Abstract
A series of experiments probed into the degree of chemosensory detection additivity exhibited by mixtures of ethyl propanoate and heptanoate in terms of their trigeminal detectability via nasal pungency (i.e., irritation) and eye irritation. Nasal pungency was tested in subjects lacking a functional sense of smell (i.e., anosmics) to avoid olfactory biases. First, we built concentration-detection functions for each chemical and sensory endpoint. Second, we used the data from the functions to prepare mixtures of the two compounds in complementary proportions, and suitable single-chemical standards, all of which should be equally detectable under a rule of complete additivity, i.e., independence of detection. Third, we compared the experimentally obtained detectability with that expected under such rule. The outcome revealed that, at a low detectability level (but still above chance), the mixtures showed complete additivity for both trigeminal endpoints. At a high detectability level (but below perfect detection), the mixtures showed complete additivity for nasal pungency but less than complete additivity for eye irritation. In the context of previous studies, the results consolidate a picture of higher degree of detection additivity at perithreshold levels in trigeminal than in olfactory chemoreception. The outcome presents another line of evidence suggesting broader chemical tuning in chemesthesis compared to olfaction.
Keywords: Trigeminal nerve, Concentration-detection functions, Eye irritation, Nasal pungency, Chemosensory irritation from mixtures
Introduction
Advances in molecular biology have provided a better understanding of some of the transduction mechanisms at play in sensory chemoreception, particularly in olfaction (Rawson and Gomez 2002) and, to a lesser extent, in chemesthesis (Bryant and Silver 2000). In the mucosae of the face, chemesthesis is principally mediated by the trigeminal nerve (Finger et al. 1999). Nociceptors present in free nerve endings of the trigeminal nerve are responsible for their chemical sensitivity. Nociceptors are present in axons belonging to C and Adelta fibers (Martin and Jessell 1991).
A relevant question in ocular and nasal trigeminal chemoreception of airborne chemicals is whether the stimulus can directly activate certain receptors or whether activation comes from release of mediators as a result of some degree of cellular injury produced by the stimulus. Mediators such as ATP, H+, and K+, among others, can then act upon specific receptors and ion channels to activate nociception (McCleskey and Gold 1999; Sutherland et al. 2000; Cook and McCleskey 2002). Alternatively, other chemicals can directly activate specific receptors in sensory neurons. Among these substances, capsaicin is probably the most widely studied (Szallasi 1994; Caterina et al. 1997; Szallasi and Blumberg 1999; Caterina and Julius 2001), but nasal trigeminal nerve endings also seem to contain specific receptors for nicotine (Alimohammadi and Silver 2000). In addition, a receptor for menthol, a compound producing a typical chemesthetic sensation of cooling or freshness, has also been recently identified in trigeminal sensory neurons (McKemy et al. 2002; Peier et al. 2002). Interestingly, the receptors for capsaicin and menthol are also activated by temperature in the warm/hot and cool/cold ranges, respectively, which broadens substantially the nature of the physiological role that these receptors play in the intact organism.
Activation of the capsaicin, nicotine, and menthol receptors, respectively, is accomplished by chemicals possessing defined structural requirements (Eccles 1994; Walpole et al. 1996; Alimohammadi and Silver 2000). In agreement with this, specific blockage of nicotinic nasal trigeminal receptors seems to leave sensitivity to unrelated volatile organic compounds (VOCs) intact (Alimohammadi and Silver 2000). A myriad of VOCs has the potential to evoke trigeminal chemosensory responses such as eye irritation and nasal pungency in humans (Cometto-Muñiz and Cain 1995). Many of these VOCs are relatively nonreactive vapors (cf. Alarie et al. 1998), unlikely to damage mucosal tissue simply upon brief vapor exposure. Still, despite their enormous diversity in chemical structure (Cometto-Muñiz 2001) and their nonreactive nature, they produce mucosal sensory irritation. We interpreted this observation as an indication that the trigeminal impact of these VOCs would rest principally on physicochemical parameters governing the transfer of the VOC from the vapor phase to a receptive biophase, i.e., the free nerve endings of the trigeminal nerve, rather than on restrictive structural requirements. The expectation was born out when a solvation equation (cf. Abraham and Weathersby 1994) that models transfer processes proved very successful in describing and predicting human nasal pungency and eye irritation thresholds (Abraham et al. 1998a; Abraham et al. 1998b; Abraham et al. 2001).
The studies on human trigeminal chemoreception mentioned above focused on single chemicals, typically members of various homologous chemical series. Studies of mixtures offer additional information on the functional characteristics of the trigeminal system and on the breadth of chemical tuning of the reception processes involved. A previous study on nasal pungency and eye irritation thresholds for mixtures of up to 9 VOCs showed agonistic sensory effects among the mixed chemicals (Cometto-Muñiz et al. 1997). This investigation measured “thresholds” but recognized the importance of measuring detectability or concentration-detection (i.e., psychometric) functions for a better characterization of trigeminal detection and a better knowledge of the physicochemical basis that underlies chemical irritation potency. These advantages can outweigh the intensive investment in labor and time that measuring such functions demand, particularly in the chemical senses. The present study of binary mixtures of two esters, ethyl propanoate and ethyl heptanoate, includes measurement of trigeminal detectability functions for the single chemicals. It explores the degree of chemesthetic detection additivity in mixtures, and its implications for understanding human trigeminal chemoreception of VOCs.
Materials and Methods
The Human Subjects Committee of the University of California, San Diego, approved the study protocol. Subjects gave written informed consent on forms approved by the Committee.
Subjects
Participants were given the CCCRC test of olfactory function to establish normosmia or anosmia (Cain 1989).
Experiment 1. Eye irritation detectability of the single chemicals.
We tested 18 normosmic subjects (i.e., normosmics) (10 females, 8 males) with an average age (±SD) of 25 (±20) years, and ranging from 19 to 53 years of age. All participants were nonsmokers.
Experiment 2. Eye Irritation detectability of binary mixtures.
We tested 20 normosmics (10 females, 10 males) with an average age (±SD) of 25 (±13) years, and ranging from 18 to 54 years of age. All participants were nonsmokers. Eight subjects (4 females, 4 males) had participated in Experiment 1.
Experiment 3. Nasal Pungency detectability of the single chemicals.
