Abstract
Recent experiments show that properly controlled recombination between homologous DNA molecules is essential for the maintenance of genome stability and for the prevention of tumorigenesis.
Exchange of sequence information between two homologous DNA molecules, referred to as homologous recombination, makes a major contribution to the repair of DNA damage and thereby contributes to the preservation of genome integrity [1]. In particular, double-strand breaks or gaps in the DNA of sister chromatids can be repaired flawlessly by homologous recombination. In addition, accurate replication of the genome is intimately coupled to homologous recombination [2], because imperfections in the DNA template often lead to arrest and breakdown of the replication fork. The resulting DNA intermediates are acted upon by homologous-recombination factors that rebuild a functional replication fork. Finally, homologous recombination contributes to the generation of genetic diversity and the faithful germline transmission of genetic information during meiosis [3].
In spite of these important biological functions, homologous recombination was for a long time considered to be a minor and rather inefficient process in mammalian cells. This view has radically changed during the past few years. The widespread role of homologous recombination in the repair of DNA damage in mammalian cells is now firmly established. Furthermore, a growing list of genome-destabilizing human genetic diseases and syndromes that confer increased susceptibility to cancer have been linked to aberrant homologous recombination [4]. Here, we highlight recent findings concerning regulatory and mechanistic aspects of homologous recombination in mammalian cells.
Homologous recombination: the core reaction
Discontinuities in double-stranded DNA, particularly DNA double-strand breaks, are prime instigators of homologous recombination. Homologous recombination restores the continuity of a broken DNA molecule by using an intact and homologous DNA molecule (usually the sister chromatid) as a template (see Figure 1) [3]. To copy information from the template, the DNA ends at the break site are first processed into single-stranded DNA tails with 3' extensions, presumably by the combined action of helicases and/or nucleases. These tails are the substrate onto which monomers of the Rad51 recombinase polymerize to form a nucleoprotein filament. This filament executes the central functions in homologous recombination: the search for a homologous template DNA and the formation of a joint heteroduplex molecule between the damaged DNA and the undamaged template. In addition to Rad51, these steps require the coordinated action of a number of other homologous-recombination proteins, including the RP-A protein, which binds single-stranded DNA, Rad52, which can bind DNA ends and anneal complementary single-stranded DNA molecules [5], and a number of Rad51 paralogs (see Table 1). The joint heteroduplex molecule provides the substrate for DNA synthesis, and this requires at least one DNA polymerase and its accessory factors to restore the missing information. The continuity of the strands is established by a DNA ligase. Migration of the branch-point of the crossed DNA strands (known as 'Holliday junctions') allows the generation of genuine heteroduplex DNA, consisting of one strand from each of the parental DNA molecules. Finally, the recombined molecules are separated into intact duplex DNAs, in a process called 'resolution' (Figure 1).
Figure 1.
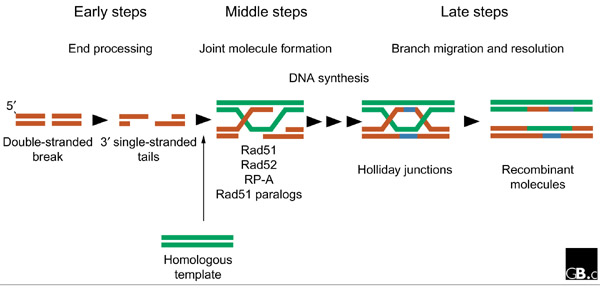
Schematic representation of the critical steps of homologous recombination. The early steps of the reaction consist of processing the substrate DNA. Indicated in red is a DNA molecule with a double-stranded break. The DNA ends are processed to form 3' single-stranded tails. During the middle steps, the tails are used by Rad51 and its accessory proteins to search for, and invade, a homologous DNA template (green). In the resulting joint molecules, extension and annealing of DNA strands by de novo synthesis (blue) restores the continuity of the broken DNA. During the late steps, the branched Holliday junctions are resolved into two duplex DNAs. For simplicity, only one outcome of the resolution process is shown.
