Summary
The arrangement of myocytes within the ventricle is critical for its contractile performance, as evidenced by significant functional impairment seen in cardiomyopathies associated with myofiber disarray or post-infarction remodelling. A review on this topic by Anderson and associates provides anatomical insight gained from a multitude of approaches, and concludes that the best concept is that of syncytial continuum with supporting collagenous matrix. The overall arrangement is in the form of several intertwined helices, and the authors find no support for a recently suggested ventricular myocardial band hypothesis. This commentary aims at providing a developmental and physiological perspective on this purely anatomical concept. Unlike some other organ systems, the developing heart has to function since very early stages to support the oxygen and nutrition demands of the growing embryo, thus putting some constraints on heart development. The ventricular myocardial architecture transforms from a single-layered tube through trabeculated stages into a mature form that relies on a multi-layered compact zone. The first evidence of helical patterns is found in trabeculated hearts during ventricular contraction, and layers with different helix pitch develop during later fetal stages as the compact zone thickens. The second major point determining ventricular contraction is the sequence of its electrical activation. The ventricular activation sequence changes concomitantly with its morphology, from slow peristaltoid through base-to-apex pattern found in looped trabeculated hearts, to mature apex-to-base direction. Thus, adult ventricular myocardial architecture is best understood when one also considers the way it developed together with its electrical activation sequence and contraction pattern.
Keywords: Ventricular contraction, Cardiac conduction system, Heart development, Activation sequence, Chick embryo
1. Introduction
The anatomical organization of ventricular myocardium has been studied over the centuries, however, the interpretation of its complex arrangement remains controversial, as illustrated by a recent series of articles published in this journal representing two conflicting view points. The point of disagreement lies in the interpretation of the higher level of organization of myocardial fibers: while both groups agree that they form a helical structure, Torrent-Guasp and associates [1] claim that this structure can be “unwrapped” to a twisted “ventricular myocardial band” (VMB), while Anderson and colleagues [2] find no support for such organization into separate muscles reminiscent of skeletal muscle, advocating the importance of supportive collagenous matrix and concept of intertwined helices. Rather than simply arguing in favor of whichever point, it might be interesting to consider the anatomical structure from developmental and physiological perspectives, asking the following questions: 1) how does the spiral myocardial architecture of the left ventricle arise during development, and 2) what is the sequence of its electrical activation and resulting contraction.
2. Developmental changes in ventricular myocardial architecture
The beating heart of higher vertebrates transforms from a single tube to a four-chambered organ while meeting continuously increasing circulatory demands of the rapidly growing embryo. For the sake of simplicity, three distinct stages can be pointed out in this continuum. At the stage of cardiac tube (initially straight, later looped), the myocardium forms a single or double cell thick mantle at the periphery, and is not yet covered by the epicardium (Figure 1). Even at that early stage, anisotropic arrangement of myocytes can be found when looking close enough. The inner cell layer is more differentiated [3], and along the length of the tube, preferential circular alignment of myofibrils is seen in the regions of the atrio-ventricular canal and the outflow tract [4]. Since there is no coronary circulation, nutrition and oxygen are supplied by diffusion from the lumen. At this stage, the heart resembles tubular structures found in some invertebrates. The next stage in ventricular morphogenesis is characterized by development of trabeculation (summarized in [5]; Figure 1). Albeit the epicardium is now covering the ventricular surface, there is still no coronary circulation, so this is the only way to increase myocardial mass without hitting the diffusion barrier. Trabecular patterns differ between the left and right ventricle [6] as well as between different species [7]. In general, their arrangement is radial, with some indication of spiraling during systole or in response to experimentally induced pressure overload [8]. During these stages, the compact layer thickens only slightly, but already starts to exhibit differences in preferential myocyte orientation across the wall. The development of spiraling can be accelerated or delayed by manipulating the loading conditions [9].
Figure 1.
