Abstract
Objective: To investigate the anti-tumor efficacy of dendritic cell (DC)-based vaccines pulsed with tumor extracts or RNA in a mouse model of intracranial G422 glioblastoma. Methods: Bone marrow-derived DCs were pulsed exvivo with tumor extracts or RNA. Ninety female mice harboring 4-day-old intracranial G422 glioblastomas and 126 normal mice were treated with three spaced one week apart subcutaneous injections either with PBS, unpulsed DCs, G422 tumor extracts, RNA, DCs pulsed with G422 tumor extracts (DC/extract) or with RNA (DC/RNA). Seven days after the third immunization of normal mice, the spleens of 36 of them were harvested for cytotoxic T lyphocyte (CTL) assays and the others were challenged in the brain with G422 tumor cells. All the treated mice were followed for survival. Some mice brains were removed and examined pathologically when they died. Results: Immunization using DC/extract or DC/RNA significantly induced G422-specific CTL responses compared with control groups (P<0.01). Vaccination with DC/extract or DC/RNA, either prior to G422 tumor challenge or in tumor-harboring mice, significantly prolonged survival compared with other control groups (P<0.01). Conclusion: DCs pulsed with tumor extracts or RNA derived from autologous tumors has potential antitumor effects via activation of cell-mediated immunity. Our results suggest a useful therapeutic strategy against gliomas.
Keywords: Dendritic cell, Glioma, Immunotherapy
INTRODUCTION
Gliomas are the most common primary tumors of the central nervous system. Despite the advances in surgery, radiation therapy and chemotherapy, the prognosis of patients with primary malignant glioma has not improved significantly over the past 20 years. Recently, due to exponential increase in genetic and molecular biological knowledge about gliomas, immunotherapy becomes more and more important strategy in the treatment of tumors. Dendritic cell (DC) presents antigen to naive or quiescent T cells and plays a central role in the induction of T and B cell immunity in vivo. Immunizations using DCs loaded with tumor antigens may represent a powerful method for inducing antitumor immunity (Schreurs et al., 2000). The present study investigated the anti-tumor efficacy of DCs-based vaccines pulsed with tumor extracts or RNA in a mouse model of intracranial G422 glioblastoma and to evaluate the feasibility of this active immunotherapy strategy for gliomas.
MATERIALS AND METHODS
Animal and cells
Female Kuming mice (6–8 weeks, about 20 gram) were purchased from the animal center of Zhejiang Provincial Institute of Drug Control. G422 glioblastoma cells were purchased from the Institute of Neurosurgery of Beijing.
Materials
Trizol RNA isolation kit and RPMI-1640 were purchased from Gibro BRL, rmGM-CSF and rmIL-4 from R&D, mitomycin C from Sigma Co., FCS from Hyclone, and DOTAP from Roche Co., Anti-I-Ek-FITC, anti-CD80-FITC and anti-CD86-FITC were purchased from PharMingen.
Bone marrow-derived DCs
Under sterile condition, bone marrow was flushed from their femurs and depleted of red cells with ammonium chloride. Cells were plated in 24-well culture plates (1×109 L−1, 3 ml/well) in RPMI-1640 medium supplemented with 20 μg/L rmGM-CSF and 2 μg/L rmIL-4. On day 3 of culture, floating cells were gently removed, and fresh medium and rmGM-CSF and rmIL-4 were added. On day 6 of culture, nonadherent cells and loosedly adherent proliferating DC aggregates were collected and replaced in 100-mm Petri dishes (1×109 cells/L, 10 ml/dish). At day 7 of culture, nonadherent cells (DCs) were removed for analysis and immunizations. Prior to vaccination, the DC phenotype was confirmed by fluorescence analysis using the following fluorescein isothiocyanate (FITC) conjugated mAbs: CD80, CD86, I-Ek. Isotype-matched mAbs conjugated to FITC were used as negative controls. The concentration of DCs was adjusted to 2×109–5×109 L−1.