Table 1.
Rad51 and its accessory proteins in different organisms
| Organism | Rad51 | Rad51-accessory proteins | |
| recombinase | Rad52 | Rad51 paralogs | |
| S. cerevisiae | Present | Present | Rad55, Rad57 |
| C. elegans | Present | Absent | Absent |
| D. melanogaster | Present | Absent | Rad51C, Rad51D |
| Chicken | Present | Present | Xrcc2, Xrcc3, Rad51B, |
| Rad51C, Rad51D | |||
| Human | Present | Present | Xrcc2, Xrcc3, Rad51B, |
| Rad51C, Rad51D | |||
This information was extracted from the Saccharomyces Genome Database [29], the C. elegans Genome Project [30], the Berkeley Drosophila Genome Project [31], and the Human Genome Resources [32]; the databases were accessed in March 2001.
Genome projects reveal species differences in the complement of recombination proteins
Although it is clear that the key player in homologous recombination, the Rad51 recombinase, is conserved from bacteriophages to humans, recent whole-genome sequencing projects have yielded a more complete insight into the conservation of Rad51-accessory proteins. Interestingly, different organisms seem to get by with a different sets of accessory proteins. In particular, the difference in the conservation of Rad52 and the Rad51 paralogs is striking (see Table 1). Although conserved in yeast and mammals, Rad52 appears to be lacking from Drosophila and Caenorhabditis elegans. Furthermore, the C. elegans genome sequence contains no candidate genes that could encode Rad51 paralogs.
The yeast Saccharomyces cerevisiae Rad52 protein and the heterodimeric complex of the Rad51 paralogs Rad55 and Rad57 have been implicated in overcoming the inhibitory effect that RP-A has on DNA-strand exchange catalyzed by Rad51 [6,7,8,9]. RP-A is required for efficient DNA-strand exchange, and it is presumed to act by removing secondary structure from the single-stranded DNA to promote efficient formation of the Rad51 nucleoprotein filament [10]. RP-A is a much more tenacious single-stranded-DNA-binding protein than Rad51, however. Because of this property, RP-A can cause inhibition of DNA-strand exchange, but this inhibition can be alleviated by Rad52 or the Rad55-Rad57 heterodimer. Human Rad52 protein has also been shown to stimulate the formation of the joint heteroduplex DNA generated by human Rad51 [11]. The role of the mammalian Rad51 paralogs in this reaction is still unclear, however, because the biochemical characterization of the mammalian Rad51 paralogs is still in its infancy.
Interestingly, the recombination defects seen in RAD52-disruption mutants are much more severe in yeast cells than in mammalian cells [3,12,13]. Conversely, whereas the recombination phenotype of yeast Rad55/Rad57 mutants is mild, mouse cells lacking Rad51-paralog genes are not viable [3,14]. Perhaps, the major contribution to assisting Rad51 in homologous recombination has shifted from Rad52 in yeast towards the Rad51 paralogs in mammals. Surprisingly, C. elegans lacks these Rad51-accessory proteins. The C. elegans Rad51 protein might have radically different biochemical properties from its mammalian or yeast counterparts, but its high degree of conservation (59% identical to human Rad51) makes that unlikely. Alternatively, C. elegans might contain Rad51-accessory proteins that have no sequence homology to Rad52 or the Rad51 paralogs. It is not unprecedented that proteins that are not related in amino-acid sequence can nevertheless perform similar functions. For example, loading of the Escherichia coli Rad51-homolog RecA onto single-stranded DNA can be stimulated by either RecBCD or RecOR, according to the particular RecA-dependent subpathway of recombination [15,16]. In sequence terms, these proteins have no similarity to each other or to Rad52 or the Rad51 paralogs.
