Abstract
To examine whether or not the regulatory sequence of chicken ovalbumin gene can drive transgene expression specifically in hen oviduct, the authors constructed an oviduct-specific expression vector (pOV), containing 3.0 kilobases (kb) of the 5′-flanking sequence and 3.0 kb of the 3′-flanking sequence of the chicken ovalbumin gene. Jellyfish green fluorescence protein (EGFP) reporter gene and bacterial LacZ reporter gene were respectively inserted into the downstream of the 5′-regulatory region. The recombinants were named as pOVEGFP and pOVLacZ. Two transfer systems, in vitro and in vivo, were used to verify the function of the vector. In vitro, the plasmid DNA pOVEGFP and pEGFP-N1 were transfected respectively by the polyethyleneimine procedure into the primary chicken oviduct epithelium (PCOE) and fibroblasts cells isolated from laying hens. In vivo, the recombinant vector pOVLacZ was injected into egg-laying hens via wing vein and the tissues were collected for RT-PCR analysis. The results showed that expression of pEGFP-N1 was achieved at low level in oviduct epithelial cells and at high level in fibroblasts, but that the recombinant vector was not expressed in both cells. RT-PCR analysis showed that the LacZ gene was transcribed in the oviduct, but not in the heart, liver, kidney and spleen of the injected hens. Accordingly, the β-galactosidase activity was only detected in the oviduct magnum (116.7 mU/ml) and eggs (16.47 mU/ml). These results indicated that the cloned regulation regions of chicken ovalbumin gene could drive exogenous gene expression specifically in the oviducts of hens. In vivo gene injection via wing vein may serve as a rapid production system of recombinant proteins in chicken eggs. In addition, the cultured primary oviduct cells from laying hens were not efficient temporary expression systems for analyzing the function of regulating elements of ovalbumin gene.
Keywords: Chicken ovalbumin gene regulatory regions, Oviduct-specific expression vector, Oviduct epithelium, In vivo expression
INTRODUCTION
The chicken oviduct bioreactor has great commercial value because of the relatively short generation gap of chicken and the large number of progeny. Recent researches revealed that very minimal high expression of foreign gene was achieved, so most examples were not suitable for commercial program. The key to improve the expression of foreign gene is the construction of specific expression vector for chicken oviduct bioreactor. The chicken ovalbumin gene is specifically expressed in the chicken oviduct controlled by steroid hormones, and its amount accounts for 55%–60% of chicken egg white, so its regulation regions are excellent for the expression of foreign genes in transgenic chicken oviduct bioreactor.
In order to determine the feasibility of the expression vector, it is highly desirable to establish a temporary expression system in which transfected gene expression can be studied. Present research on the expression characteristics of control elements of chicken ovalbumin gene mainly focuses on the primary oviduct epithelial cells derived from immature chicken which had been fed large dose of diethylstilbestrol for a long time (Sanders and McKnight, 1988; Haecker et al., 1995; Park et al., 1995; Yu et al., 2001). That involves long time with low efficiency transfection. Localized in vivo gene transfer using electroporation was also used to transfer the recombinant vector into the magnum of laying hens (Park and Muramatsu, 1999; Hiroshi et al., 1998; 2002), but a lot of factors influenced the experiment so it was hard to get satisfactory results.
The present work was aimed to study the function of oviduct-specific expression vector by using oviduct epithelial cells from laying hens and injection of pOVLacZ plasmid DNA into the wing vein of egg-laying hens.
MATERIALS AND METHODS
Experimental animals
White Leghorn laying hens at 17 months of age obtained from the Poultry Research Institute of the China Agriculture Science Academy and having average egg production rate of about 70% were used in the experiment.
Construction of oviduct-specific expression vector
Both 5′- and 3′-regulatory regions of chicken ovalbumin gene were amplified from chicken genome DNA by high fidelity PCR. The 3.0 kb PCR products were subcloned into pGEM-T vector (Promega) and the correctness of the sequences was confirmed by sequence analysis. The 5′- and 3′-regulatory regions were subcloned into cosmid pHC20 (saved by our lab) and the resulted vector was named as pOV. Then, the EGFP and LacZ reporter genes were respectively subcloned at the down-stream of the 5′-regulatory region as a Xho I fragment and the resulted recombinant vector was called pOVEGFP and pOVLacZ (Fig.1).