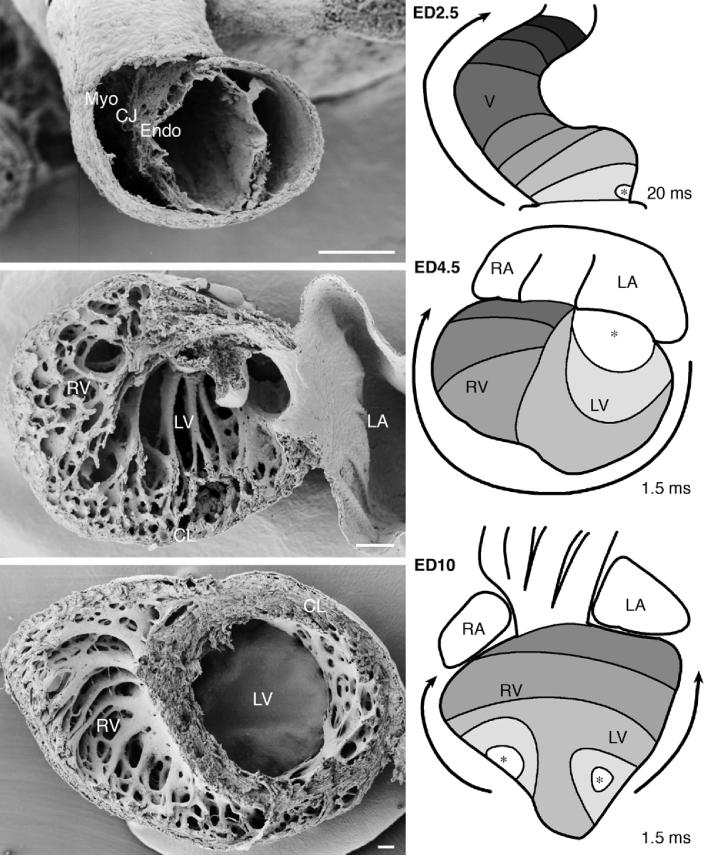
Development of ventricular myocardial architecture and is activation sequence. Top panels: The tubular heart. A cross section through embryonic day (ED) 2.5 chick ventricle reveals the outer myocardial mantle (Myo), separated from the endocardium (Endo) by cardiac jelly (CJ). Electrical activation at these stages proceeds in a slow and fairly isotropic fashion from the venous pole towards the aortic sac. Middle panels: Trabeculated pre-septation heart. Ventricular lumen at ED4.5 is filled with three-dimensional trabecular network in both left (LV) and right (RV) ventricles. The outside compact layer (CL) is very thin. Electrical activation of these ventricles is much more rapid in comparison with the previous stage, but still proceeds from the preferentially left-sided atrio-ventricular junction towards the right-sided outflow tract. The bottom panels show the mostly compacted nature of the left ventricular myocardium at ED10, although the three spiral layers are not yet very distinct. Activation proceeds in a mature apex-to-base pattern, revealing functionality of both bundle branches. Scale bars are 100 micrometers; asterisks mark the earliest activated ventricular region and arrows indicate the direction of electrical impulse propagation. Original data generated in context of studies cited in the text [5, 16, 19].
The bulk of compact myocardium is formed by the process of trabecular compaction, which coincides with functional deployment of coronary circulation [10, 11]. The typical three-layered structure, with innermost longitudinal, middle circular, and subepicardial oblique preferential orientation, matures during fetal development [12, 13]. Formation of the compact myocardium is critical for cardiac performance, as evidenced by fetal lethality of mouse mutants with complete failure of compaction (reviewed in [7, 14]), or contractile dysfunction in patients with isolated ventricular non-compaction [15].
