Abstract
Novel bacterial blight (BB) resistance gene(s) for rice was (were) introduced into a cultivated japonica rice variety Oryza sativa (cv. 8411), via somatic hybridization using the wild rice Oryza meyeriana as the donor of the resistance gene(s). Twenty-nine progenies of somatically hybridized plants were obtained. Seven somatically hybridized plants and their parents were used for AFLP (amplified fragment length polymorphism) analysis using 8 primer pairs. Results confirmed that these plants were somatic hybrids containing the characteristic bands of both parents. The morphology of the regenerated rice showed characters of both O. sativa and O. meyeriana. Two somatic hybrids showed highest BB resistance and the other 8 plants showed moderate resistance. The new germplasms with highest resistance have been used in the rice breeding program for the improvement of bacterial blight resistance.
Keywords: Oryza sativa L., Oryza meyeriana L., Somatic hybridization, Rice bacterial blight resistance
INTRODUCTION
There are abundant resources of rice species in China including a variety of wild rice that possess desirable agricultural traits. For instance, Oryza officinalis has high resistance to rice planthopper, O. minuta is resistant to both rice blast and planthopper (Peng et al., 1996), while O. meyeriana shows high immunity to bacterial blight. Introducing specific genes of the wild rice into cultivars has been the goal pursued by research workers for a long time. However, problems such as hybrid sterility between cultivars and wild rice have posed major obstacles.
O. sativa (AA type chromosomes) is readily hybridized with common AA-type wild rice, but not with non-AA types (such as O. officinalis of CC type and O. meyeriana of GG type). In the latter cases, conventional crossing methods have failed to generate fertile hybrids. However, this problem can be overcome by subsequent embryo-rescue of hybrids (Jena and Khush, 1990). Because the non-AA type wild rice generally possess desirable traits not found in cultivated rice or closely related wild rice species, it is important to develop techniques to generate hybrid plants between O. sativa and non-AA types of wild rice species.
The ability to produce and fuse rice protoplasts, has allowed the generation of inter-species hybrid clones between the wild rice and O. sativa (Hayashi et al., 1988) as well as inter-generic hybrid clones between rice and Panicum maximum (Zhang et al., 1999), rice and Hordeum vulgare L. (Kisaka et al., 1998), and rice and Porteresia coarctata (Jelodar et al., 1999). This demonstrates the great potential of cell fusion in increasing the variety of rice. In this work, we obtained hybrids via asymmetric somatic hybridization between Oryza sativa L. ssp. japonica (cv. 8411) and O. meyeriana L. Furthermore, a new rice breed with resistance to bacterial blight was obtained through selection.
MATERIALS AND METHODS
Plant materials
Oryza meyeriana L. with high resistance to bacterial blight, was provided by Professor Shen at Zhejiang University. Oryza sativa L. ssp. japonica (cv. 8411) provided by Jiaxing Academy of Agricultural Sciences (cv. 8411) was high-yielding, and of good grain quality, but susceptible to bacterial blight pathogens.
Mature seeds of O. sativa (cv. 8411) and O. meyeriana were surface sterilized, and pre-cultured in the dark for 24 h at 26±1 °C in LS medium (Linsmaier and Skoog, 1965) containing 2.5 mg/L of 2,4-D, 0.1 mg/L 6BA. The swelling granules were selected, and the embryos were surgically extracted and cultured in the same medium to induce callus formation. Subculturing was performed under the same conditions once every 30 days to obtain embryo-derived calli.
Establishment of embryo-derived suspension cell lines
Calli (1.0 g) derived from the embryos of O. sativa (cv. 8411) and O. meyeriana were inoculated into 15–20 ml AA liquid medium containing 2.5 mg/L of 2,4-D, 0.1 mg/L 6-BA and cultured in the dark at 26±1 °C. The medium was replaced once every 3 days and after about one and a half months, 4 ml small granular suspension cells were inoculated into 35 ml of fresh medium which was replaced once every 9 days. When the suspension cells became pale yellow, fast-growing, cytoplasmically dense, well dispersed, and roughly identical in cell size, they were suited for protoplast culture and somatic hybridization.