We tested 5 anosmic subjects (i.e., anosmics) (2 females, 3 males) with an average age (±SD) of 44 (±20) years, and ranging from 20 to 64 years of age. All participants were nonsmokers. They included three congenital anosmics (2 males, 1 female), one head-trauma anosmic (male), and one idiopathic anosmic (male).
Experiment 4. Nasal pungency detectability of binary mixtures.
We tested 4 anosmics (2 females, 2 males) with an average age (±SD) of 51 (±9) years, and ranging from 45 to 64 years of age. Three of them (1 female, 2 males) had participated in Experiment 3. All participants were nonsmokers. They included three congenital anosmics (2 females, 1 male) and one idiopathic anosmic (male).
Stimuli and Equipment
Experiment 1. Eye irritation detectability of the single chemicals.
Stimuli comprised ethyl propanoate (97+%) and ethyl heptanoate (98+%). Mineral oil (Light, Food Chemical Codex quality) served as solvent and blank. For both stimuli we prepared duplicate dilution series made in 2-fold steps. The series for ethyl propanoate ranged from 1% to 0.0625% v/v. The series for ethyl heptanoate ranged from 10% to 0.625% v/v. Vapor stimuli were stored and delivered (at 4 l/min) from glass vessels (1,900 ml capacity) containing 200 ml of solution. Briefly described, by means of compressed air (Medical air, USP quality) an aliquot of the headspace of the vessel was delivered for 3 sec to a conical container (25 ml) where the eye was exposed (Cometto-Muñiz et al. 2001).
Vapor concentrations in the headspace of vessels were measured weekly using gas chromatography (flame ionization detector, FID), via direct sampling with a gas-tight syringe. The relationship between liquid-phase (% v/v) and vapor-phase (ppm by volume) concentration was given by the following equations (see Cometto-Muñiz et al. 2003b):
| (1) |
| (2) |
where “y” represents log ppm and “x” represents log % v/v. The coefficient of variation across dilution steps for the weekly measurements averaged (±SD) 6.4% (±2.9) for ethyl propanoate and 6.4% (±0.6) for ethyl heptanoate. Chromatographic readings were converted into concentration units (ppm by volume) by reference to a calibration curve created by repetitive liquid injections of known masses of each chemical into the gas chromatograph (Cometto-Muñiz et al. 2003b).
Experiment 2. Eye irritation detectability of binary mixtures.
The chemicals were identical to those used in Experiment 1. The glass vessels adapted for ocular testing were also identical to those employed to test the single chemicals. The stimuli used in this experiment, however, included both single chemicals and binary mixtures of ethyl propanoate and ethyl heptanoate.
To prepare the binary mixtures, we used the psychometric function obtained for each chemical in Experiment 1 (cf. Figure 1). Detection probability or detectability (P) was corrected for chance (Macmillan and Creelman 1991) and adjusted to a scale ranging from 0.0, i.e., chance detection, to 1.0, i.e., perfect detection. Two levels of detection probability (“P” values) were selected: A relatively high one (P=0.80) and a relatively low one (P=0.40). “P” values, expressed as z-scores, depict a linear relationship with concentration. The corresponding linear equations can then be used to calculate the concentration of each chemical producing any pre-determined detection probability (previously transformed into the equivalent z-score). To create the five stimulitied to P=0.80 we used the linear equations to calculate the concentration of each chemical producing detection probabilities of 0.20, 0.40, and 0.60, that is, P values corresponding to 1/4, 1/2, and 3/4 of 0.80. Next, we assembled a set of five stimuli as follows: 1) the concentration of ethyl propanoate producing P=0.80 (labeled EP0.80); 2) a mixture of the concentration of ethyl propanoate producing P=0.60 (i.e., 3/4 of 0.80) and that of ethyl heptanoate producing P=0.20 (i.e., 1/4 of 0.80) (labeled EP0.60+EH0.20); 3) a mixture of the concentration of ethyl propanoate producing P=0.40 (i.e., 1/2 of 0.80) and that of ethyl heptanoate also producing P=0.40 (labeled EP0.40+EH0.40); 4) a mixture of the concentration of ethyl propanoate producing P=0.20 (i.e., 1/4 of 0.80) and that of ethyl heptanoate producing P=0.60 (i.e., 3/4 of 0.80) (labeled EP0.20+EH0.60); and 5) the concentration of ethyl heptanoate producing P=0.80 (labeled EH0.80). In this way, the set of five stimuli contained two that comprised a single chemical (stimuli # 1 and 5) and three that comprised binary mixtures of the two compounds in varying but complementary proportions (stimuli # 2, 3, and 4). Thus, the mixtures were prepared in such a way that the sum of the detection probabilities of the two constituent chemicals would always produce P=0.80, the same level of detectability as that of the two stimuli that comprised only a single chemical. Under an assumption of complete additivity of detection between the chemicals, all five stimuli should approximate equal detection (see Data analysis).
Figure 1.

Psychometric (i.e., detectability) functions (left y-axis) and confidence ratings (right y-axis) for eye irritation evoked by increasing concentrations of ethyl propanoate and heptanoate. Each point represents the average of 360 trials (half with each eye) made by 18 normosmics. Bars, hidden by the symbol in the case of detectability functions, indicate standard errors (SE).
To create the five stimuli tied to P=0.40, we again used the psychometric functions in Figure 1 to calculate the concentration of each chemical producing detection probabilities of 0.10, 0.20, and 0.30, that is, P values corresponding to 1/4, 1/2, and 3/4 of 0.40. Thus, following the same strategy and notation described above we assembled another set of five stimuli labeled: 1) EP0.40, 2) EP0.30+EH0.10, 3) EP0.20+EH0.20, 4) EP0.10+EH0.30, and 5) EH0.40. Under an assumption of complete additivity of detection between the chemicals, these five stimuli too should approximate equal detection (see Data analysis) albeit at a lower level than the previous five.
To minimize the possibility of depletion of the headspace vapor in any vessel (as a result of excessive repetitive sampling through a testing session) each stimulus described above was prepared in quintuplicate. Chromatographic samples were taken weekly from alternate stimuli and replicas to monitor stability.
Experiment 3. Nasal pungency detectability of the single chemicals.