DCs were pulsed with tumor extracts or tumor RNA
Tumor extracts were obtained by sonicating tumor cells in Opti-MEM (2×1010 cells/L) using a special ultrasonic cleaner. Tumor extracts (500 μl) and DOTAP (125 μg in 500 μl Opti-MEM) were mixed in a 12 mm×75 mm polystyrene tube at room temperature for 20 min. DCs were then added to the mixture and incubated at 37 °C for 4 hours; the concentration was adjusted to 2×109 tumor extract-pulsed DCs/L PBS. Tumor RNA was extracted using Trizol RNA isolation kit according to the manufactures’ specifications. Tumor RNA (25 μg in 250 μl Opti-MEM, 250 μl) and DOTAP (50 μg in 250 μl Opti-MEM, 250 μl) were mixed in the 12 mm×75 mm polystyrene tube at room temperature for 20 min. DCs were then added to the mixture and incubated at 37 °C for 4 hours; the concentration was adjusted to 2×109 tumor RNA-pulsed DCs/L PBS.
Experimental protocols
One-hundred and twenty-six female mice were treated with three spaced one week apart subcutaneous injections with different agents (100 μl/mouse, 21 mice/group) at random. Group I: PBS; Group II: unpulsed DC (2×105/mouse); Group III: tumor extracts (1×106 tumor cell extracts/mouse); Group IV: tumor RNA (2.5 μg/mouse); Group V (DC/extract): DC pulsed with tumor extracts (2×105 tumor extract-pulsed DCs/mouse); Group VI (DC/RNA): DCs pulsed with tumor RNA (2×105 tumor RNA-pulsed DCs/mouse). Groups I to IV were control groups. Seven days after the third immunization, spleens of 36 mice (6 mice/group) were harvested for cytotoxic T lyphocyte (CTL) assays and the other 90 mice (15 mice/group) were challenged in the brain with G422 tumor cells and were followed for survival. Another 90 female mice harboring 4-day-old intracranial G422 glioblastomas were also divided into six groups (15 mice/group) at random in terms of different subcutaneous injections as described above. These mice were also followed for survival.
Intracranial G422 glioblastomas implantation
Intracranial tumor implantation was performed by microinjector 2 mm anterior and 2 mm lateral to the junction of the sagittal to lambdoid suture. G422 tumor cells (1×105, 5 μl) were injected into the deep area of the parietal lobe.
Cytotoxicity assay
Splenocytes were obtained from immunized mice and after restimulation in vitro, incubated at 37 °C with 5% CO2 for 5 days vitro with mitomycin C-treated G422 cells. Cytotoxic activity was tested against G422 cells. Lactic dehydrogenase assay was used to evaluate the activity of CTL. The cytotoxicity was calculated using the following formula: [(experimental release−background release)/(maximum release−background release)]×100.
Histopathological examinations of tumors
Some brains from mice in different treatment groups were removed soon after death for histopathological examination with hematoxylin and eosin staining.
Statistical analysis
Survival estimates and median survivals were determined using the Kaplan and Merier method. The activity of CTL cytotoxicity was compared using the method of Student-Newman-Keuls. Statistical significance was set at the level of P<0.05.
RESULTS
Bone marrow-derived DC phenotype
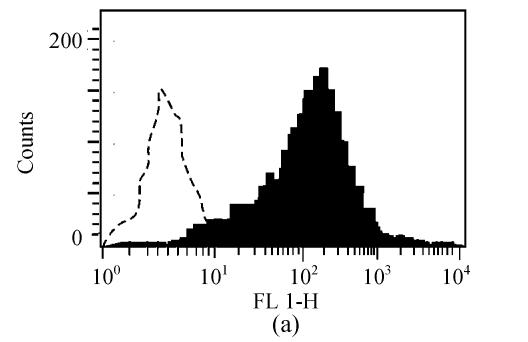
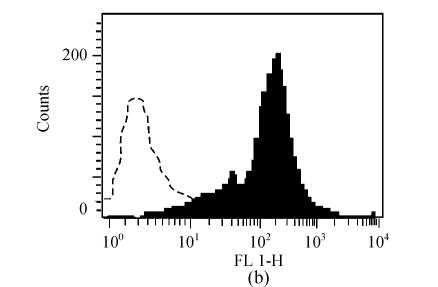
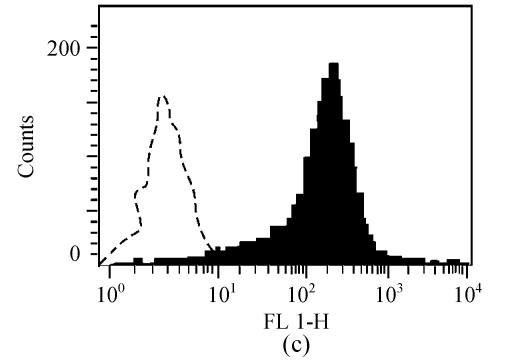
Prior to vaccination, the phenotype of DCs was confirmed. As shown in Fig.1, DCs demonstrated increased expression compared with isotype controls of CD80, CD86, I-Ek.