Isolation and culturing of the protoplasts
Isolation and culturing of the protoplasts followed the methods proposed by Abdullah et al.(1986). Six days after subculturing, protoplasts were isolated from the suspension cells. The enzyme solution was composed of 1.5% (W/V) cellulase Onozuka RS, 0.1% (W/V) pectolyase Y-23, 5 mmol/L MES, 13% Mannotal in CPW solution, pH 5.6 (27.2 mg/L KH2PO4, 101.0 mg/L KNO3, 2.6 g/L CaCl2·2H2O, 246 mg/L MgSO4·7H2O, 0.16 mg/L KI, 0.02 mg/L CuSO4·5H2O) (Frearson et al., 1973). One g of fresh cells was mixed with 5 ml of enzyme solution and incubated in a 6-cm Petri dish at 26 °C in the dark. The dish was initially shaken at 50 r/min for 3 h, and then allowed to be still for 2 h. At the end of incubation, the mixture was filtered with 85 μm-nylon sieve and the filtrate was collected, centrifugated at 300 r/min to harvest the protoplasts, washed twice by CPW containing 13% of Mannitol and washed once by KpR (Kao, 1997) Protoplast medium.
The protoplasts resuspended in KpR medium were incubated in a 45 °C water bath for 5 min and then in ice-water bath for 10 seconds, and subsequently mixed with KpR containing 1.2% sea plaque agarose to a density of (0.6~1) ×106 ml−1 and each 3.5-cm petri dish was inoculated with 0.3 ml of the mixture. After the agarose solidified, it was cut into 4 parts, and was added to 0.5 ml KpR liquid medium in a 3.5 cm Petri dish, sealed with parafilm and cultured in the dark at 26±1 °C. Nine days later, cells regenerated from the protoplasts began to divide for the first and the second time. At this time, adding KpR medium (similar to KpR, but containing 1.5% glucose and 1.5% sucrose) was necessary to maintain cell division, presumably by maintaining a balance of osmotic pressure. When the regenerated cells formed cell clusters containing 32–64 cells, AA liquid medium with the concentration of 2,4-D raised to 2.5 mg/L was added to maintain a balance of osmotic pressure and to promote regenerated cells development into embryo cells (Yan et al., 2002). When the regenerated cells formed large cell clusters or small calli organization visible to the naked eye, they were transferred into N6 (Zhu et al., 1975) solid medium. When they differentiated into plantlets, they were transferred into 1/2 MS containing 0.3 mg/L NAA and 0.1 mg/L IBA to promote the growth of plantlets.
Fusion of the protoplasts
To establish the screening system of hybrid clones, protoplasts of O. sativa L. ssp. japonica (cv. 8411) were treated with 2.5 mmol/L IOA (Iodoacetamide) for 15 min to inactivate their cytoplasts prior to fusion, then washed twice with CPW 13 Mannitol (Frearson et al., 1973) to remove residual IOA. Protoplasts of O. meyeriana were treated with 60 krd soft X-ray to inactivate the nucleus (Akagi et al., 1989). The two types of protoplasts were mixed in a 1:1 proportion and resuspended in F solution (140 mmol/L NaCl, 5 mmol/L KCl, 0.75 mmol/L NaHPO4, 125 mmol/L CaCl2·2H2O, 110 mmol/L Mannitol, pH=7.0), and the same volume of 4% PEG (MW=8.000) was added subsequently to fuse the protoplasts (Yang et al., 1988). Thirty min later, fusion products were diluted gradually by F solution. When the solution volume became 12 times as much, fusion products were collected through low speed centrifugation and washed 2 times with CPW 13 Mannitol solution followed by 5 times with KpR medium. Fusion protoplasts were cultured in the same way as protoplasts.
Determination of chromosome number
The chromosome number of the hybrid lines was determined from root-tip cells according to Levan et al.(1964) for each plant, at least 30 intact cells at metaphase were counted.
Hybrid confirmation
Total genomic DNA isolation was carried out according to the CTAB method (Porebski et al., 1997). Regenerated plants and their parents were analyzed for AFLP patterns using the procedure adapted from Vos et al.(1995) using an AFLP DNA analysis system-I kit (Life Technologies) according to the manufacturer’s instruction. Briefly, 0.5 μg genomic DNA was digested with EcoRI and MseI and ligated with EcoRI and MseI adapters for selective PCR amplification. The DNA fragments generated by PCR-amplification of selected genomic restriction fragments were separated by electrophoresis on a denaturing sequencing acryl amide gel and visualized with silver staining.