The chemicals were identical to those used in Experiment 1. For both stimuli we prepared duplicate dilution series made in 2-fold steps. The series for ethyl propanoate ranged from 0.50% to 0.031% v/v. The series for ethyl heptanoate ranged from 40% to 1.25% v/v. Vapor stimuli were stored and delivered from the same type of glass vessels as in Experiment 1, containing 200 ml of solution, but adapted with two nosepieces as described previously (Cometto-Muñiz et al. 2000). As in Experiment 1, vapor concentrations in the headspace of vessels were measured weekly to monitor stability. For the stimuli used in this experiment, the average coefficient of variation (±SD) for the gas chromatographic measurements was found to be 8.2% (±2.9) for ethyl propanoate and 5.3% (±1.6) for ethyl heptanoate.
Experiment 4. Nasal pungency detectability of binary mixtures.
The chemicals were identical to those used in Experiment 1. The glass vessels were the same as those employed in Experiment 3. The stimuli used in this experiment, however, included both single chemicals and binary mixtures of ethyl propanoate and ethyl heptanoate.
To prepare the binary mixtures, we used the psychometric function obtained for each chemical in Experiment 3 (cf. Figure 3). To prepare the mixtures (and single chemicals) for studying nasal pungency we followed exactly the same strategy and procedure as in the case of eye irritation (described in Experiment 2). Thus, we prepared a set of five stimuli (two single chemicals and three mixtures) for a relatively high level of nasal pungency detectability (i.e., P=0.80), and another analogous set of five stimuli for a relatively low level of nasal pungency detectability (i.e., P=0.40). Again, each stimulus was prepared in quintuplicate and chromatographic samples were taken weekly from alternate stimuli and replicas to monitor stability.
Figure 3.
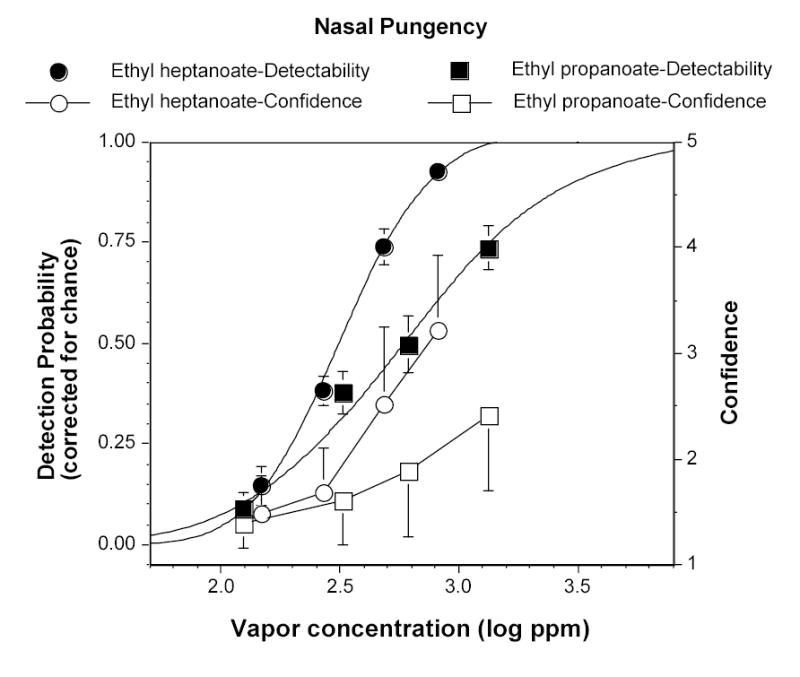
Psychometric (i.e., detectability) functions (left y-axis) and confidence ratings (right y-axis) for nasal pungency evoked by increasing concentrations of ethyl propanoate and heptanoate. Each point represents the average of 100 trials made by 5 anosmics. Bars indicate standard errors (SE).
Procedure
Experiment 1. Eye irritation detectability of the single chemicals.
To obtain concentration-detection (psychometric) functions for eye irritation from the single chemicals, we utilized a 3-alternative, forced-choice procedure with presentation of ascending concentrations and a 3-sec stimulation time. This method required participants to select which of the 3 vessels in a trial produced a sensation different from the other two. Subjects were not aware that, in a trial, 2 vessels contained blanks (mineral oil) and one contained a target chemical stimulus at some concentration. Position of blanks and stimulus were randomized. In a test series, dilution steps of a chemical were presented to the participant twice (once to each eye), in ascending order of concentration. Since we prepared duplicate vessels per dilution step, each vessel was presented only once in a series, thus minimizing depletion of headspace vapor. The inter-trial interval was at least 45 sec.
To avoid detection of the stimulus by smell, subjects wore nose clips during testing of each triad of vessels. Participants were told that, before removing the nose clips, they had to indicate verbally which vessel was different and had to rate their level of confidence in the choice. Confidence was rated on a scale ranging from “1” (not confident) to “5” (extremely confident).
Subjects participated in 2 to 5 sessions of 1 to 4 hours to complete a total of 10 test series per chemical (20 presentations per concentration, 10 in each eye). Within a test series, subjects alternated the left and right eye so that at the end of the series each eye had been exposed to every concentration. The chemical presented first and the eye tested first in a series followed an irregular order across series for the same subject and across subjects. The data from all series for each chemical were averaged, first, within individuals and, then, across individuals to obtain group data.
Experiment 2. Eye irritation detectability of binary mixtures.
We used the same methods and instructions to subjects as those employed in Experiment 1, i.e., a 3-alternative forced-choice procedure, randomized position of blanks and stimulus, an inter-trial interval of at least 45 sec, use of nose clips, confidence ratings, etc. In addition, order of presentation of the five stimuli in the P=0.80 series and the five in the P=0.40 series was randomized and each stimulus was prepared in quintuplicate.
Subjects participated in 1 to 4 sessions of 1 to 3 hours to complete a total of 20 trials per stimulus in the P=0.80 series and another 20 per stimulus in the P=0.40 series. (Two subjects, a male and a female, only completed 10 trials per stimulus on each series.) Participants first completed the P=0.80 series and then the P=0.40 series. The eye tested alternated from right to left. Individual and group averages were calculated as before.
Experiment 3. Nasal pungency detectability of the single chemicals.
We used a procedure parallel to that described in Experiment 1, but using nosepiece attachments on the vessels to deliver the vapors to both nostrils simultaneously on a trial. Anosmics participated in 2 to 5 sessions of 1 to 3 hours each to complete 10 series for each chemical (20 presentations per concentration) using the same mode of testing as in Experiment 1.
Experiment 4. Nasal pungency detectability of binary mixtures.
