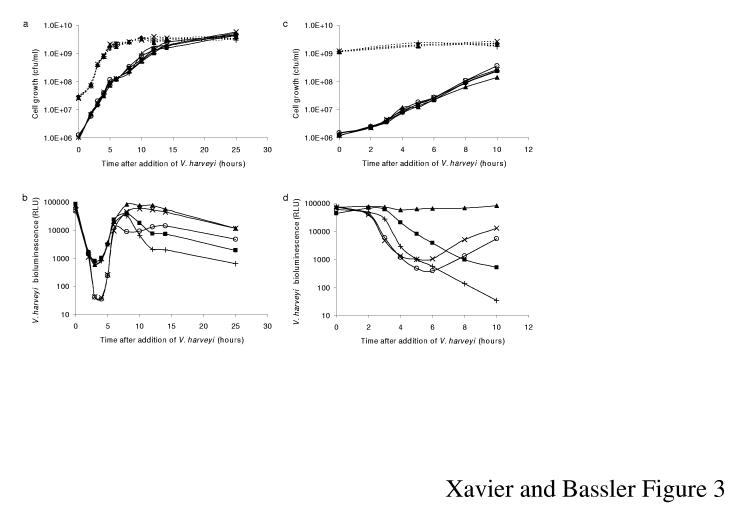
Dilution of V. harveyi in pure culture causes autoinducer levels to fall below that required for detection, and bioluminescence expression terminates at low cell densities. During subsequent growth, autoinducers are released. Upon achieving a threshold concentration, they are detected, and the cells respond by inducing a rapid increase in light production. Our premise is that in mixed cultures, early production and later consumption of AI-2 by E. coli should inversely affect the V. harveyi quorum-sensing response. To test this idea, we measured V. harveyi bioluminescence during growth in co-culture with E. coli. Both species were diluted to low cell density and combined under conditions allowing each to grow exponentially (Fig. 3a). V. harveyi growing with the non-AI-2-producing, non-AI-2-importing E. coli LuxS-, LsrK- strain shows the characteristic initial decline in bioluminescence followed by the rapid increase to the maximal, pre-dilution level (Fig. 3b; ×). In the presence of all AI-2+ E. coli strains tested, the initial decrease in bioluminescence is significantly reduced because V. harveyi rapidly initiates quorum sensing in response to the AI-2 supplied by E. coli (■,+,▲). At later times, corresponding to post-lsr induction, all E. coli strains that have LsrK, and are therefore capable of transporting AI-2, consume the AI-2 (■,+,○). This has the consequence of decreasing V. harveyi light output by almost 100-fold compared to when V. harveyi is grown in the presence of LsrK- strains that are unable to internalize AI-2 (▲,×). Mixing V. harveyi with the E. coli LsrR- strain results in the most pronounced reduction in light production presumably due to the constitutive removal of AI-2 (+).
Figure 3.
The E. coli Lsr system interferes with V. harveyi quorum sensing. a, Growth curves for strains in b. Solid and dotted lines denote V. harveyi and E. coli cfu, respectively. b, Light production for LuxN- V. harveyi in co-culture with E. coli strains: WT (■), LsrR- (+), LsrK- (▲), LuxS- (○), and LsrK- LuxS- (×). c, Growth curves for strains in d; see legend for a. d, LuxN- V. harveyi was diluted into E. coli cultures entering stationary phase. E. coli strains are identical to those in b. Relative light units (RLU) are cpm ml-1 103/(V. harveyi cfu ml-1).
A more dramatic effect on V. harveyi quorum sensing occurs when exponential phase V. harveyi encounters E. coli in stationary phase (Fig. 3c). In this case, at the time of mixing, WT E. coli is induced for lsr expression, and the cells are actively internalizing AI-2. An almost immediate and continuous decrease in V. harveyi bioluminescence occurs (Fig. 3d; ■), which is further exaggerated in the mixture containing the LsrR- E. coli (+). In stark contrast, in co-culture with stationary phase LsrK- E. coli, V. harveyi steadily maintains maximal light production (▲) due to the presence of AI-2 produced by the transport deficient E. coli mutant. As expected, in the presence of the LsrK-, LuxS- E. coli, V. harveyi displays density dependent bioluminescence (×). Mixing V. harveyi with LuxS- E. coli initially has no effect on V. harveyi quorum sensing because LuxS- E. coli has not been exposed to AI-2 prior to the addition of V. harveyi, so the Lsr transporter is repressed (○). During co-incubation, the V. harveyi-produced AI-2 induces LuxS- E. coli to express the Lsr transporter. E. coli consumes AI-2, causing a reduction in V. harveyi light production. Therefore, at late times, light output from the V. harveyi strain mixed with the E. coli LuxS- strain falls below that of V. harveyi co-incubated with the E. coli LsrK-, LuxS- strain.