Regulation of homologous recombination in mammalian cells
Whereas the core homologous-recombination machinery is conserved between yeast and humans, it is now clear that additional important homologous-recombination proteins, such as BRCA1 and BRCA2, are present only in mammalian cells. Germline mutations in the BRCA1 and BRCA2 genes were first identified because they predispose carriers to breast cancer [17]. Early on, associations between both BRCA proteins and human Rad51 were demonstrated, suggesting a link between homologous recombination and the BRCA proteins [17]. The involvement of the BRCA proteins in homologous recombination has now been firmly established by recent experiments from a number of laboratories.
The involvement of mouse Brca1 in homologous recombination was established by the Jasin [18] and Koller [19] laboratories. They used homologous gene-targeting assays to measure the efficiency of homologous recombination. They found that gene targeting in Brca1-deficient mouse embryonic stem cells was reduced by more than 20-fold compared to Brca1-containing cells. Recently, Moynahan et al. [20] showed that gene-targeting efficiency was also reduced in Brca2-deficient cells, but only by a factor of two. Interestingly, even though the efficiencies of homologous gene targeting showed a 10-fold difference in Brca1- and Brca2-deficient cells, the cells were equally deficient in repair of a site-specific chromosomal DNA double-strand break [18,20]. Perhaps, Brca1 and Brca2 contribute differentially to the various subpathways of homologous recombination [20].
Because of their absence from yeast, the BRCA proteins are probably not required for the core steps of homologous recombination. Instead, they might be important in mammalian cells to help solve logistical problems of DNA repair in a larger genome. An organizational or regulatory role for BRCA2 in homologous recombination is suggested by recent experiments from the Stasiak, Venkitaraman and West laboratories [21]. The formation of joint intermediate molecules made up of the processed broken DNA ends and the double-stranded template DNA, which is mediated by Rad51, is at the core of most homologous recombination (see Figure 1), and so may be a critical target for regulation. Knowing that BRCA2 can physically interact with Rad51, Davies et al. [21] explored the biological relevance of this protein-protein interaction. Using peptides derived from the Rad51-interaction domain of BRCA2, they showed that interaction with BRCA2 controls DNA binding by Rad51. When bound to some of the BRCA2-derived peptides, Rad51 was no longer able to self-associate and to assemble nucleoprotein filaments on single-stranded extensions of broken DNA molecules. Given that the assembly of these filaments is an absolute prerequisite for the strand invasion reaction that is catalyzed by Rad51 (see middle steps in Figure 1), the results of Davies et al. [21] point to BRCA2 as an excellent candidate for a key regulator of homologous recombination. The authors suggest that BRCA2 sequesters Rad51 into an inactive form that can be relocalized to sites of DNA damage and activated upon receiving the appropriate signal. Consistent with this notion, locally increased concentrations of Rad51, found at sites of DNA damage in BRCA2-containing cells [22], occur much less efficiently in BRCA2-deficient cells [17].
Although a decrease in homologous recombination was found in BRCA-deficient cells, an increase in homologous recombination has recently been linked to tumor susceptibility as well. The Shultz and Bradley laboratories generated mice that were deficient in the Bloom syndrome gene [23]. This gene encodes a DNA helicase, BLM, of the E. coli RecQ family, which is implicated in many aspects of DNA metabolism [24]. Cells from Bloom-syndrome patients have an increase in homologous recombination, and the patients are predisposed to cancer [24,25]. Luo et al. [23] found that BLM-deficient mice were also cancer-prone. Using polymorphic microsatellite markers, they provided evidence that the underlying mechanism of the cancer predisposition is a loss of heterozygosity resulting from increased homologous recombination. These studies of BRCA-deficient cells and BLM-deficient mice underline the importance of accurate regulation of the levels of homologous recombination in mammalian cells.