We used an analogous procedure to that described in Experiment 2 as regard to testing the stimuli but using the nosepiece attachments and testing both nostrils simultaneously as mentioned under Experiment 3. Anosmics participated in 4 to 6 sessions of 1 to 3 hours each to complete a total of 20 to 40 trials per stimulus in the P=0.80 series and 20 to 30 trials per stimulus in the P=0.40 series.
Data analysis
Plots of detection probability as a function of stimulus concentration (in log ppm by volume) summarized the outcome. Detection probability was corrected for chance (Macmillan and Creelman 1991) and adjusted to a scale ranging from 0.0 for chance detection to 1.0 for perfect detection. A repeated measures analysis of variance (ANOVA) (SuperANOVA v.1.11, Abacus Concepts, Inc.) as described under Results served to test for significance. The following formula was used to calculate the theoretical values of detectability under an assumption of complete additivity of detection, i.e., independence of detection, for the individual chemicals (Feller 1968–1971):
In this formula, Pdet.EP,EH = Probability of detection of the binary mixture of ethyl propanoate and ethyl heptanoate, Pdet.EP = Probability of detection of ethyl propanoate alone, and Pdet.EH = Probability of detection of ethyl heptanoate alone.
Results
Experiment 1. Eye irritation detectability of the single chemicals.
Figure 1 depicts the psychometric (i.e., detectability) functions for eye irritation from ethyl propanoate and heptanoate. In agreement with previous data (see review in Cometto-Muñiz 2001), trigeminal chemosensory potency (in this case, eye irritation) increases with carbon chain length within a homologous series. This reflects itself in the function for ethyl heptanoate lying to the left (toward lower concentrations) of that for ethyl propanoate. In addition, the function for heptanoate is steeper than that for propanoate Also shown are the confidence ratings associated with each concentration and detectability. As expected, confidence ratings increased with detectability for both chemicals. Detection probabilities can be converted into corresponding z-scores and the ogival functions become linear, producing the following equations:
| (3) |
| (4) |
where “y” represents z-score and “x” represents vapor concentration in log ppm.
Experiment 2. Eye irritation detectability of binary mixtures.
Figure 2a shows the eye irritation detectability of the five stimuli per preselected level (i.e., P=0.8 and P=0.4) and their associated confidence rating, whereas Figure 2b compares the experimental detectability obtained with the theoretical values expected from complete additivity. The two single stimuli selected based on the results of Experiment 1 to produce a detectability of 0.8, produced detectabilities close to 0.8 (ethyl propanoate) and to 0.6 (ethyl heptanoate), not far from target albeit slightly unbalanced. The corresponding mixtures for the P=0.8 series tended to fall short of complete additivity of detection, particularly as the composition of the mixtures gained in the proportion of heptanoate present, creating a “U” with a skewed appearance. The two single stimuli selected based on the results of Experiment 1 to produce a detectability of 0.4, produced detectabilities between 0.2 and 0.3, lower than target but very close to each other, i.e., balanced. In contrast to the outcome for the P=0.8 series, the corresponding mixtures for the P=0.4 series depicted complete additivity of detection, irrespective of the relative proportion of the components. For all stimuli, confidence ratings followed tightly the trend seen with actual detectability. The results of a repeated measures ANOVA including the factors target detectability (two levels: P=0.8 and P=0.4), stimulus (five levels: two single chemicals and three mixtures), and their interaction, gave statistical support to the trends observed: 1) detectability of stimuli at target P=0.8 was significantly higher than at target P=0.4 (F(1,19) = 90.07, p < 0.0001); 2) detectability among the five stimuli was significantly different (F(4,76) = 15.00, p < 0.0001); and 3) there was a significant interaction between target detectability level (P=0.8 or P=0.4) and the five stimuli (F(4.76) = 14.12, p < 0.0001), indicating that the trends observed (skewed “U” for the P=0.8 series and a horizontal line for the P=0.4 series) differed significantly.
Figure 2.

a) Detectability (left y-axis) and confidence ratings (right y-axis) for the eye irritation evoked by the five stimuli in the P=0.8 series and the five stimuli in the P=0.4 series (see text). Each point represents the average of 400 trials (half with each eye) made by 20 normosmics. Bars indicate standard errors (SE). b) Same detectability data as in a) but compared with the theoretical detectability expected from complete additivity of detection of the individual chemicals. Bars indicate standard errors (SE).
Experiment 3. Nasal pungency detectability of the single chemicals.
Figure 3 depicts the psychometric (i.e., detectability) functions for nasal pungency from ethyl propanoate and heptanoate. Also shown are the confidence ratings corresponding to each concentration and detectability, for both compounds. The outcome roughly parallels that obtained for eye irritation: The function for heptanoate is shifted to the left and steeper than that for propanoate, and confidence ratings increase with detectability for both substances. Nevertheless, confidence ratings given by the anosmic group for nasal pungency tended to be lower than those given by the normosmic group for eye irritation at comparable detectabilities. Conversion of detection probabilities into z-scores produced the following linear functions for nasal pungency:
| (5) |
| (6) |
where “y” represents z-score and “x” represents vapor concentration in log ppm. The slope value differed between chemicals but was very close to that obtained for eye irritation from the same chemical. Also, another difference emerged between the shorter and the longer homolog in terms of their comparative trigeminal chemosensory potency in the ocular and nasal mucosae: As shown in Figure 4, whereas for ethyl propanoate the eyes and the nose were equally sensitive, for ethyl heptanoate the eyes were uniformly more sensitive than the nose across the entire detectability range.
Figure 4.

Showing how, for ethyl propanoate, the ocular and nasal trigeminal chemosensitivity are virtually identical, whereas, for ethyl heptanoate, ocular chemosensitivity is uniformly higher (i.e., displaced to the left, towards lower concentrations) than nasal chemosensitivity. Bars, sometimes hidden by the symbols, indicate standard errors (SE).
Experiment 4. Nasal pungency detectability of binary mixtures.