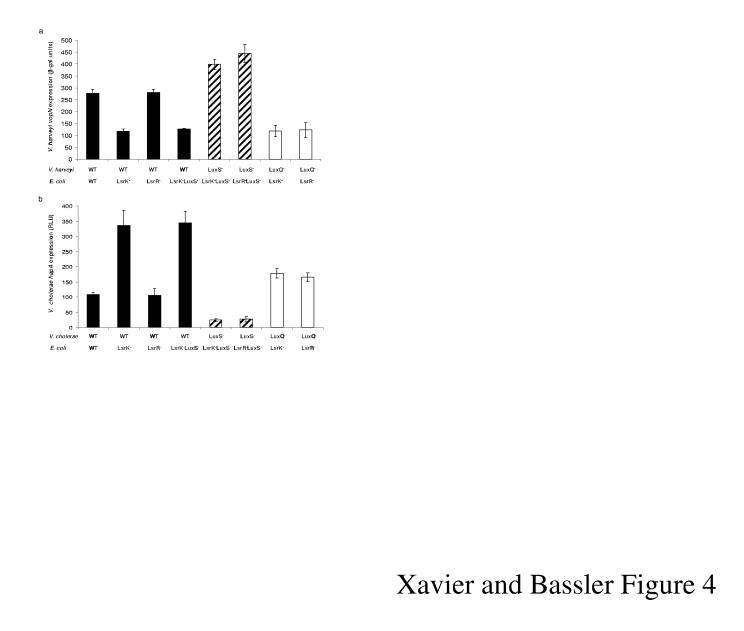
The V. harveyi quorum-sensing regulon contains many genes, and in consortia, all are presumably susceptible to effects of AI-2 production and consumption. To examine this possibility, the TTS gene vopN was measured in co-cultures of E. coli and V. harveyi. TTS genes are repressed by autoinducers at high cell density in V. harveyi (Fig. 1a)9. Production of AI-2 by E. coli incapable of importing AI-2 does not affect vopN expression showing that AI-2 supplied by V. harveyi is sufficient for maximal repression (Fig. 4a; black bars, compare LsrK- to LsrK-, LuxS-). Growth with E. coli strains that consume AI-2 (black bars, WT and LsrR-) results in de-repression of vopN. Full de-repression only occurs in combinations in which neither V. harveyi nor E. coli produces AI-2 (striped bars) demonstrating that in the mixtures depicted by the black bars, E. coli has not internalized all of the AI-2. As in Fig. 2a, AI-2 production and consumption does not affect vopN expression if V. harveyi lacks LuxQ (white bars).
Figure 4.
V. harveyi TTS and V. cholerae H/A protease production are regulated by E. coli Lsr-mediated AI-2 transport. a, V. harveyi TTS expression was measured using a vopN-lacZ fusion (18 h co-culture). b, V. cholerae hapA expression was measured with a hapA-lux fusion (16 h co-culture). Activities were measured in the following vibrio strains: WT, black bars; LuxS-, striped bars; LuxQ-, white bars. Each vibrio strain was grown in co-culture with the following E. coli strains: WT, LsrR-, LsrK-, and LuxS-, LsrK- double mutant. β-gal units and RLU are as in Fig. 2 and 3. Error bars denote the standard deviations.
We recognize that V. harveyi, which routinely lives in mixed-species marine communities, is not likely to co-exist in environments with E. coli. In contrast, V. cholerae must co-exist with E. coli during pathogenic associations with humans. To determine whether E. coli AI-2 consumption could interfere with V. cholerae signalling, we measured expression of the quorum-sensing-activated gene hapA, encoding the H/A protease. Indeed, our results mimic those acquired with TTS in V. harveyi except that the pattern of regulation is reversed (Fig. 4b). Induction occurs in mixtures containing E. coli strains incapable of AI-2 transport (LsrK- and LsrK-, LuxS-) and no induction occurs in mixtures containing E. coli strains that do transport AI-2 (WT, and LsrR-).
AI-2 consumption has a more modest effect on vopN and hapA regulation than on regulation of bioluminescence. Many environmental factors are known to control H/A protease production and TTS10,12. AI-2 interference likely has more subtle effects on H/A protease and TTS than on bioluminescence because, in the former cases, additional regulators exert control over the outputs, and regulation by these factors remains unchanged in our experiments.