Resolution of recombination intermediates
Upon joint-molecule formation and DNA synthesis, branched DNA structures called Holliday junctions can form as late intermediates in homologous recombination (see Figure 1). Holliday junctions can slide, or 'branch-migrate', along the joined DNAs. Branch migration extends the heteroduplex DNA region between identical recombination partners and might thereby provide a mechanism to prevent recombination between repetitive sequences that are dispersed throughout the genome. Branch migration can be promoted by specialized helicases such as the bacterial RuvAB proteins [26]. Completion of recombination requires the resolution of Holliday junctions, in order to separate the recombining partners. One well-characterized way of resolving Holliday junctions requires the enzymatic action of a resolvase, such as the bacterial RuvC protein, which interacts with the RuvAB proteins to form a 'resolvasome' [26]. Efforts to identify yeast and mammalian homologs of the RuvABC proteins by sequence-homology searches have not led to the identification of a mammalian resolvasome. Mammalian protein preparations containing an activity analogous to the RuvC resolvase have been described in the past [27], however, suggesting at least a functional conservation of this mode of resolution in mammalian cells; but no eukaryotic branch migration activity had been reported until recently.
Now, the West laboratory [28] has pushed the biochemical characterization of the mammalian resolvase activity a step forward. Constantinou et al. [28] re-isolated the mammalian Holliday-junction-resolvase activity, from calf testis, but this time they managed to co-purify an ATP-dependent branch-migration activity analogous to the RuvAB activity. This mammalian resolvasome was active not only on synthetic oligonucleotide-based substrates but also on genuine strand-exchange intermediates. In addition, the branch migration and resolution activities operated in a concerted manner, just as in the RuvABC resolvasome. The identity of the proteins involved remains to be determined, however. The BLM and Werner-syndrome genes, both of which encode helicases that can catalyze branch migration in vitro [24], were potential candidates, but they have been ruled out because extracts from cell lines isolated from Bloom or Werner syndrome patients were still able to carry out branch migration and resolution [28].
The study of homologous recombination has traditionally been the domain of basic biological sciences. Elegant genetic and biochemical experiments using bacteriophages, bacteria and fungi resulted in the identification of the major players and pathways in homologous recombination. A sophisticated level of understanding of both the protein machinery and the complex DNA gymnastics underpinning homologous recombination has come from the study of these model organisms. Recently, however, homologous recombination has moved into the domain of medical science with the realization of the importance of properly controlled homologous recombination in providing genome stability in mammalian cells and its involvement in the prevention of carcinogenesis. In the next few years, we can expect considerable new insights to come from biochemical and cell biological approaches, as well as from large-scale genomic and proteomic analyses of DNA damage responses in mammalian cells.
Acknowledgments
Acknowledgements
We thank Claire Wyman for comments on the manuscript. Work in our laboratory is supported by the Netherlands Organization of Scientific Research (NWO), the Dutch Cancer Society (KWF), and the Association for International Cancer Research.
References
- Jasin M. Chromosome breaks and genomic instability. Cancer Invest. 2000;18:78–86. doi: 10.3109/07357900009023065. [DOI] [PubMed] [Google Scholar]
- Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. doi: 10.1016/S0014-5793(97)00080-X. [DOI] [PubMed] [Google Scholar]
- Pâques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genes and Disease http://www.ncbi.nlm.nih.gov/disease/Cancer.html
- Hiom K. DNA repair: Rad52 - the means to an end. Curr Biol. 1999;9:R446–R448. doi: 10.1016/s0960-9822(99)80278-4. [DOI] [PubMed] [Google Scholar]
- Sung P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 1997;11:1111–1121. doi: 10.1101/gad.11.9.1111. [DOI] [PubMed] [Google Scholar]
- Sung P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J Biol Chem. 1997;272:28194–28197. doi: 10.1074/jbc.272.45.28194. [DOI] [PubMed] [Google Scholar]