Identification of regenerated plantlets that are resistant to bacterial blight
Twenty-nine hybrid plants and the parents were grown in the experiment field of the Ningbo Academy of Agricultural Sciences, according to the rice blast grade criterion of the International Rice Research Institute (0–9 grades). We evaluated the average blast-grade of the inoculated leaves of the 29 hybrid plants. Ten leaves of each line (67 days old) with one of four bacterial blight strains (Chinese pathotypes III, IV, V and VI) of Xanthomonas Oryzae pv. oryza at inoculum concentration of 3×108 cells per milliliter using the leaf clipping method (Kauffman et al., 1973). Thirty-six days following inoculation the lesion of the parental species O. sativa (cv. 8411) and O. meyeriana were examined.
RESULTS AND DISCUSSIONS
Induction of calli and establishment of embryo-derived suspension cell lines
Seeds of O. meyeriana were treated at 45 °C for 48 h before inoculation, which enables gradual calli induction 28 days later, presumably due to the long period of dormancy of the wild rice. The percentage of O. meyeriana embryos induced to form calli was only 27.5%, much less than that of O. sativa cv. 8411 (93%).
Some of the calli originated from the first time induction were non-embryonic. When the final concentrations of sugar (3.0%) and agarose increased to 1.2% and glutamine (500 mg/L) was added to the subculture medium, part of the calli became small-granular and fragile embryo-derived calli. The embryo-derived calli consisting of loose, frangible, and small granular cell clusters were cultured in AA liquid medium and subcultured twice every week. About 4 months later, it became an embryo-derived suspension cell line that was well-dispersed, bright-yellow, densely-cytoplasmic, and vigorously dividing. If the subculture were maintained for too long, the embryo-derived characteristics of the suspension cell line would decrease (part of the cell clusters disaggregated, cells at the surface of cell clusters became rough, irregular granular protuberance appeared, and some