3. Ventricular activation sequence reflects the changes in blood flow and geometry
Concomitant with changes in the ventricular geometry and myoarchitecture is shifting the ventricular activation sequence (reviewed in [16, 17]; Figure 1). In general, the activation follows the direction of blood flow, and thus proceeds towards the outflow portion of the ventricle. In the tubular hart, the excitation is relatively slow (Figure 1), resulting in a peristaltoid contraction pattern very different from cardiac mechanics at later stages. With trabecular development, heterogeneities in conduction properties within the ventricular myocardium emerge. The trabeculae, located on the inside, are activated first [18, 19], and spread the excitation radially towards the outside. These structures are the precursors of the specialized conduction network (His-Purkinje system) of the adult heart. It is the geometry of the ventricle that helps explain the transition from apparent base-to-apex to mature apex-to-base adult pattern. At the earlier stages, the two ventricles are widely connected, and the inflow and outflow portion are widely separated since the heart still resembles a tube rather than an ellipsoid. By the completion of looping and ventricular septation, both inflow and outflow of the left ventricle are found closely aligned at the “top” of the ventricle. Thus, it is necessary to start activation from the apex (Figure 1) to move the blood towards the aortic outlet, while back flow is prevented by closure of the mitral valve during systole.
4. Conclusions
What do the above-mentioned facts mean for our understanding of adult ventricular myoarchitecture? Starting with the activation sequence, we can plainly see that the activation pattern of descending segment first, ascending segment next in the VMB hypothesis [1] could be traced in the tubular heart, but does not extend past the trabeculated stage. The ventricular activation is brought by a system of the His bundle, its left and right branches, and Purkinje fiber network, resulting in activation and contraction patterns starting from the apex and proceeding towards the base where the aortic outlet is located [19-22]. The systolic twisting of the ventricular apex [23, 24] is consistent with the spiral systems illustrated by Anderson and colleagues in their review [2] and reflects the active movement of the ventricular mass towards the base, which is anchored to the body wall by pericardial reflections along the great arteries and veins. During development, the myocardial wall matures from a single-layered epithelium to a complex multilayered structure supplied by epicardially-derived vasculature and is reinforced by collagenous supporting network while continuously pumping blood. The cell layers are added to both the inside (by compaction of trabecular network) and the outside (by continued myocyte proliferation). At no point could there be traced a sheet-like continuity resembling the hypothetic VMB.
There are, however, still important gaps in our understanding of the anatomy of ventricular syncytium and its electrical wiring. As we have seen, activation patterns change during development, and we still do not know how the patterning of the Purkinje-myocyte junctions is controlled, or how mechanical and electrical connections among working myocytes providing functional coupling across the layers are remodelled. With current non-invasive in vivo imaging methods and increasing computing power, we are able to image the cardiac structure and function in both space and time, and construct more realistic mathematical models, which might in turn help us design surgical procedures respecting myocardial architecture and predict their effects on the restoration of left ventricular performance.
Acknowledgements
Author's research is currently supported by NIH (RR16434 and HD39946).
References