- New JH, Sugiyama T, Zaitseva E, Kowalczykowski SC. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature. 1998;391:407–410. doi: 10.1038/34950. [DOI] [PubMed] [Google Scholar]
- Shinohara A, Ogawa T. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature. 1998;391:404–407. doi: 10.1038/34943. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Zaitseva EM, Kowalczykowski SC. A single-stranded DNA-binding protein is needed for efficient presynaptic complex formation by the Saccharomyces cerevisiae Rad51 protein. J Biol Chem. 1997;272:7940–7945. doi: 10.1074/jbc.272.12.7940. [DOI] [PubMed] [Google Scholar]
- Benson FE, Baumann P, West SC. Synergistic actions of Rad51 and Rad52 in recombination and DNA repair. Nature. 1998;391:401–404. doi: 10.1038/34937. [DOI] [PubMed] [Google Scholar]
- Rijkers T, Van Den Ouweland J, Morolli B, Rolink AG, Baarends WM, Van Sloun PP, Lohman PH, Pastink A. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol Cell Biol. 1998;18:6423–6429. doi: 10.1128/mcb.18.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Sonoda E, Buerstedde JM, Bezzubova O, Morrison C, Takata M, Shinohara A, Takeda S. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. Mol Cell Biol. 1998;18:6430–6435. doi: 10.1128/mcb.18.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker J. A surfeit of RAD51-like genes? Trends Genet. 1999;15:166–168. doi: 10.1016/s0168-9525(99)01733-3. [DOI] [PubMed] [Google Scholar]
- Anderson DG, Kowalczykowski SC. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a chi-regulated manner. Cell. 1997;90:77–86. doi: 10.1016/s0092-8674(00)80315-3. [DOI] [PubMed] [Google Scholar]
- Umezu K, Kolodner RD. Protein interactions in genetic recombination in Escherichia coli. Interactions involving RecO and RecR overcome the inhibition of RecA by single-stranded DNA-binding protein. J Biol Chem. 1994;269:30005–30013. [PubMed] [Google Scholar]
- Welcsh PL, Owens KN, King MC. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 2000;16:69–74. doi: 10.1016/s0168-9525(99)01930-7. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Gowen LC, Latour AM, Mohn AR, Xiao A, DiBiase L, Koller BH. BRCA1 deficient embryonic stem cells display a decreased homologous recombination frequency and an increased frequency of non-homologous recombination that is corrected by expression of a brca1 transgene. Oncogene. 1999;18:7900–7907. doi: 10.1038/sj/onc/1203334. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell. 2001;7:273–282. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- Tashiro S, Walter J, Shinohara A, Kamada N, Cremer T. Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J Cell Biol. 2000;150:283–291. doi: 10.1083/jcb.150.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Santoro IM, McDaniel LD, Nishijima I, Mills M, Youssoufian H, Vogel H, Schultz RA, Bradley A. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat Genet. 2000;26:424–429. doi: 10.1038/82548. [DOI] [PubMed] [Google Scholar]
- Karow JK, Wu L, Hickson ID. RecQ family helicases: roles in cancer and aging. Curr Opin Genet Dev. 2000;10:32–38. doi: 10.1016/s0959-437x(99)00039-8. [DOI] [PubMed] [Google Scholar]
- Wang W, Seki M, Narita Y, Sonoda E, Takeda S, Yamada K, Masuko T, Katada T, Enomoto T. Possible association of BLM in decreasing DNA double strand breaks during DNA replication. EMBO J. 2000;19:3428–3435. doi: 10.1093/emboj/19.13.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SC. The RuvABC proteins and Holliday junction processing in Escherichia coli. J Bacteriol. 1996;178:1237–1241. doi: 10.1128/jb.178.5.1237-1241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborough KM, West SC. Resolution of synthetic Holliday junctions in DNA by an endonuclease activity from calf thymus. EMBO J. 1990;9:2931–2936. doi: 10.1002/j.1460-2075.1990.tb07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou A, Davies AA, West SC. Branch migration and Holliday junction resolution catalyzed by activities from mammalian cells. Cell. 2001;104:259–268. doi: 10.1016/s0092-8674(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Saccharomyces Genome Database http://genome-www.stanford.edu/Saccharomyces/
- The C. elegans Genome Project http://www.sanger.ac.uk/Projects/C_elegans/
- Berkeley Drosophila Genome Project http://www.fruitfly.org/
- Human Genome Resources http://www.ncbi.nlm.nih.gov/Sitemap/index.html#HumanGenome