cells lysed). Such suspension cells were not suitable for deriving protoplasts. Under this condition, a few suspension cells should be transferred into LS solid medium first and then into AA liquid medium, so that well dispersed, bright-yellow, densely-cytoplasmic, and vigorously dividing cell lines can be obtained again in the future. It should be emphasized that both cv. 8411 and wild rice suspension cell lines should be adjusted to their optimized states before protoplasts preparation.
Production of asymmetric somatic hybrids
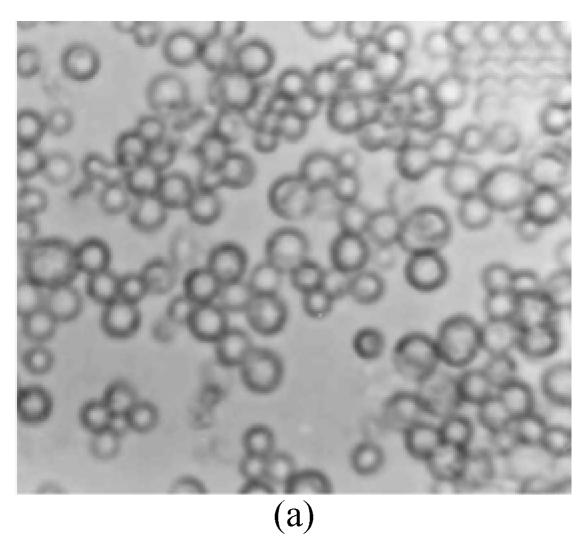
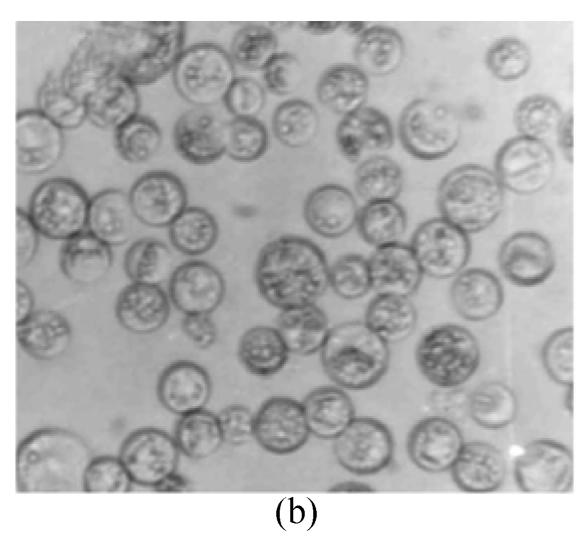
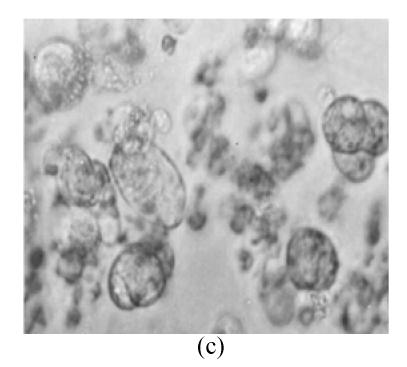
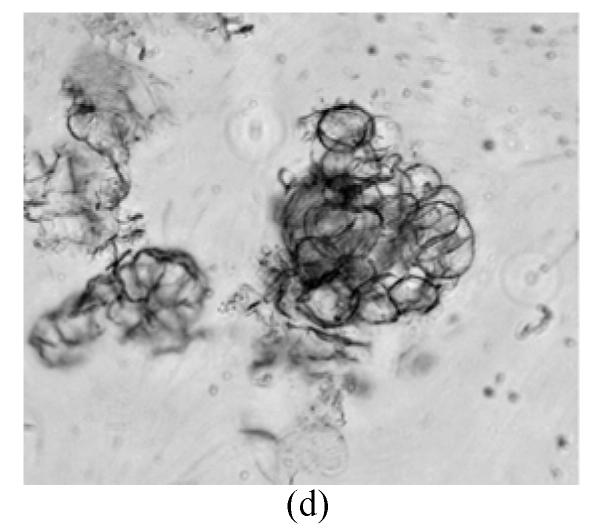
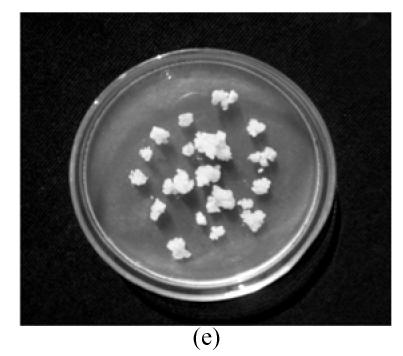
The protoplasts of O. sativa (cv. 8411) (Fig.1a) were treated with IOA to inactivate their cytoplasts. The protoplasts of O. meyeriana (Fig.1b) were treated with soft X-ray to inactivate their nucleus. Thus, neither could divide after the treatments. In contrast, the fusion cells originated from the two types of treated protoplasts could divide continuously and differentially into plantlets in differentiation medium. Compared with the parental protoplasts, the fusion protoplasts showed decreased numbers of swelling ones and an induced rate of division. The first division of the hybrid cells was usually delayed by 1–2 days. The full and shining fusion cells could usually go on dividing to form small cell clusters which would develop in the same way as the ones from rice protoplasts. The fusion protoplasts commonly divided for the first time on the 7th day, for the second time on the 11th day (Fig.1c), continued to divide to form small cell clusters on the 28th day (Fig.1d), to form densely structured calli (Fig.1e) on the 50th day, and to form regenerated plantlets about 3 months later (Fig.1f). We obtained 29 regenerated plantlets possessing morphological traits of both the cultivar (cv. 8411) and the wild rice (such as small size, low fruiting rate, straight sword-shaped leaves, and strong tillering ability).
Fig. 1.
