Abstract
A solution with different Cu supply levels was cultured to investigate gama-aminobutyric acid (GABA) accumulation in Elsholtzia splendens, a native Chinese Cu-tolerant and accumulating plant species. Increasing Cu from 0.25 to 500 μmol/L significantly enhanced levels of GABA and histidine (His), but considerably decreased levels of aspartate (Asp) and glutamate (Glu) in the leaves. The leaf Asp level negatively correlated with leaf Cu level, while leaf GABA level positively correlated with leaf Cu level. The leaf Glu level negatively correlated with leaf GABA level in Elsholtzia splendens. The depletion of leaf Glu may be related to the enhanced synthesis of leaf GABA under Cu stress.
Keywords: Accumulation, Copper toxicity, Elsholtzia splendens, Gama-aminobutyric acid (GABA)
INTRODUCTION
Plants have developed metal tolerance traits to adapt to heavy metal contamination in soil by allowing absorption of essential micronutrients to satisfy normal physiological requirements, but avoiding excessive absorption of non-essential or toxic elements in the plant cell (Baker and Proctor, 1990; Verkleij and Schat, 1990). Elsholtzia splendens growing widely over Cu mining areas along the middle and lower streams of the Changjiang River has been identified as Cu-tolerant and accumulating plant species native to China (Yang et al., 1998; Jiang et al., 2002). The excess of free Cu ions not only induces generation of free radicals and oxidative stress in cells, but also inactivates enzymes and increases membrane permeability by binding to the sulfhydryl groups of enzymes and cytoplasm membrane proteins, thus destroying metabolic homeostasis (Van Assche and Clijsters, 1990).
The responses of plants to metal exposure have received considerable attention in toxicological and evolutionary contexts. However, little is known about metal-tolerance mechanisms based on the pathways by which metal “signals” in the environment elicit biochemical responses in E. splendens. GABA levels in plant tissues are typically low, ranging from 0.03 to 2.00 μmol/g fresh weight (FW), but increase several fold in response to various stimuli, including heat shock, mechanical stimulation, hypoxia and phytohormones (Shelp et al., 1999). There is some evidence that GABA acts as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. (Beuve et al., 2004). The aim of this study was to determine whether changes in the levels of leaf amino acids are related to Cu supply levels in solution and Cu levels in leaf of E. splendens.
MATERIALS AND METHODS
Seeds of E. splendens were surface-sterilized and rinsed to establish plant seedlings for 14 d pre-culture in a basal nutrient solution as described by Yang et al.(2002). Cu at treatment concentrations of 0.25, 12.5, 50, 100 and 500 μmol/L was added as CuSO4·5H2O. The experiment was set up in a completely randomized design with six replicates for each treatment. The culture solution was continuously aerated and maintained at pH 5.5±0.3, and adjusted daily with 0.1 mol/L NaOH or 0.1 mol/L HCl, and was renewed every 4 d during the 12 d experiment in a greenhouse with temperature ranging from (28±2) °C (day) to (15±2) °C (night), without supplementary light. At harvest, leaf samples were collected, and rinsed thoroughly with deionized water, blotted dry. For amino acid detection, the 4th leaf sample from the top of the plant were frozen immediately in liquid N2, lyophilized under vacuum and kept at −60 °C. Amino acids were assayed following the methods of Schaeffer and Sharpe (1997), and determined by HPLC with an PE LS-50B fluorescence detector and an AccQ-Taq amino acid column by using AccQ-Taq Amino acids quantitation methods (Waters Alliance 2690). Plant leaves were dried at 65 °C, ground and passed through a 60-mesh sieve for elemental analyses by an Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES, Model IRAS-AP, TJA).
All data were presented as mean values of at least six replicates. SPSS statistical software package (Version 11.0) was used for one-way Analysis of variance (ANOVA) to evaluate whether the means were significantly different at P≤0.05.
RESULTS AND DISCUSSION
Results in Table 1 show drastic increase of leaf Cu with the addition of 100 and 500 μmol/L Cu as compared to 0.25 μmol/L Cu. Yang et al.(2002) has observed the stimulated growth of E. splendens at 50 μmol/L Cu, but that significantly inhibited at 100 and 500 μmol/L Cu. Amino acids are involved in immobilization and detoxification of heavy metals in plant cells, which is attributed mainly to its carboxyl, amido, sulphydryl and phenol groups that can be bonded with heavy metals in cytosol. Xylem Ni accumulation in a Ni hyperaccumulator Alyssum lesbiacum was positively correlated with xylem His level when exposed to 10−3~10 mmol/L Ni. EXAFS technique detected the binding of xylem Ni with xylem His (Krämer et al., 1996).
Table 1.