Our results show that AI-2 can foster two-way communication between bacterial species because, when E. coli and V. harveyi are grown in co-culture, AI-2 production by either species can regulate light production in V. harveyi and trigger lsr induction in E. coli. Thus, AI-2 production allows each species to include the other in the ‘census’. Because E. coli and V. harveyi respond to AI-2s with different structures2,4, cross-communication implies that the signal released by one species must convert into that used by the other species. Presumably, these transformations occur in the medium between the sender and receiver cells. This is especially interesting given that the vibrios detect an AI-2 molecule containing boron whereas there is no boron in the AI-2 signal recognized by enterics2,4. Our results demonstrate that these drastic chemical interconversions are occurring on a time scale that promotes major effects on gene expression.
In model V. harveyi-E. coli mixtures, induction of lsr genes in E. coli results in assembly of the AI-2 transporter and subsequent consumption of AI-2. This has the consequence of inhibiting light production by V. harveyi demonstrating that E. coli interferes with AI-2-mediated communication by causing V. harveyi to prematurely terminate its quorum-sensing behaviors. The impact of the E. coli AI-2-Lsr system is not restricted to interference with activation of bioluminescence because repression of Type III secretion is also affected. These findings imply that interference with AI-2 signalling influences the expression of entire quorum-sensing regulons.
Lsr-mediated interference with AI-2 signalling also occurs in co-cultures of E. coli and V. cholerae, two bacteria that certainly jointly colonize the human intestine during V. cholerae disease. We do not expect these interactions to be restricted to enteric bacteria; indeed disappearance of AI-2 in stationary phase has been reported for diverse bacterial species indicating that, either Lsr transporters exist in these species, or additional mechanisms exist to eliminate AI-213. Any species of bacteria that relies on AI-2-mediated communication and inhabits niches containing another bacterial species that produces and/or consumes AI-2 could be similarly affected. Whether quorum sensing is enhanced or inhibited will depend on the growth status of the different species when they encounter one another. Complex pro- and anti-AI-2-mediated interactions could be taking place in natural niches. Possibly, eukaryotes have capitalized on this by evolving specific associations with bacteria that use or manipulate AI-2 signalling; and these interactions could have important human consequences in the maintenance of the normal human gut microflora as well as in the prevention of bacterial diseases.
Methods
Bacterial Strains and Growth Conditions. Bioluminescence was measured in V. harveyi strain BB120 (WT)1, MM30 (luxS)14, BB170 (luxN)15, and BB960 (luxQ)5. E. coli strains are derivatives of MG165516. The E. coli strains used in Fig. 2, 3, and supplementary Fig. 1S, and 2S contain a lacZYA deletion and an lsr-lacZ fusion integrated at the att site. These strains are KX1123 (WT), KX1218 (luxS), KX1186 (lsrK), KX1322 (lsrR), and KX1372 (lsrK, luxS)8. To measure vopN expression, V. harveyi strains JMH385 (WT), KX1530 (luxS), JMH669 (luxQ), containing a chromosomal vopN::mini-MulacZ insertion were used9. The final two strains were obtained by introduction of luxS::Tn514, and luxQ::Tn55 onto the chromosome of JMH385, respectively. E. coli strains used in TTS experiments are KX1102 (WT), KX1479 (lsrK), KX1477 (lsrR), and KX1526 (luxS, lsrK) and each was constructed by the method reported8. V. cholerae strains are derivatives of El Tor C6706str2, a streptomycin-resistant isolate of C670617, and all have a hap-luxCDABE transcriptional fusion cloned into a plasmid containing a chlroamphenicol resistance (CmR) marker. The hap-luxCDABE transcriptional fusion was constructed as reported18 except that a PCR-amplified fragment containing the promoter region of V. cholerae hapA was cloned into unique SpeI to BamHI sites. The V. cholerae strains used are: BH1220 (WT), BH1312 (luxS), and BH1253 (luxQ). Details of their construction will be reported elsewhere (B. K. Hammer, B. L. Bassler, manuscript in preparation). E. coli strains used for co-culture with V. cholerae are identical to those described for Fig. 3 except for KX1583 (WT) contains a CmR marker downstream of the luxS gene. Addition of Cm was required in E. coli-V. cholerae co-cultures to maintain the plasmid harboring the hapA-luxCDABE fusion in V. cholerae. Co-cultures were grown in LM5 in experiments with V. harveyi, and in LB supplemented with Cm (10 mg/L) in experiments with V. cholerae. All cultures were incubated at 30°C with aeration.