Protoplasts fusion between Oryza sativa (cv. 8411) and O. meyeriana. (a) protoplast of cv. 8411 from cell suspension culture (×200); (b) protoplast of O. meyeriana from cell suspension culture (×200); (c) the second division of the fused cells (×100); (d) small cell colony formed from the fused cells (×50); (e) calli formed from fused cells; (f) Plantlets regenerated from fused cells
Confirmation of somatic hybrid lines
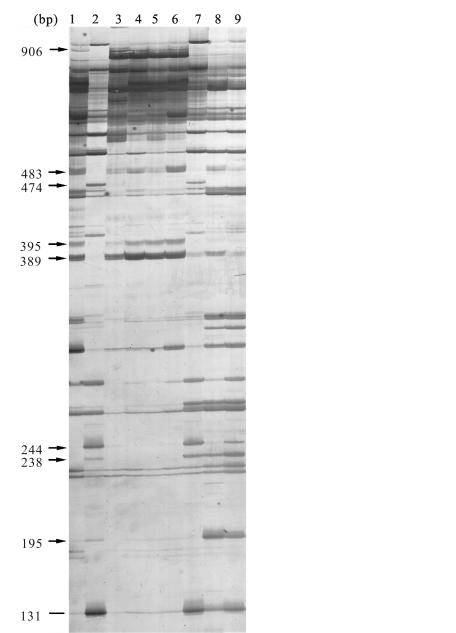
To examine whether these regenerated plants were hybrids between O. sativa (cv. 8411) and O. meyeriana, we carried out AFLP analysis on seven of the regenerated plants representative of different phenotype at the R1 stage and their parental species. Among eight primer pairs used in this study, four of these polymorphic primer pairs showed polymorphic bands among the parents and the hybrids. EcoRI-AAT/MesI-GGC primer pairs produced the greatest number of clear polymorphic bands (Fig.2). Approximately 10 bands from O. meyeriana samples (Lane 2) were not present in O. sativa (cv. 8411), while O. sativa (cv. 8411) (Lane 1) had 3 unique bands. Of these seven regenerated lines analyzed (Lanes 3–9), 5 had O. meyeriana AFLP patterns while 2 had hybrid patterns. These results indicated that the regenerated plants were hybrids between O. meyeriana and O. sativa (cv. 8411). All these seven regenerated lines were previously classified as putative hybrids of O. meyeriana and O. sativa based on their phenotype, two individuals were resistant to bacterial blight (Lanes 7 and 9) (Fig.3), two plants were susceptible to bacterial blight pathogens (Lanes 4 and 5) and 3 showed moderate resistance (Lanes 3, 6 and 8) (Table 1).
Fig. 2.
Part of an amplified fragment length polymorphism (AFLP) gel with analysis of asymmetric somatic hybrid (SH) lines and their parental species. AFLP banding patterns using the primer E-AAT/M-GGC of Oryza sativa cv. 8411 (Lane 1), O. meyeriana (Lane 2), and somatic hybrids from O. meyeriana and Oryza sativa cv. 8411 (Lanes 3–9), arrows indicate the bands of somatic hybrids progeny differing from that of their parents
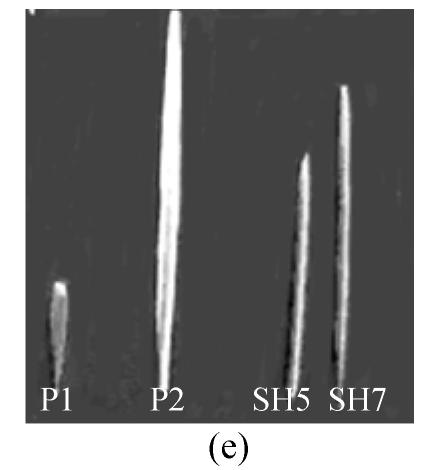
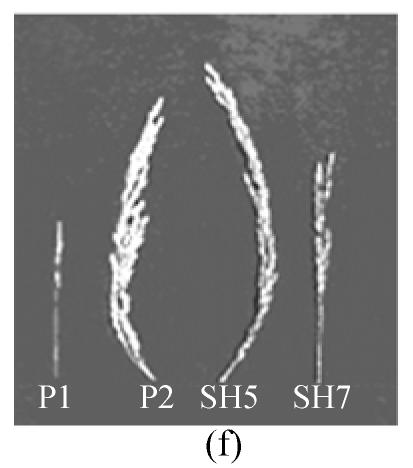
Fig. 3.
Morphology of somatic hybrids (SH5, SH7) and parental species (a) Oryza meyeriana, (b) O. sativa cv 8411, (c) somatic hybrids of blight resistant bacterial strain SH5, (d) somatic hybrids of blight resistant bacterial strain SH7, (e) Leaf morphology of somatic hybrids SH5, SH7 and their parental species, (P1) O. meyeriana indicated high resistance against bacterial blight, (P2) Oryza sativa cv 8411 indicated susceptibility, (SH5) somatic hybrids indicated resistance against bacterial blight, (SH7) somatic hybrids indicated resistance against bacterial blight, (f) Panicle morphology of somatic hybrids SH7, SH5 and their parental strain
Table 1.