Fig. 1.
Strategy for construction of recombinant vector
Preparation and purification of plasmid DNA
Preparation of plasmid DNA was by alkaline lysis with sodium dodecyl sulphate (SDS), and purification of the plasmid DNA by polyethylene glycol (PEG) precipitation. The plasmid DNA was dissolved in 5% glucose buffer.
Preparation of primary oviduct cells
A laying hen was decapitated and the magnum portion of the oviduct was taken aseptically. The tissue was finely minced with scissors in phosphate buffered saline (PBS), washed several times with the same buffer and finally suspended in DMEM containing 10 mmol/L HEPES as well as collagenase at a concentration of 0.5 mg/ml. The enzymatic disintegration of the oviduct was performed in a 100 ml glass bottle in a total volume of 20 ml by incubating the vessel for 1 h in a shaking bed at 37 °C. The tissue residue was removed by filtering the dissociation mixture with layers of gauze. Epithelial cells were collected by centrifugation at 600 rpm for 2 min. The cells were washed two or three times with DMEM containing 10% fetal bovine serum, penicillin at 50 μg/ml, streptomycin at 50 μg/ml. Then the primary oviduct cells were transfected immediately or grown at 37 °C or 41 °C, 5% CO2 in DMEM supplemented with 10 mmol/L HEPES at pH 7.4, and antibiotics, 8% chicken serum, 2% fetal calf serum, 10−7 mol/L 17β-estradiol (Sigma, USA), 10−6 mol/L corticosterone (Sigma, USA), and 50 μg/L insulin (Sigma, USA).
In vitro DNA transfection
Primary oviduct cells were transiently transfected by polyethyleneimine procedure. Four microlitre 10 mmol/L polyethyleneimine was added to two micrograms test plasmid DNA and mixed, and then cultured for 15 min at room temperature. Then the mixture was pipetted into 106 cells plated on a 35 mm plastic culture dish in 1 ml of basal medium DMEM supplemented with 1% fetal calf serum. After the cells were cultured at 37 °C or 41 °C in 5% CO2 for about 4 h, the medium was changed with DMEM supplemented with 10 mmol/L HEPES at pH 7.4, 8% chicken serum, 2% fetal calf serum, and antibiotics, 10−7 mol/L 17β-estradiol, 10−6 mol/L corticosterone, 50 μg/L insulin. The cells were examined at 48 h after transfection.
In vivo transfection
Plasmid DNA was prepared according to the above procedure. Ten laying hens were divided into three groups. Control A (2 hens) was injected with 5% glucose; control B (4 hens) was injected with pOV plasmid DNA; the experiment group (4 hens) was injected with pOVLacZ plasmid DNA. In control B and experiment group every hen was injected with 1 mg test plasmid DNA every day via wing vein for two days. Then, eggs were collected from the third day. After laying 1 egg, the hens were killed and their hearts, livers, spleens, kidneys and oviduct magnums were taken and stored in liquid nitrogen.
RT-PCR detection
The total RNA was isolated from the above tissues using acid-phenol-guanidinium thiocyanate-chloroform extraction and treated with Dnase I to ensure no contamination of plasmid DNA. RT-PCR was then performed according to the manufacturer’s recommendations (AccessQuic™ RT-PCR System, Promega) with LacZ gene specific primers, by which a 900 bp PCR product could be obtained. The whole reaction volume was 50 μl. The reverse transcription was at 48 °C for 45 min. The PCR profile was as follows: 94 °C for 2 min, 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min for 40 cycles, and 72 °C for 7 min for 1 cycle.
β-galactosidase assay
The above tissue samples were homogenized and washed with ice cold PBS buffer. Then double volume of reporter lysis buffer was added to the homogenate. After centrifugation, the supernatant was transfered to a new tube for β-galactosidase assay with β-galactosidase Enzyme Assay System (Promega).