Figure 5a shows the nasal pungency detectability of the five stimuli per preselected level (i.e., P=0.8 and P=0.4) and their associated confidence rating, whereas Figure 5b compares the experimental detectability obtained with the theoretical values expected from complete additivity. The two single stimuli selected based on the results of Experiment 3 to produce a detectability of 0.8, actually produced a detectability of 0.8, right on target. The detectabilities of the mixtures for the P=0.8 series did not depart from the theoretical values expected from complete additivity (Figure 5b). This marked a contrast with the trend seen for the mixtures in the eye irritation experiment (Figure 2b, target P=0.8). The two single stimuli selected based on the results of Experiment 3 to produce a detectability of 0.4, produced detectabilities between 0.25 and 0.38, somewhat lower than target but close to each other. The corresponding mixtures for the P=0.4 series also did not depart from the theoretical values expected from complete additivity (Figure 5b). Thus, the trend for this series was similar to that for eye irritation (Figure 2b, target P=0.4). For stimuli on both series, confidence ratings followed the trend seen with actual detectability, an outcome also observed regarding eye irritation albeit absolute values of confidence of detection were, again, lower for the nasal than for the ocular response. The results of a repeated measures ANOVA analogous to the one performed on the eye irritation data confirmed that nasal detectability of stimuli at target P=0.8 was significantly higher than at target P=0.4 (F(1,3) = 48.56, p = 0.006) and that the differences in detectability among the five stimuli and the interaction between target detectability level and the five stimuli failed to reach significance.
Figure 5.
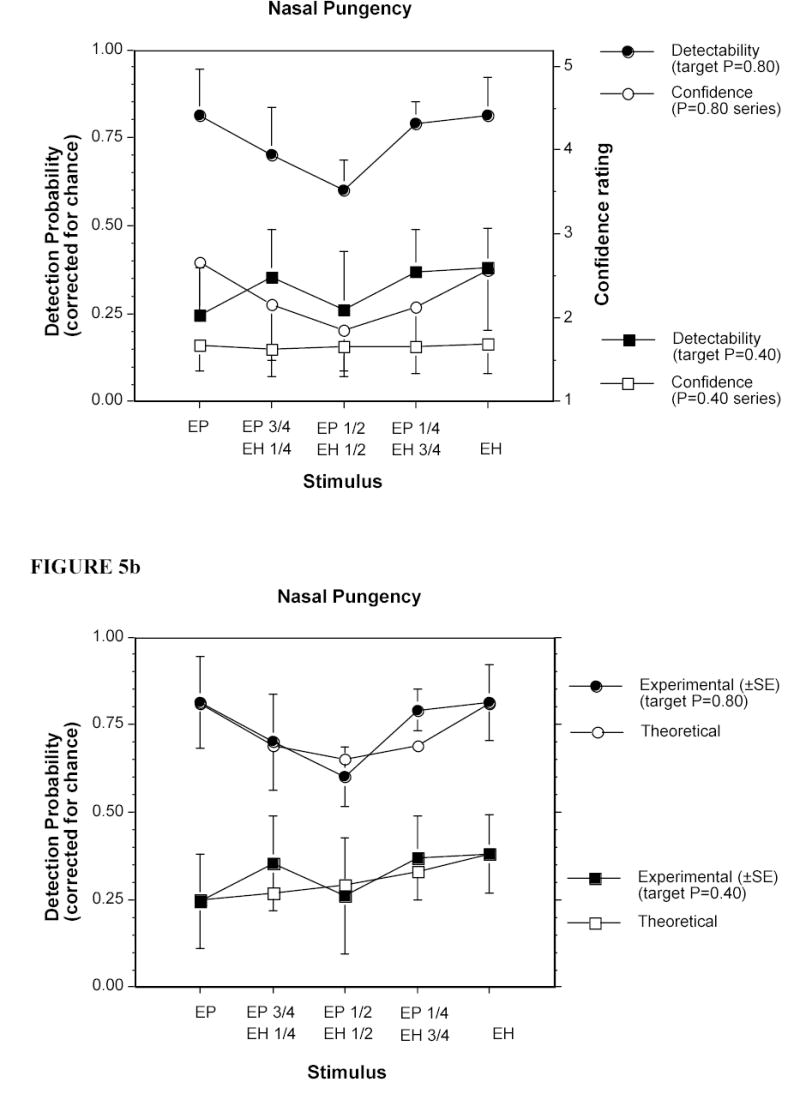
a) Detectability (left y-axis) and confidence ratings (right y-axis) for the nasal pungency evoked by the five stimuli in the P=0.8 series and the five stimuli in the P=0.4 series (see text). Each point represents the average of 133 trials in the P=0.8 series and 100 trials in the P=0.4 series, made by 4 anosmics. Bars indicate standard errors (SE). b) Same detectability data as in a) but compared with the theoretical detectability expected from complete additivity of detection of the individual chemicals. Bars indicate standard errors (SE).
Comparability of vapor concentrations in single stimuli and mixtures.
Since we measured the actual vapor concentrations of both single and mixed stimuli, we can compare the values obtained in the headspace of the same liquid dilution when the stimulus was presented singly (Experiments 1 and 3) or in a mixture (Experiments 2 and 4). Also, as described above, we included testing of single chemicals (acting as ‘references” or ‘standards”) when the mixtures were tested. In these cases we can compare the vapor concentration of the same liquid dilution of a single chemical prepared and tested in one (i.e., single) or the other (i.e., mixtures) experimental context. Table 1 presents such comparisons as the ratio of vapor concentrations (in log ppm) “Single/Mixed” corresponding to the same liquid dilution measured in the context of testing single chemicals or in the context of testing mixtures. The outcome showed that all ratios equal or are very close to one. This meant that the vapor concentration from a certain liquid dilution of a chemical remained unchanged whether the chemical was singly or in a mixture. It also served as an internal control to show that repetitive preparations of the same liquid dilution for different experiments produced the same vapor concentration. Thus, changes in chemosensory detectability of stimuli in Experiments 2 and 4 cannot be attributed to variability of presented vapor-phase concentrations.
Table 1.