β-Galactosidase Assays. Overnight cultures of E. coli and V. harveyi strains were diluted 1:1000 into LM and grown for 8 h after which lsr-lacZ expression was measured. When vopN-lacZ expression was measured, V. harveyi strains were diluted 1:5000 into LM and 1:100 dilutions of overnight cultures of E. coli were added. Cultures were grown for 18 h because expression of TTS genes is maximal in stationary phase. Cells from 1 ml of culture were resuspended in 1 ml of Z buffer for β-galactosidase assays19. The values shown represent the average of triplicates.
Bioluminescence Assays. Overnight cultures of V. harveyi strains were diluted 1:5000 into LM. In co-incubations, 1:100 dilutions of overnight cultures of E. coli were added. In the experiments shown in Fig 2b bioluminescence was measured after 12 h of incubation. In experiments in Fig. 3c and d the V. harveyi strains were added to E. coli cultures that had been pre-grown for 4 h in LM. At the reported times, aliquots were plated to determine cfu, and bioluminescence was measured using a liquid scintillation counter (Wallac model 1409).
Analysis of hapA-luxCDABE Expression. Overnight cultures of E. coli and V. cholerae were diluted 1:1000 into LB supplemented with Cm and grown for 16 h. Aliquots were plated to determine cfu, and luciferase activity from the hapA-luxCDABE fusion was measured in a liquid scintillation counter. Values shown represent the average of triplicates.
Measurements of cell number in co-cultures. In all co-culture experiments, the mixtures of cells were plated on two different types of media. The media conditions were chosen so that they either permitted growth of only one of the two species in the mixture or the colonies of each species could be distinguished visually by morphology. This strategy allowed us to determine the cell numbers of both species in each co-culture experiment. V. harveyi cfu were assessed following overnight incubation at 30° on LM agar supplemented with ampicillin (100 mg/L). V. cholerae cfu were counted after incubation at 37°C on LB agar containing 50 U/L polymyxin B. E. coli cfu were counted following incubation at 37°C on LB. V. harveyi does not grow at 37°C and V. cholerae colonies are translucent under this condition whereas E. coli colonies are opaque.
Supplementary Material
Acknowledgements
This research was supported by NIH and NSF grants (B. L. Bassler) and a Praxis XXI postdoctoral fellowship, Portugal (K. B. Xavier). We thank the members of the Bassler lab, as well as Drs. Ned Wingreen and Frederick Hughson for insightful discussions. We are grateful to Dr. B. Hammer for V. cholerae strains and to Dr. J. Henke for V. harveyi TTS mutants.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
References
- 1.Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–5. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–9. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 3.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–76. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller ST, et al. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15:677–87. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–86. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–14. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 7.Taga ME, Semmelhack JL, Bassler BL. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol Microbiol. 2001;42:777–93. doi: 10.1046/j.1365-2958.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- 8.Xavier KB, Bassler BL. Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J Bacteriol. 2005;187:238–48. doi: 10.1128/JB.187.1.238-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henke JM, Bassler BL. Quorum Sensing Regulates Type III Secretion in Vibrio harveyi and Vibrio parahaemolyticus. J Bacteriol. 2004;186:3794–805. doi: 10.1128/JB.186.12.3794-3805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva AJ, Benitez JA. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS. J Bacteriol. 2004;186:6374–82. doi: 10.1128/JB.186.19.6374-6382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taga ME, Miller ST, Bassler BL. Lsr-mediated transport and processing of AI-2 in Salmonella typhimurium. Mol Microbiol. 2003;50:1411–27. doi: 10.1046/j.1365-2958.2003.03781.x. [DOI] [PubMed] [Google Scholar]
- 12.Hacker J, Kaper JB. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol. 2000;54:641–79. doi: 10.1146/annurev.micro.54.1.641. [DOI] [PubMed] [Google Scholar]
- 13.Xavier KB, Bassler BL. LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol. 2003;6:191–7. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 14.Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A. 1999;96:1639–44. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–86. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 16.Daniels DL, Plunkett G, Burland V, Blattner FR. Analysis of the Escherichia coli genome: DNA sequence of the region from 84.5 to 86.5 minutes. Science. 1992;257:771–8. doi: 10.1126/science.1379743. [DOI] [PubMed] [Google Scholar]
- 17.Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–6. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz DH, et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Slauch JM, Silhavy TJ. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991;173:4039–48. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.