RESULTS
Primary culture of oviduct epithelium
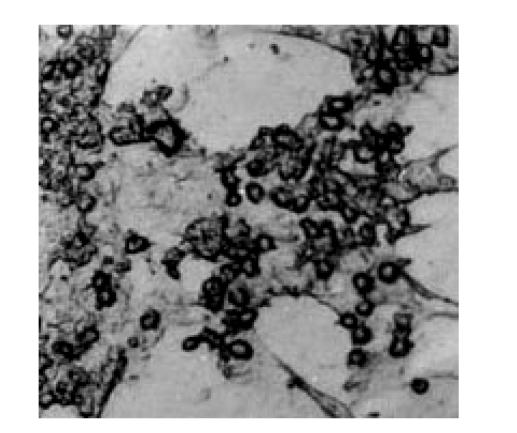
Selective digestion of chicken oviduct tissue resulted in the isolation of two primary cultures with distinct cellular morphology and proliferation rates. Primary cultures were obtained after 1 h digestion in collagenase containing a heterogeneous population of cells which included “fibroblast-like” spindle-shaped cells as well as stellate and circular shape cells. After continued culture for 5–6 d, cell clusters attached to the bottom of the plastic dishes and organized into lumen-like structures with characteristics of epithelial cells (Fig.2).
Fig. 2.
Primary culture of chicken oviduct epithelium, day 5 (10×40)
Temporal expression of transfected EGFP gene in PCOE
The cells were observed under fluorescent microscope at 48 h after the transfection. The expression level of the control plasmid pEGFP-N1 was low in PCOE and higher in fibroblasts cells (Fig.3). In addition, transfection efficiency peaked on the third day of culture, but the expression of the plasmid pOVEGFP was not attained in either cell.
Fig. 3.

The EGFP gene expression in the plasma of cells
(a) Oviduct epithelial cells; (b) Oviduct fibroblasts cells
The transcription of pOVLacZ in vivo in chicken
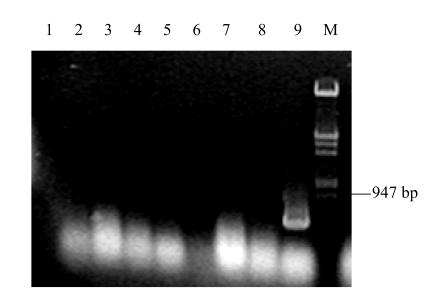
The LacZ transcript was detected only in the oviduct magnum of the experiment group, while LacZ transcript was not detected in other tissues. The LacZ transcript was not detected in any tissues of the control groups (Fig.4).
Fig. 4.
RT-PCR detection of laying hens tissues. The hens were injected with pOVLacZ plasmid and pOV plasmid
Lane 1, negative control; Lanes 2–5, the heart, liver, spleen, oviduct of control B; Lanes 6–9, the heart, liver, spleen, oviduct of experiment group; Lane M, λDNA/Hind III+EcoRI Marker
Detection of β-galactosidase activity
β-galactosidase activity of tissues and egg white was detected with β-galactosidase Enzyme Assay System with reporter lysis buffer (Promega). The oviduct magnum and egg white of hens injected with pOVLacZ plasmid DNA showed β-galactosidase activity (Fig.5 and Fig.6), while other tissues showed no difference from those of the control groups (Fig.5).
Fig. 5.
β-galactosidase activity in different tissues of laying hens in control B and experiment group
Fig. 6.
β-galactosidase activity in eggs of laying hens in control B and experiment group
DISCUSSION
In preliminary experiments, tests of various transfection procedures including electroporation, use of liposome and polyethyleneimine showed that transfection mediated by polyethyleneimine produced the highest efficiency and also revealed that efficiency was highest on the third day, then the 2nd day, by comparing different transfection periods including just after the preparation of PCOE, the 2nd, 3rd and 6th day, when no reporter proteins were identified. This result indicated that primary oviduct cells cultured for 2–3 d were fit to be transfected and that the vigor of cells weakened after the 3rd day.
In order to increase the purity of epithelial cells, the fibroblasts from the epithelium were isolated. But after isolation, the epithelial cells rapidly became weak, compared with the cells co-culture. Perhaps the fibroblasts functioned as feed cells facilitating the growth of epithelium.
The chicken ovalbumin gene promoter controls gene expression not only in a cell-type specific manner, but also in the presence of steroid hormones (Bruno and Gunther, 1989). It has been gradually recognized that DNA transfection using chicken oviduct cells is problematic (Hiroshi et al., 1998). Firstly, it depends on the primary culture, which is not easily transfected due to unavailability of cell line. Secondly, the expression of endogenous chicken ovalbumin gene was strictly limited to the oviduct epithelial cells in the laying season. This kind of specific expression system is regulated by various factors including steroid hormones, cell-substratum, and cell-cell interactions (Masami and Takami, 1990). Thirdly, after steroid treatment, the oviduct cells from immature chicks had clearer induction of ovalbumin mRNA than those from laying hens (Muramatsu et al., 1995). The oviduct epithelial cells prepared from laying hens probably lost the inductivity of related hormones which play an important role in expression of the ovalbumin gene in vivo.