Ratios of vapor concentrations (log ppm) corresponding to the same liquid dilution of a stimulus when it was prepared singly (Experiments 1 and 3) or in a mixture context (Experiments 2 and 4). EP(s): ethyl propanoate, singly; EP(m): ethyl propanoate, mixture context; EH(s): ehtyl heptanoate, singly; EH(m): ethyl heptanoate, mixture context.
| Modality tested | Target detectability series (P) | Stimulus | Ratio EP(s)/ EP(m) | Ratio EH(s)/ EH(m) |
|---|---|---|---|---|
| Eye irritation | 0.8 | EP | 0.98 | |
| 3/4EP - 1/4EH | 0.98 | 1.04 | ||
| 1/2EP - 1/2EH | 0.99 | 1.02 | ||
| 1/4EP - 3/4EH | 1.01 | 1.03 | ||
| EH | 1.02 | |||
| 0.4 | EP | 0.96 | ||
| 3/4EP - 1/4EH | 1.01 | 1.01 | ||
| 1/2EP - 1/2EH | 1.05 | 1.03 | ||
| 1/4EP - 3/4EH | 1.01 | 1.08 | ||
| EH | 1.01 | |||
| Nasal pungency | 0.8 | EP | 1.01 | |
| 3/4EP - 1/4EH | 1.01 | 1.04 | ||
| 1/2EP - 1/2EH | 1.00 | 1.00 | ||
| 1/4EP - 3/4EH | 1.02 | 1.03 | ||
| EH | 1.07 | |||
| 0.4 | EP | 0.98 | ||
| 3/4EP - 1/4EH | 0.98 | 0.99 | ||
| 1/2EP - 1/2EH | 0.95 | 0.99 | ||
| 1/4EP - 3/4EH | 0.98 | 1.00 | ||
| EH | 1.02 |
Discussion
A previous investigation measuring trigeminal (nasal and ocular) and olfactory thresholds for the detection of single and mixed chemicals indicated the existence of various degrees of sensory agonism in the mixtures, with chemesthesis showing somewhat more agonism than olfaction (Cometto-Muñiz et al. 1997). A later study on mixtures of 1-butanol and 2-heptanone looked into the issue in more detail by measuring complete concentration-detection functions for eye irritation, nasal pungency, and odor of the chemicals singly and mixed (Cometto-Muñiz et al. 1999). The approach used with this alcohol/ketone mixture focused on testing whether a general trend of dose-addition between individual chemicals would hold along the continuous range of detectability, from slightly above chance to near perfect detection. As a first approximation, the outcome lent support to such a trend for the three chemosensory responses. Two subsequent studies on mixtures that addressed, respectively, ocular and nasal chemesthesis (Cometto-Muñiz et al. 2001), and olfaction (Cometto-Muñiz et al. 2003a) tested the structurally more dissimilar compounds butyl acetate and toluene. They also resorted to measure complete concentration-detection functions for the single chemicals but, as in the present study, focused on testing a hypothesis of complete additivity of detection between individual chemicals presented in mixtures of varying but complementary proportions, at low and high detectability. The results showed that, for the three sensory endpoints, the detection of mixtures followed a rule of complete additivity at relatively low levels of detectability, i.e., 0.0<P<0.5, but fell short of complete additivity at relatively high levels of detectability, i.e., 0.5<P<1.0. A further comparative look at the results revealed that the degree of departure from complete additivity at high detectability levels was small for nasal pungency, larger for eye irritation, and largest for odor (Cometto-Muñiz et al. 2002; Cometto-Muñiz et al. 2003a).
The pair of esters tested here are structurally much closer than the ester/aromatic pair tested earlier, yet they still lie far enough apart within the same homologous series to expect differences in chemosensory potency. In terms of trigeminal detection of single chemicals, the expected differences came out in the direction predicted by previously measured chemesthetic thresholds within members of homologous chemical series (see review in Cometto-Muñiz 2001): For both eye irritation and nasal pungency, the homolog with the longer chain-length was detected at lower vapor concentrations than that with the shorter chain-length. In terms of trigeminal detection of mixtures, a comparison of results between the present pair ethyl propanoate/ethyl heptanoate and the previous pair butyl acetate/toluene revealed that: 1) neither pair, at relatively high detectabilities, could achieve complete additivity of detection for eye irritation (Figure 6, top), and 2) the present pair achieved complete additivity for nasal pungency, even at high detectabilities, whereas the previous pair fell barely short of that (Figure 6, bottom). Despite this small difference probably resting on the closer resemblance in structure and chemical functionality between members of the present pair, the overall outcome is comparable for both the similar (i.e., present) and the dissimilar (i.e., previous) pair, an indication of the relatively wide breadth of chemical tuning in chemesthesis (see below).
Figure 6.
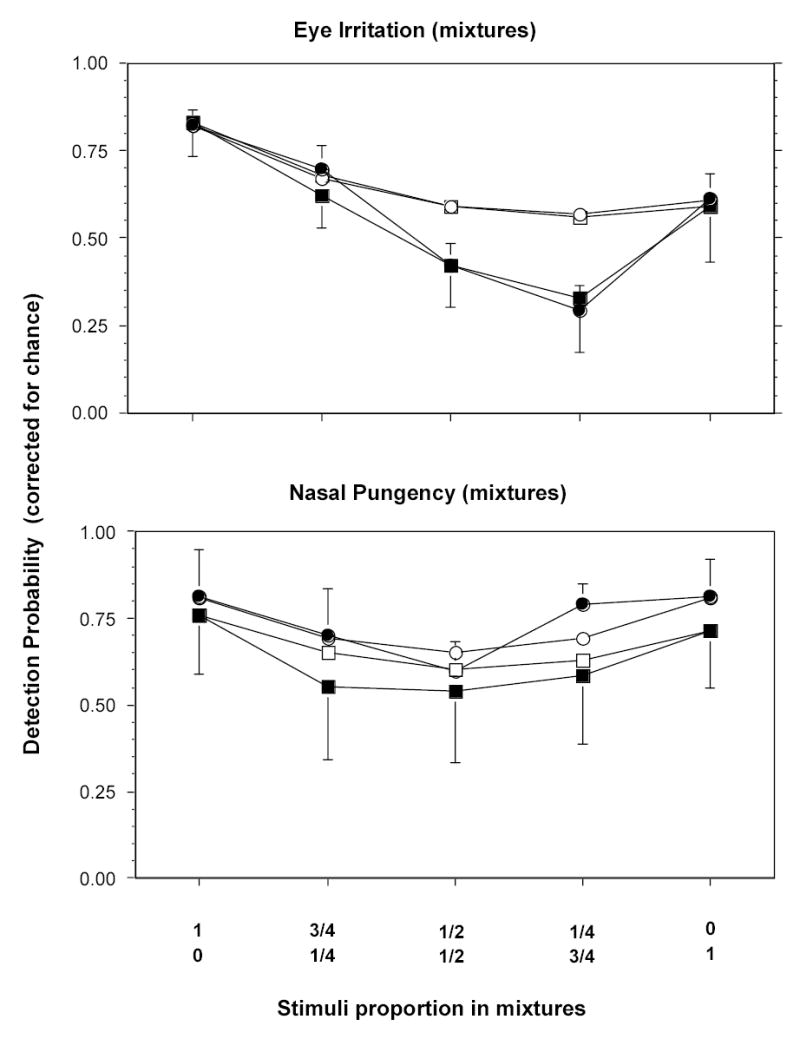
Top. Showing, for eye irritation, how detection of mixtures of varying proportions of ethyl propanoate/ethyl heptanoate (filled circles) and toluene/butyl acetate (filled squares), at equivalent detectability of the single chemicals, compare with the theoretical functions representing complete additivity of detection of the same mixtures (empty circles and empty squares, respectively). Note that both kind of mixtures tend to fall short of complete additivity of detection. Bars indicate standard errors (SE). Bottom. Analogous to above but for nasal pungency. Note that whereas the mixture ethyl propanoate/ethyl heptanoate (filled circles) achieves complete additivity (empty circles), the mixture toluene/butyl acetate (filled squares) falls slightly short of complete additivity (empty squares). Bars indicate standard errors (SE).