The levels of macro- and micronutrients in the leaves of E. splendens at different Cu supply levels
| Element | Leaf Cu concentration (μmol/L) |
LSD0.05 | ||||
| 0.25 | 12.5 | 50 | 100 | 500 | ||
| Ca (g/kg) | 3.2 | 3.4 | 3.2 | 3.3 | 3.2 | 0.17 |
| K (g/kg) | 20 | 22 | 19 | 19 | 17 | 1.20 |
| Mg (g/kg) | 2.9 | 3.4 | 3.0 | 3.2 | 2.6 | 0.20 |
| P (g/kg) | 6.5 | 6.9 | 5.3 | 5.7 | 4.7 | 0.20 |
| S (g/kg) | 4.1 | 3.8 | 3.3 | 4.3 | 4.6 | 0.18 |
| Cu (μg/g) | 48.1 | 52.2 | 55.1 | 84.8 | 274.1 | 3.87 |
| Zn (μg/g) | 72 | 136 | 335 | 235 | 105 | 15.3 |
| Fe (μg/g) | 817 | 830 | 834 | 754 | 606 | 39.1 |
| Mn (μg/g) | 78 | 101 | 183 | 143 | 53 | 4.1 |
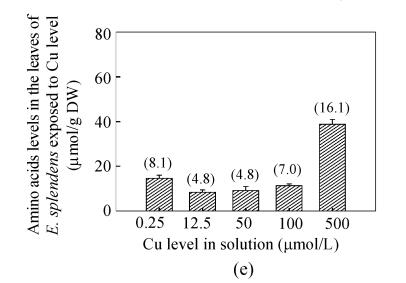
In this study, of 18 amino acids, Asp, Ser, Glu, His, Val and GABA constituted 80%–92% of the total amino acids in the leaves of E. splendens, each with the proportion of >5% at different Cu supply levels. The levels of both leaf Ser and leaf Val were not correlated with the leaf Cu level or the Cu level in solution, and their proportion changed slightly as Cu increased from 0.25 to 500 μmol/L (Fig.1). Leaf Asp was negatively correlated with both the leaf Cu level and Cu level in solution (Table 2). The fast degradation of Asp might decrease leaf Asp level when E. splendens accumulated high levels of Cu in the leaves. The leaf His levels were positively related to both the leaf Cu level and the Cu level in solution (Table 2). For example, leaf His increased 3.4-fold with the increase in Cu from 50 to 500 μmol/L. The positive correlation between leaf His levels with leaf Cu levels indicated that His accumulation in the plant might be involved in Cu uptake and accumulation in the plants. In both the vacuole and cytoplasm of Ni hyperaccumulator Thlaspi goesingense, Ni was mainly bonded with citric acid and His detected by X-ray Absorption Spectroscopy (XAS) techniques (Krämer et al., 1997). But whether and how Cu is bonded with His in leaf cells of E. splendens is unknown.
Fig. 1.
Free amino acids in the leaves of E. splendens exposed to different Cu level. Values in the bracket represent the proportion of total free amino acids (%). (a) Asp; (b) Glu; (c) Val; (d) Ser; (e) His; (f) GABA
Table 2.
Regression and correlation between leaf amino acid and Cu levels in solution or in the leaves of E. splendens
| Leaf amino acid level | Cu levels | Regression equation | Correlation coefficient (R2) |
| Leaf Asp | Cu in solution | Y=31.06−0.04X | 0.912* |
| Leaf Asp | Leaf Cu | Y=34.42−0.09X | 0.967* |
| Leaf His | Cu in solution | Y=3.89X+0.13 | 0.962* |
| Leaf His | Leaf Cu | Y=3.89X+0.13 | 0.962* |
| Leaf Glu | Leaf Cu | Y=103.25−2.87X+0.22X2 | 0.978* |
| Leaf GABA | Cu in solution | Y=26.99+0.23X−3.07X2 | 0.879* |
| Leaf GABA | Leaf Cu | Y=17.91+0.35X−6.33X2 | 0.842* |
Correlation is significant at the 0.05 levels. Regression coefficients were tested with the analysis of covariance by SPSS statistical software package (Version 11.0)
As compared to 0.25 μmol/L Cu, leaf Glu level did not significantly change at 50 μmol/L Cu, but considerably decreased at 100 and 500 μmol/L Cu (Fig.1). Significantly quadratic and negative correlations between the leaf Glu level and the leaf Cu level were observed (Table 2), and the leaf Glu level had considerably negative correlation with the leaf GABA level (Y=61.84−0.85X, R 2=0.929, X and Y represent leaf GABA level and leaf Glu level, respectively). The leaf GABA level was increased pronouncedly at 100 and 500 μmol/L Cu, whereas no marked difference was observed at 50 μmol/L Cu as compared with 0.25 μmol/L Cu (Fig.1). Positively quadratic relations were found between the leaf GABA levels and the leaf Cu level or the Cu level in solution (Table 2).
It has been suggested that in plant cell stress-induced GABA synthesis is the result of cytosolic acidosis and the consequent stimulation of glutamate decarboxylase (GAD) (Snedden et al., 1992). GAD activity catalysing the formation of GABA from Glu is regulated by Glu levels as well as Ca2+/calmodulin and H+, which result from the stresses of mechanical damage, heat shock, cold shock, hypoxia, and drought stimulation. In this study, the decreased Glu level in the plant may be due to the increased conversion of Glu to GABA under Cu toxicity, which inhibits glutamine synthesis or reduces protein synthesis (Satyanarayan and Nair, 1990; Scott-Taggart et al., 1999).