Distribution of BB resistance grades among the 29 somatic hybridized lines (SH1–SH29) and the parents
| Plant | Grade | Plant | Grade | Plant | Grade | Plant | Grade |
| O. meyeriana | 0.8 | SH7 | 2.8 | SH15 | 4.9 | SH23 | 7.7 |
| O. sativa (cv. 8411) | 8.1 | SH8 | 6.5 | SH16 | 8.8 | SH24 | 6.1 |
| SH1 | 4.5 | SH9 | 7.1 | SH17 | 7.3 | SH25 | 5.9 |
| SH2 | 6.8 | SH10 | 4.6 | SH18 | 5.2 | SH26 | 4.8 |
| SH3 | 7.6 | SH11 | 5.9 | SH19 | 6.0 | SH27 | 5.1 |
| SH4 | 3.8 | SH12 | 7.2 | SH20 | 5.4 | SH28 | 5.7 |
| SH5 | 3.0 | SH13 | 3.7 | SH21 | 6.4 | SH29 | 6.8 |
| SH6 | 3.7 | SH14 | 6.5 | SH22 | 4.7 |
Scores of more than 5.1, less than 5.1 but more than 3.1, and less than 3.1 indicate low, intermediate, and high resistance, respectively
Examination of the chromosome numbers of SH3–SH9, SH13, SH18 at the R1 stage showed that no additional chromosomes were present in these hybrid lines. Some genetic material from O. meyeriana must have been integrated into the recipient from cell through either chromosome recombination or chromosome substitution. It was obvious that this occurred in hybrid lines (Lane 7 SH5 and Lane 9 SH7), which possessed the characteristic AFLP band (Fig.2) of O. meyeriana. In addition, these two hybrid lines exhibited high bacterial blight resistance derived from O. meyeriana.
Somatic hybrids were resistant to bacterial blight pathogen
We tested 29 SH lines of regenerated plants at the BC1F2 stage (backcrossed with O. sativa cv.8411). All of these SH lines (SH1–SH29) were tested for resistance to X. oryzae pv. oryzae strains using Chinese pathotype III, IV, V and VI. The majority of these hybrid lines exhibited intermediate resistance to the bacterial blight pathogens, compared to the susceptible O. sativa (cv. 8411) [mean bacterial blight resistance score of 8.1 on a scale of 1 (resistant)–9 (susceptible) and resistant O. meyeriana score of 0.8]. Ten hybrid lines had scorces of less than 5.1 and were more resistant to the bacterial blight pathogen than cv. 8411. Two hybrid lines (SH5, SH7) exhibited high resistance to the bacterial blight pathogen and had scores of less than 3.1 (Table 1, Fig.3c–3f. Table 2 shows data on the lesion lengths of O. sativa, O. meyeriana and hybrid lines (SH5 and SH7) in response to inoculation with the Chinese pathotypes III, IV, V, VI. SH5 and SH7 lines showed reduced lesion lengths relative to the susceptible O. sativa cv. 8411.
Table 2.
The leaf lesion of two asymmetric somatic hybrid lines in comparison with their parental species after inoculation with Xanthomonas oryzae pv. oryzae
| Plant | Leaf lesion length (cm) |
|||
| Pathotype: III | IV | V | VI | |
| O. meyeriana | 0.6±0.12 | 0.8±0.14 | 0.73±0.15 | 0.42±0.21 |
| O. sativa (cv. 8411) | 11.3±0.21 | 16.7±0.2 | 15.4±0.26 | 18.1±0.17 |
| SH5 | 2.7±0.29 | 3.0±0.11 | 2.5±0.31 | 2.6±0.18 |
| SH7 | 2.9±0.13 | 2.8±0.14 | 2.4±0.22 | 2.2±0.41 |
Values are means (n=10) and the standard deviations, measured at 36 days after inoculation with X. o. pv. oryzae
CONCLUTION
The availability of O. meyeriana bacterial blight resistance genes in the hybrid rice plants provides additional genes to the current resistance gene pool in commercial cultivars. These new bacterial blight resistance genes from O. meyeriana can be used to further improve rice resistance to a broad spectrum of bacterial blight pathogens by crossing these hybrid lines with other bacterial blight-resistant rice genotypes.