Mixture addition at low vs. at high detectability, contrast between the ocular and nasal mucosae
The present finding of a higher degree of mixture addition at low vs. at high detectability for eye irritation but not for nasal pungency presents a contrast between the chemesthetic response of the two mucosae. At relatively high levels of chemosensory stimulation, a decrease from complete addition between the mixture components can be expected as the concentration of both chemicals in the receptor environment increases, transforming what might have began as agonism into a process of competitive agonism between the stimulating molecules impinging into an array of receptors. There is ample precedent for such phenomenon from previous studies in mice and rats dealing with nasal irritation from binary, ternary, and more complex mixtures (Kane and Alarie 1978; Nielsen et al. 1988; Cassee et al. 1996; Kasanen et al. 1999). Our experiments, though, involve relatively low levels of stimulation considering that, even in cases of “high detectability”, detection of the stimulus is below 100%. One can argue that, under such conditions, the number of available receptors has not yet become a limiting factor, and mixture components sharing substantial chemical similarity can achieve response-addition as was the case for nasal pungency from the present mixture. Why, then, might the situation be different in the ocular mucosa for that same mixture? Perhaps the answer relates to an issue of homogeneity of receptors or receptor environments. Recent psychophysical studies have indicated differences in sensitivity (including chemical sensitivity) between the cornea, more sensitive, and the conjunctiva, less sensitive (Acosta et al. 2001; Feng and Simpson 2003). Such lack of homogeneity in the ocular mucosal environment might have contributed to the failure to achieve complete response-addition, even for the chemically similar mixture tested here.
Response-addition vs. dose-addition
The experiments reported here directly address the issue of response- addition in the trigeminal detectability of these binary mixtures. A direct test of dose-addition would require measurement of families of psychometric functions with one chemical as the independent variable and the other as the parameter (and viceversa) (cf. Cometto-Muñiz et al. 1999). Nevertheless, in an indirect way, the present approach can suggest trends of compliance or departure from a rule of dose-addition among the mixtures studied.
We have shown that the vapor concentration from a fixed liquid-dilution of ethyl propanoate or heptanoate remains constant whether it comes from a single stimulus or a mixture, and also that has remained constant across the various experiments (Table 1). Thus, the changes in detectability observed from single stimuli common to Experiments 1 and 2, and from those common to Experiments 3 and 4 have to originate from variability between the respective testing conditions and/or group of subjects. For example, it is possible that the repetitive presentations of “ascending concentration test series” employed in Experiments 1 and 3 could have produced, for essentially the same stimuli, slightly higher detectability than the “random presentation” employed in Experiments 2 and 4. From this it follows that it might be misleading to apply directly the psychometric functions obtained in Experiments 1 and 3 to the calculation of trends in dose-addition in Experiments 2 and 4. Nevertheless, from the latter experiments, we have two detectability points from each chemical that can be used to estimate their respective linear psychometric functions (via z-scores) under the conditions and subjects tested in those experiments (i.e., 2 and 4). Admittedly, relying on only 2 experimental points to derive a linear function is far from ideal but if the new functions fall into register with those obtained in Experiments 1 and 3 the calculated dose-addition trends rest on firmer ground and can be considered, at least, preliminary. This preliminary analysis of the data indicate agreement between response- and dose- addition.
Breadth of chemical tuning in chemesthesis
As mentioned in the Introduction, recent studies have explored the nature and characteristics of chemesthetic receptors responding to capsaicin, menthol, and nicotine. The specificity of each of these receptors is difficult to assess since at least the first two of them also respond to temperature in the warm/hot and cool/cold range, respectively. Capsaicin receptors can be modulated by H+ and activated by chemicals structurally distinct from vanilloids (Szallasi et al. 1996) and even by an unrelated VOC such as ethanol (Trevisani et al. 2002). In fact, a diverse family of GPCRs has been found to be expressed in nociceptive sensory neurons (Dong et al. 2001). These findings, along with data on comparative structure-activity relationships between chemesthetic and olfactory thresholds for VOCs (Abraham et al. 2001) reinforce the concept of a narrower chemical tuning in olfactory than in trigeminal detection. Such narrower tuning could underlie the larger break-down in detection additivity of mixtures, at high detectabilities, observed for olfaction compared to chemesthesis (Cometto-Muñiz et al. 2003a). In addition, another contributing factor could include a more prominent role of inhibitory circuits in the olfactory pathway than in the chemesthetic pathway, even at perithreshold levels. There is ample evidence for such inhibition in olfaction although its interpretation is open to debate (Laurent 1999; Mori et al. 1999). The immediate follow-up of the present experiments will consist on testing mixtures of ethyl propanoate/ethyl heptanoate but regarding olfactory detection additivity.
Acknowledgments
The work described in this article was supported by research grant number R01 DC 02741 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. Thanks are due to J.M. Snell and A.J. Gorzeman for excellent technical assistance. Thanks are also due to the following students for their help in various stages of the study: Y.T. Wong, O. Del Valle, and B. Flores.