Fig. 1.
Characterization of cell surface molecule expressions in DCs. The filled region represents DCs staining with FITC-conjugated CD80 (a), CD86 (b) and I-Ek (c). Isotype-matched mAbs conjugated to FITC were used as negative controls
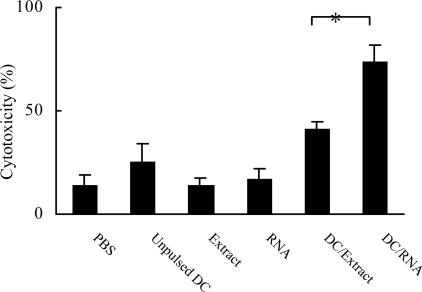
DC/extract or DC/RNA induced tumor-specific CTL
As shown in Fig.2, G422-specific CTL responses were significantly higher after immunization with DC/extract or DC/RNA compared with immunization with DCs pulsed with controls (P<0.01).
Fig. 2.
Vaccination with bone marrow-derived DCs pulsed with tumor extracts or tumor RNA induced tumor-specific CTL (*P<0.01 vs other groups)
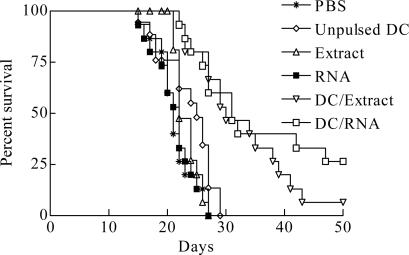
DC/extract or DC/RNA prolonged survival of mice with G422 tumor challenge in brain after immunization
As shown in Fig.3, no mouse in the control groups, immunized using PBS, unpulsed DC, tumor extracts or RNA, survived for more than 30 days, and the survival time were 21.40±0.87, 23.33±1.18, 23.27±0.52 and 21.40±0.91 days, respectively. In contrast, significantly prolonged survival was observed in DC/extract (32.67±2.13 days) and DC/RNA (35.47±2.91 days) groups compared with other control groups (P<0.01), with 53.33% and 60.00% mice surviving for more than 30 days, respectively.
Fig. 3.
Prolonged survival of mice with G422 tumor challenge in CNS after immunization using DCs with tumor extracts or tumor RNA (P<0.01)
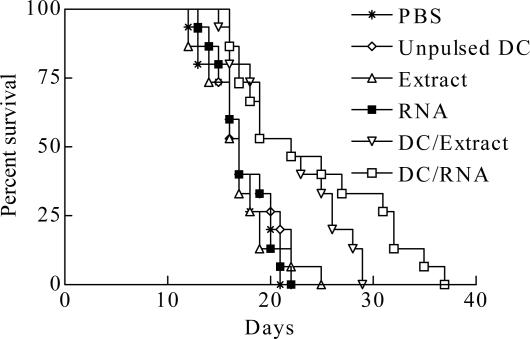
DC/extract or DC/RNA prolonged survivals of mice bearing intracranial G422 tumor
As shown in Fig.4, mice treated with PBS, unpulsed DCs, tumor extracts or RNA survived for 17.00±0.76, 17.67±0.80, 16.93±0.90 and 17.53±0.69 days, respectively. In contrast, mice in DC/extract or DC/RNA groups had a significantly longer survival time of 22.00±1.27 and 24.20±1.94 days compared with other control mice (P<0.01). 40.00% in DC/extract group and 46.67% in DC/RNA groups survived for 25 days whereas none of the control mice survived for 25 days.