The ovalbumin gene includes several control elements related to oviduct specific expression. The study results showed that the regulation region can not only drive reporter gene specific expression in the oviduct but also has high expression. Injection via wing vein provides a simple and convenient means of gene transfer. This approach facilitates detection of other tissues (except for oviduct) and objective estimation of the recombinant construction. Above all, by this means, the expression product from eggs of experiment hens can be tested, which facilitates study of the active change of gene expression and determination of the physical and chemical character of the expression product. The research also indicated that using PCOE (as recipient cells) to study oviduct-specific expression vector is not a good choice.
Footnotes
Project (No. JH01-066) supported by Science Research Funding of Jiangsu provincial Education Department, China
References
- 1.Bruno L, Gunther S. Cell-type specificity of regulatory elements identified by linker scanning mutagenesis in the promoter of the chicken lysozyme gene. Nucleic Acids Research. 1989;17(21):8451–8463. doi: 10.1093/nar/17.21.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haecker SA, Muramatsu T, Sensenbaugh KR, Sanders MM. Repression of the ovalbumin gene involves multiple negative elements including a ubiquitous transcriptional silencer. Mol Endocrinol. 1995;9(9):1113–1126. doi: 10.1210/mend.9.9.7491104. [DOI] [PubMed] [Google Scholar]
- 3.Hiroshi O, Hyi-Man P, Akihiro N, Ryuzo S, Jun-Ichi O, Tatsuo M. Synthesis of human erythropoietin in vivo in the oviduct of laying hens by localized in vivo gene transfer using electroporation. Poultry Science. 1998;77:299–302. doi: 10.1093/ps/77.2.299. [DOI] [PubMed] [Google Scholar]
- 4.Hiroshi T, Hisako W, Yasushige O, Hyi-Man P, Tatsuo M. Human alkaline phosphatase expression and secretion into chicken eggs in vivo gene electroporration in the oviduct of laying hens. Biochemical and Biophysical Research Communications. 2002;292:88–93. doi: 10.1006/bbrc.2002.6604. [DOI] [PubMed] [Google Scholar]
- 5.Liu YB, Yu MZ, Shen XZ. The establishment of a temporary expression system in chicken oviduct epithelium. Developmental & Reproductive Biology. 2001;10(1):13–19. [Google Scholar]
- 6.Masami Y, Takami O. Transfection of β-casein chimeric gene and hormonal induction o its expression in primary murine mammary epithelial cells. Proc Natl Acad Sci USA. 1990;87:3670–3674. doi: 10.1073/pnas.87.10.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muramatsu T, Hiramatsu H, Okumura J. Induction of ovalbumin mRNA by ascorbic acid in primary cultures of tubular gland cells of the chicken oviduct. Biochemistry and Molecular Biology. 1995;112:2209–2216. doi: 10.1016/0305-0491(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 8.Park HM, Okumura J, Muramatsu T. Modulation of transcriptional activity of the chicken ovalbumin gene promoter in primary cultures of chicken oviduct cell: effects of putative regulatory element the 5′-flanking region. Biochem Mol Biol Int. 1995;36(4):811–816. [PubMed] [Google Scholar]
- 9.Park HM, Muramatsu T. In vivo manipulation of foreign gene expression by steroid administeration in the oviduct of laying hens. Journal of Endocrinology. 1999;163:173–179. doi: 10.1677/joe.0.1630173. [DOI] [PubMed] [Google Scholar]
- 10.Sanders MM, McKnight GS. Positive and negative regulatory elements control the steroid-responsive ovalbumin promoter. Biochemistry. 1988;27:6550–6557. doi: 10.1021/bi00417a053. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Zhao J, Zhang YL. Construction of the expressing vector of 5′-flanking regulatory regions o the chicken ovalbumin gene and its transient expression in chicken primary oviduct cell and chicken fibroblasts cell cultures. Chinese Journal of Veterinary Science. 2001;21(1):21–24. (in Chinese) [Google Scholar]