References
- Abraham MH, Gola JMR, Cometto-Muniz JE, Cain WS. The correlation and prediction of VOC thresholds for nasal pungency, eye irritation and odour in humans. Indoor Built Environ. 2001;10:252–257. [Google Scholar]
- Abraham MH, Kumarsingh R, Cometto-Muñiz JE, Cain WS. An algorithm for nasal pungency thresholds in man. Arch Toxicol. 1998a;72:227–232. doi: 10.1007/s002040050493. [DOI] [PubMed] [Google Scholar]
- Abraham MH, Kumarsingh R, Cometto-Muñiz JE, Cain WS. Draize eye scores and eye irritation thresholds in man can be combined into one quantitative structure-activity relationship. Toxicol in Vitro. 1998b;12:403–408. doi: 10.1016/s0887-2333(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Abraham MH, Weathersby PK. Hydrogen bonding. 30 Solubility of gases and vapors in biological liquids and tissues. J Pharm Sci. 1994;83:1450–1456. doi: 10.1002/jps.2600831017. [DOI] [PubMed] [Google Scholar]
- Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001;42:2063–2067. [PubMed] [Google Scholar]
- Alarie Y, Nielsen GD, Abraham MH. A theoretical approach to the Ferguson principle and its use with non-reactive and reactive airborne chemicals. Pharmacol Toxicol. 1998;83:270–279. doi: 10.1111/j.1600-0773.1998.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25:61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- Bryant B, Silver WL (2000) Chemesthesis: The Common Chemical Sense. In: Finger TE, Silver WL, Restrepo D (eds) The Neurobiology of Taste and Smell. 2nd Edition. Wiley-Liss, New York, pp 73–100
- Cain WS. Testing olfaction in a clinical setting. Ear Nose Throat J. 1989;68:316–328. [PubMed] [Google Scholar]
- Cassee FR, Arts JHE, Groten JP, Feron VJ. Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Arch Toxicol. 1996;70:329–337. doi: 10.1007/s002040050282. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE (2001) Physicochemical basis for odor and irritation potency of VOCs. In: Spengler JD, Samet J, McCarthy JF (eds) Indoor Air Quality Handbook. McGraw-Hill, New York, pp 20.1–20.21
- Cometto-Muñiz JE, Cain WS. Relative sensitivity of the ocular trigeminal, nasal trigeminal, and olfactory systems to airborne chemicals. Chem Senses. 1995;20:191–198. doi: 10.1093/chemse/20.2.191. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH (2002) Sensory detection of VOCs singly and in mixtures: Odor and sensory irritation. In: Levin H (ed) Indoor Air 2002. Vol. II. Indoor Air 2002, Monterey, pp 237–242
- Cometto-Muñiz JE, Cain WS, Abraham MH. Dose-addition of individual odorants in the odor detection of binary mixtures. Behav Brain Res. 2003a;138:95–105. doi: 10.1016/s0166-4328(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chem Senses. 2003b;28:467–477. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH, Gola JMR. Chemosensory detectability of 1-butanol and 2-heptanone singly and in binary mixtures. Physiol Behav. 1999;67:269–276. doi: 10.1016/s0031-9384(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH, Gola JMR. Ocular and nasal trigeminal detection of butyl acetate and toluene presented singly and in mixtures. Toxicol Sci. 2001;63:233–244. doi: 10.1093/toxsci/63.2.233. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Hiraishi T, Abraham MH, Gola JMR. Comparison of two stimulus-delivery systems for measurement of nasal pungency thresholds. Chem Senses. 2000;25:285–291. doi: 10.1093/chemse/25.3.285. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Hudnell HK. Agonistic sensory effects of airborne chemicals in mixtures: Odor, nasal pungency, and eye irritation. Percept Psychophys. 1997;59:665–674. doi: 10.3758/bf03206014. [DOI] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Feller W (1968–1971) An introduction to probability theory and its applications. Wiley, New York
- Feng Y, Simpson TL. Nociceptive sensation and sensitivity evoked from human cornea and conjunctiva stimulated by CO2. Invest Ophthalmol Vis Sci. 2003;44:529–532. doi: 10.1167/iovs.02-0003. [DOI] [PubMed] [Google Scholar]
- Finger TE, Silver WL, Bryant B (1999) Trigeminal nerve. In: Adelman G, Smith BH (eds) Encyclopedia of Neuroscience. Vol. II. Elsevier, Amsterdam, pp 2069–2071
- Kane LE, Alarie Y. Evaluation of sensory irritation from acrolein-formaldehyde mixtures. Am Ind Hyg Assoc J. 1978;39:270–274. doi: 10.1080/0002889778507758. [DOI] [PubMed] [Google Scholar]
- Kasanen J-P, Pasanen A-L, Pasanen P, Liesivuori J, Kosma V-M, Alarie Y. Evaluation of sensory irritation of w3-carene and turpentine, and acceptable levels of monoterpenes in occupational and indoor environment. J Toxicol Environm Health Part A. 1999;57:89–114. doi: 10.1080/009841099157809. [DOI] [PubMed] [Google Scholar]
- Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD (1991) Detection theory: A user’s guide. Cambridge University Press, Cambridge
- Martin JH, Jessell TM (1991) Modality coding in the somatic sensory system. In: Kandel ER, Schwartz JH, Jessell TM (eds) Principles of Neural Science. 3rd Edition. Elsevier, New York, pp 341–352
- McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Kristiansen U, Hansen L, Alarie Y. Irritation of the upper airways from mixtures of cumene and n-propanol. Mechanisms and their consequences for setting industrial exposure limits. Arch Toxicol. 1988;62:209–215. doi: 10.1007/BF00570142. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Gomez G. Cell and molecular biology of human olfaction. Microsc Res Tech. 2002;58:142–151. doi: 10.1002/jemt.10132. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Cook SP, McCleskey EW. Chemical mediators of pain due to tissue damage and ischemia. Prog Brain Res. 2000;129:21–38. doi: 10.1016/S0079-6123(00)29003-1. [DOI] [PubMed] [Google Scholar]
- Szallasi A. The vanilloid (capsaicin) receptor: receptor types and species differences. Gen Pharmacol. 1994;25:223–243. doi: 10.1016/0306-3623(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Jonassohn M, Acs G, Biro T, Acs P, Blumberg PM, Sterner O. The stimulation of capsaicin-sensitive neurones in a vanilloid receptor-mediated fashion by pungent terpenoids possessing an unsaturated 1,4-dialdehyde moiety. Br J Pharmacol. 1996;119:283–290. doi: 10.1111/j.1476-5381.1996.tb15983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Walpole CS, Bevan S, Bloomfield G, Breckenridge R, James IF, Ritchie T, Szallasi A, Winter J, Wrigglesworth R. Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J Med Chem. 1996;39:2939–2952. doi: 10.1021/jm960139d. [DOI] [PubMed] [Google Scholar]