Fig. 4.
Prolonged survival of mice bearing intracranial G422 glioblastomas and treated using DCs with either tumor extracts or tumor RNA (P<0.01)
DC/extract or DC/RNA did not induce demonstrable allergic encephalitis
Vaccination with DC/extract or DC/RNA induced large areas of hemorrhage and necrosis with associated severe inflammatory infiltration in brain tissue compared with other control groups. Outside the peritumoral regions, the brain parenchyma appeared histologically normal. No allergic encephalitis was found in the brain tissue.
DISCUSSION
It has long been recognized that patients harboring malignant gliomas exhibit depressed cellular immune responses compared with healthy individuals (Parney et al., 2000). Although gliomas may contain immunogenic antigens, glioma cells are known to be poor antigen presenters to the immune system, in part because of down-regulation of B7 co-stimulatory molecules required for direct tumor cell activation of T cells and secretion of immunosuppressive cytokines such as TGF-β. Professional antigen-presenting cells (APCs) are needed to efficiently internalize, process, and present gliomas antigens to T cells and then to induce antitumor immune response against gliomas. DCs are bone marrow-derived cells similar to monocyte/macrophages that function as the most potent APCs for priming T cells in vivo and vitro. DCs are attractive candidates for innovative gliomas immunotherapy by virtue of their ability to function as powerful APCs and elicit potent antitumor cytotoxic immune responses (Yamanaka et al., 2003a; Fecci et al., 2003).
Successful brain tumor immunotherapy with DCs has been reported in several animal models. Mice with intracranial C3 tumors treated with DCs pulsed with the tumor-specific peptide E7 showed long term survival. It indicated a pivotal role of specific CD8+ T-cell responses in mediating the anti-tumor effect (Okada et al., 1998). In a murine melanoma model, immunization with DCs mixed with tumor-specific peptide resulted in an antigen-specific immunological response and long-lasting antitumor immunity against intracerebral tumors (Heimberger et al., 2002). Due to the advent of monoclonal antibody technology, it has been suggested that there are few true tumor-specific antigens. Therefore without a relatively specific tumor-associated antigen identified for human gliomas, targeting a single tumor-specific peptide may not be entirely realistic in the clinical setting. Furthermore, it has been suggested that an optimum host antitumor T-cell response against certain cancers may require a broad spectrum of epitopes rather than responses restricted to a single tumor-associated determinant. Vaccination with syngeneic DC pulsed with acid-eluted peptides derived from autologous tumors prolonged survival for rats harboring preestablished intracranial 9L gliosarcomas through enhancing CD8+ cell infiltration into the tumors and induction of 9L-specific cytotoxic T lymphocytes (Liau et al., 1999). Vaccination with tumor homogenate also protects against syngenic intracerebral glioma (Heimberger et al., 2000). The advantages of vaccinating with total tumor-derived material such as acid-eluted peptides, tumor extracts, tumor homogenate, or RNA, are that the identity of the tumor antigen need not be known and that the presence of multiple tumor antigens reduces the risk of antigen-negative escape mutants (Vierboom et al., 1997). In these cases, the vaccine contains multiple antigens, increasing the probability of inducing immunity relative to one associated antigen. Vaccination with unfractionated tumor-derived antigens, such as extracts or homogenate may lead to potentially disastrous consequences such as autoimmune encephalitis, which may limit the use of such vaccine. A potential advantage of using RNA rather than protein or peptides as source of unfractionated tumor antigen is that sufficient amount of antigen can be generated from very small amounts of tumor tissue using PCR amplification techniques. Moreover, if autoimmunity becomes a problem, subtractive hybridization can reduce the contribution of non tumor-specific antigens (Boczkowski et al., 1996).