- 1.Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F, Flotats A, Cosin-Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005;27:191–201. doi: 10.1016/j.ejcts.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RH, Ho SY, Redmann K, Sanchez-Quintana D, Lunkenheimer PP. The anatomical arrangemenr of the myocardial cells making up the ventricular mass. Eur J Cardiothorac Surg. 2005;XX:XX. doi: 10.1016/j.ejcts.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 3.Shiraishi I, Takamatsu T, Fujita S. Three-dimensional observation with a confocal scanning laser microscope of fibronectin immunolabeling during cardiac looping in the chick embryo. Anat Embryol (Berl) 1995;191:183–189. doi: 10.1007/BF00187817. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RP, Reckova M, DeAlmeida A, Bigelow M, Stanley CP, Spruill JB, Trusk T, Sedmera D. The oldest, toughest cells in the heart. In: Chadwick DJ, Goode J, editors. Development of the cardiac conduction system. Wiley; Chichester: 2003. pp. 157–176. [PubMed] [Google Scholar]
- 5.Sedmera D, Pexieder T, Hu N, Clark EB. Developmental changes in the myocardial architecture of the chick. Anat Rec. 1997;248:421–432. doi: 10.1002/(SICI)1097-0185(199707)248:3<421::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Wenink AC, Gittenberger-de Groot AC. Left and right ventricular trabecular patterns. Consequence of ventricular septation and valve development. Br Heart J. 1982;48:462–468. doi: 10.1136/hrt.48.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Tobita K, Garrison JB, Li JJ, Tinney JP, Keller BB. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec. 2005;283:193–201. doi: 10.1002/ar.a.20133. [DOI] [PubMed] [Google Scholar]
- 10.Rychter Z, Ostadal B. Fate of “sinusoidal” intertrabecular spaces of the cardiac wall after development of the coronary vascular bed in chick embryo. Folia Morphol. 1971;19:31–44. [PubMed] [Google Scholar]
- 11.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn. 1997;208:338–348. doi: 10.1002/(SICI)1097-0177(199703)208:3<338::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.McLean M, Prothero J. Myofiber orientation in the weanling mouse heart. Am J Anat. 1991;192:425–441. doi: 10.1002/aja.1001920410. [DOI] [PubMed] [Google Scholar]
- 13.Jouk PS, Usson Y, Michalowicz G, Grossi L. Three-dimensional cartography of the pattern of the myofibres in the second trimester fetal human heart. Anat Embryol (Berl) 2000;202:103–118. doi: 10.1007/s004290000103. [DOI] [PubMed] [Google Scholar]
- 14.Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics. 2003;15:165–176. doi: 10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- 15.Varnava AM. Isolated left ventricular non-compaction: a distinct cardiomyopathy? Heart. 2001;86:599–600. doi: 10.1136/heart.86.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedmera D, Reckova M, Bigelow MR, DeAlmeida A, Stanley CP, Mikawa T, Gourdie RG, Thompson RP. Developmental transitions in electrical activation patterns in chick embryonic heart. Anat Rec. 2004;280A:1001–1009. doi: 10.1002/ar.a.20107. [DOI] [PubMed] [Google Scholar]
- 17.Sedmera D, Reckova M, Rosengarten C, Torres MI, Gourdie RG, Thompson RP. Optical mapping of electrical activation in developing heart. Microscop Microanal. 2005;11:209–215. doi: 10.1017/S1431927605050452. [DOI] [PubMed] [Google Scholar]
- 18.de Jong F, Opthof T, Wilde AA, Janse MJ, Charles R, Lamers WH, Moorman AF. Persisting zones of slow impulse conduction in developing chicken hearts. Circ Res. 1992;71:240–250. doi: 10.1161/01.res.71.2.240. [DOI] [PubMed] [Google Scholar]
- 19.Reckova M, Rosengarten C, deAlmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93:77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 20.Durrer D, Buller J, Graaff P, Lo GI, Meyler FL. Epicardial excitation pattern as observed in the isolated revived and perfused fetal human heart. Circ Res. 1961;9:29–38. doi: 10.1161/01.res.9.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Witkowski FX, Clark RB, Larsen TS, Melnikov A, Giles WR. Voltage-sensitive dye recordings of electrophysiological activation in a Langendorff-perfused mouse heart. Can J Cardiol. 1997;13:1077–1082. [PubMed] [Google Scholar]
- 22.Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, Jalife J, Fishman GI. Visualization and functional characterization of the developing murine cardiac conduction system. Development. 2001;128:1785–1792. doi: 10.1242/dev.128.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbons Kroeker CA, Ter Keurs HE, Knudtson ML, Tyberg JV, Beyar R. An optical device to measure the dynamics of apex rotation of the left ventricle. Am J Physiol. 1993;265:H1444–1449. doi: 10.1152/ajpheart.1993.265.4.H1444. [DOI] [PubMed] [Google Scholar]
- 24.Taber LA, Yang M, Podszus WW. Mechanics of ventricular torsion. J Biomech. 1996;29:745–752. doi: 10.1016/0021-9290(95)00129-8. [DOI] [PubMed] [Google Scholar]