The levels of macro- and micronutrients in E. splendens at different Cu supply levels were listed in Table 1. Compared to 0.25 μmol/L Cu, for macronutrients, Ca was not changed, while K, Mg and P were decreased, and S was increased at Cu supply level of 500 μmol/L, whereas for micronutrients, Cu and Zn were enhanced but Fe and Mn were decreased at 500 μmol/L Cu. 500 μmol/L Cu was observed toxic to plant growth and considerably decreased levels of leaf Fe. At 100 μmol/L Cu, no marked change was found for macronutrients uptake, but increased uptake of Cu, Zn and Mn and minimum uptake for Fe were observed as compared to 0.25 μmol/L Cu.
Kinnersley and Fang (2000) reported that the increase in biomass of Lemna was accompanied by an increase in mineral content of GABA-treated plants, except for the decrease in Fe2+ levels, as GABA levels increased from 1 to 10 mmol/L. However, the function of GABA in the plant is unclear. Beuve et al.(2004) demonstrated that N stress or critical N-demand for sustaining shoot growth during bolting period could result in accumulation of GABA, which in turn would induce increase of nitrate uptake. In our experiment, the increased accumulation of GABA in E. splendens at 100 and 500 μmol/L Cu was observed. But the metabolite or signal transduction functions of GABA accumulation in E. splendens in response to Cu toxicity need to be further investigated.
Footnotes
Projects supported by the National Basic Research Program (973) of China (No. 2002CB410804) and the National Natural Science Foundation of China (No. 29977017)
References
- 1.Baker AJM, Proctor J. The influence of cadmium, copper, lead, and zinc on the distribution and evolution of metallophytes in British Isles. Plant Systematics and Evolution. 1990;173:91–108. [Google Scholar]
- 2.Beuve N, Rispail N, Laine P, Cliquet JB, Ourry A, Deunff EL. Putative role of γ-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell and Environment. 2004;27:1035–1046. [Google Scholar]
- 3.Jiang LY, Shi WY, Yang XE, Fu CX, Chen WG. Cu hyperaccumulators in mining area. Chinese Journal of Applied Ecology. 2002;13(7):906–908. (in Chinese) [PubMed] [Google Scholar]
- 4.Kinnersley AM, Fang L. Receptor modifiers indicate that 4-aminobutyric acid (GABA) is a potential modulator of ion transport in plants. Plant Growth Regulation. 2000;32:65–76. [Google Scholar]
- 5.Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC. Free histidine as a metal chelator in plants that accumulate nickel. Nature. 1996;379:635–638. [Google Scholar]
- 6.Krämer U, Smith RD, Wenzel WW, Raskin I, Salt DE. The role of metal transport and tolerance in nickel hyperaccumulation by Thlaspi goesingense Halacsy. Physiologia Plantarum. 1997;115:1641–1650. doi: 10.1104/pp.115.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satyanarayan V, Nair PM. Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry. 1990;29:367–375. [Google Scholar]
- 8.Schaeffer GW, Sharpe FT. Free and bound amino acids and proteins in developing grains of rice with enhanced lysine/proteins. Theoretical and Applied Genetics. 1997;94:878–881. [Google Scholar]
- 9.Scott-Taggart CP, Cauwenberghe VOR, McLean MD, Shelp BJ. Regulation of gama-aminobutyric acid synthesis in situ by glutamate availability. Physiologia Plantarum. 1999;106:363–369. [Google Scholar]
- 10.Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gama-aminobutyric acid. Trends in Plant Science. 1999;4(11):446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- 11.Snedden WA, Chung I, Pauls RH, Bown AW. Proton/L-glutamate symport and the regulation of intracellular pH isolated mesophyll cells. Plant Physiology. 1992;99:665–671. doi: 10.1104/pp.99.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Assche F, Clijsters H. Effects of metals on enzyme activity in plants. Plant Cell and Environment. 1990;13:195–206. [Google Scholar]
- 13.Verkleij JAC, Schat H. Mechanisms of Metal Tolerance in Higher Plants. In: Shaw AJ, editor. Heavy Metal Tolerance in Plants: Evolutionary Aspects. Boca Raton, FL: CRC Press; 1990. pp. 179–193. [Google Scholar]
- 14.Yang XE, Shi WY, Fu CX, et al. Copper Hyperaccumulators of Chinese Native Plants: Characteristics and Possible Use for Phytoremediation. In: Bassam NEL, editor. Sustainable Agriculture for Food, Energy and Industry. London: James & James, Science Publishers Ltd.; 1998. pp. 484–489. [Google Scholar]
- 15.Yang MJ, Yang XE, Roemheld V. Growth and nutrient composition of Elsholtzia splendens nakai under copper toxicity. Journal of Plant Nutrition. 2002;25(7):1359–1375. [Google Scholar]