Footnotes
Project (No. 98001) supported by Ningbo Agriculture Key Scientific Research Foundation, China
References
- 1.Abdullah R, Cocking EC, Thompsom A. Efficient plant regeneration from rice protoplasts through somatic embryogenesis. Bio/Technology. 1986;4:1087–1090. [Google Scholar]
- 2.Akagi H, Sakamoto M, Negishi T, Fujimura T. Construction of rice hybrid plants. Mol. Gen. Genet. 1989;215:501–506. [Google Scholar]
- 3.Frearson EM, Power JB, Cocking EC. The isolation culture and regeneration of Petunia leaf protoplasts. Dev. Biol. 1973;l33:130–137. doi: 10.1016/0012-1606(73)90169-3. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi Y, Kyozuka J, Shimamoto K. Hybrids of rice (Oryza sativa L.) and wild Oryza species obtained by cell fusion. Mol. Gen. Genet. 1988;214:6–10. [Google Scholar]
- 5.Jena KK, Khush GS. Introgression of genes from Oryza officinalis well to cultivated Oryza sativa L. Theor. Appl. Genet. 1990;80:737–745. doi: 10.1007/BF00224186. [DOI] [PubMed] [Google Scholar]
- 6.Jelodar NB, Blackhall NW, Hartman TPV, Brar DS, Khush G, Davey MR, Cocking EC. Intergeneic somatic hybrids of rice (Oryza sativa L.) and Porteresia coarctata (Roxb.) Tateok. Theor. Appl. Genet. 1999;99:570–577. doi: 10.1007/s001220051270. [DOI] [PubMed] [Google Scholar]
- 7.Kao KN. Chromosomal behaviour in somatic hybrids of soybean-Nicotiana glauca . Mol. Gen. Genet. 1997;150:225–230. [Google Scholar]
- 8.Kauffman HE, Reddy APKS, Hsieh PY, Nerca SD. An improved technique for evalution of resistance of rice varieties to Xanthomonas Oryzae . Plant Dis. Rep. 1973;57:537–541. [Google Scholar]
- 9.Kisaka H, Kisaka M, Kanno A, Kameya T. Intergeneric somatic hybridization of rice (Oryza sativa L.) and barley (Hordeum vulgare L.) by protoplast fusion. Plant Cell Rep. 1998;17:362–367. doi: 10.1007/s002990050407. [DOI] [PubMed] [Google Scholar]
- 10.Levan A, Fredya K, Sandberg A. Nomenclature for centromeric position on chromosome. Hereditas. 1964;54:201–220. [Google Scholar]
- 11.Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiologia Plantarum. 1965;18:100–127. [Google Scholar]
- 12.Peng SQ, Liu EM, Huang FY, Xiao FG, Fan KC, Luo LM, Chen Y. Research on durable resistance to rice blast disease. Acta Phytophylacica Sinina. 1996;23:293–299. (in Chinese) [Google Scholar]
- 13.Porebski S, Bailey G, Baum BR. Modification of CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997;15:8–15. [Google Scholar]
- 14.Vos P, Hogers R, Bleeker M, Reijans M, Van De Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan CQ, Yan QS, Qian KX, et al. Plant Regeneration from Somatic Hybridization between Oryza Meyerana and O. Sativa cv.8411; International Rice Congress Abstract; 2002. pp. 267–268. [Google Scholar]
- 16.Yang ZQ, Shiknai T, Yamada Y. Asymmetric fertile rice (Oryza sativa L.) protoplast. Theor. Appl. Genet. 1988;76:801–808. doi: 10.1007/BF00273664. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XQ, Yan QS, Teng S. Study on transfer of apomixes gene of Panicum maximum into rice plant. Chinese Science. 1999;5:1579–1580. (in Chinese) [Google Scholar]
- 18.Zhu ZQ, Wang JJ, Shen JS, Xu Z, Zhu ZY, Yang GC, Bei FY. Establish rice anther culture medium by nitrogen content. Sci China (B) 1975;5:484–490. (in Chinese) [Google Scholar]