T cell-mediated immunity specifically recognizes foreign antigens. CTL can acquire cytolytic potential to lyse infected cells or tumor cells. Induction of a primary CTL response is one of the most stringent tests of antitumor immunity (Liau et al., 1999). In the present study, animals treated with DCs pulsed with either G422 tumor extracts or tumor RNA showed significantly increased primary anti-tumor CTL response in vitro, suggesting the ability of DCs pulsed with either G422 tumor extracts or tumor RNA to express a specific tumor antigen and induce immune response against intracranial tumors. Our in vivo studies showed that treatment of tumor-bearing mice with DCs pulsed with either G422 tumor extracts or tumor RNA led to a dramatic reduction in the mortality rate, indicating that DCs vaccine is capable of inducing antitumor response against syngenic murine gliomas within the immunologically privileged brain and CNS may not be an absolute barrier to DC-based immunotherapy. Moreover, DC-based immunization can lead to immuniologic memory with protection against subsequent tumor challenge. The fact that DC-cured mice are protected from future tumor rechallenge is suggestive of an anti-tumor memory which is a specific and long lasting immune-mediated response. DC therapy proved to be safe in both animal models and clinical trials (Yamanaka et al., 2003b). No serious side effects and no evidence of autoimmune toxicity occurred. More complete evaluation needs further studies, but these initial results appear promising. The future success of clinical trials will depend on the optimization and standardizing of procedures for DC generation, loading, and administration.
In conclusion, DCs pulsed with tumor extracts or RNA derived from autologous tumors represent a promising approach to the immunotherapy of gliomas, which can specifically activate antitumor T cells and lead to significantly prolonged survival in tumor-bearing animals. This may therefore be a useful therapeutic strategy against gliomas in humans in the future.
Footnotes
Project supported by the Scientific Research Foundation for Returned Overseas Chinese Scholars, State Education Ministry, China
References
- 1.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vito. J Exp Med. 1996;184(4):465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fecci PE, Mitchell DA, Archer GE, Morse MA, Lyerly HK, Bigner DD, Sampson JH. The history, evolution, and clinical use of dendritic cell-based immunization strategies in the therapy of brain tumors. J Neurooncol. 2003;64(1-2):161–176. doi: 10.1007/BF02700031. [DOI] [PubMed] [Google Scholar]
- 3.Heimberger AB, Crotty LE, Archer GE, McLendon RE, Friedman A, Dranoff G, Bigner DD, Sampson JH. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103(1):16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 4.Heimberger AB, Archer GE, Crotty LE, McLendon RE, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective agaist murine intracerebral melanoma. Neurosurgery. 2002;50(1):158–164. doi: 10.1097/00006123-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Liau LM, Black KL, Prins RM, Sykes SN, DiPatre PL, Cloughesy TF, Becker DP, Bronstein JM. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90(6):1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 6.Okada H, Tahara H, Shurin MR. Bone marrow-derived dendritic cells pulsed with a tumor-specific peptide elicit effective anti-tumor immunity against intracranial neoplasms. Int J Cancer. 1998;78(2):196–201. doi: 10.1002/(sici)1097-0215(19981005)78:2<196::aid-ijc13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Parney IF, Hao C, Petruk KC. Glioma immunology and immunotherapy. Neurosurgery. 2000;46(4):778–786. doi: 10.1097/00006123-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Schreurs MW, Eggert AA, Punt CJ, Figdor CG, Adema GJ. Dendritic cell-based vaccines: from mouse models to clinical cancer immunotherapy. Crit Rev Oncog. 2000;11(1):1–17. [PubMed] [Google Scholar]
- 9.Vierboom MP, Nijman HW, Offringa R, van der Voort EI, van Hall T, van den Broek L, Fleuren GJ, Kenemans P, Kast WM, Melief CJ. Tumor eradication by wild-type p53-specific cytotoxic Tlymphocytes. J Exp Med. 1997;186(5):695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka R, Yajima N, Abe T, Tsuchiya N, Homma J, Narita M, Takahashi M, Tanaka R. Dendritic cell-based glioma immunotherapy. Int J Oncol. 2003;23(1):5–15. [PubMed] [Google Scholar]
- 11.Yamanaka R, Abe T, Yajima N. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89(7):1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]