Abstract
Screens for suppressors of lin-12 hypermorphic alleles in C. elegans have identified core components and modulators of the LIN-12/Notch signaling pathway. Here we describe the recovery of alleles of six new genes from a screen for suppressors of the egg-laying defect associated with elevated lin-12 activity. The molecular identification of one of the new suppressor genes revealed it as bre-5, which had previously been identified in screens for mutations that confer resistance to Bt toxin in C. elegans. bre-5 is the homolog of D. melanogaster brainiac. BRE-5/Brainiac catalyzes a step in the synthesis of glycosphingolipids, components of lipid rafts that are thought to act as platforms for association among certain kinds of membrane-bound proteins. Reducing the activity of several other genes involved in glycosphingolipid biosynthesis also suppresses the effects of constitutive lin-12 activity. Genetic analysis and cell ablation experiments suggest that bre-5 functions prior to ligand-induced ectodomain shedding that activates LIN-12 for signal transduction.
RECEPTORS of the LIN-12/Notch family mediate many cell-cell interactions that cause cells with equivalent potentials to adopt distinct fates. These receptors undergo three proteolytic processing events (reviewed in Greenwald 2005). They undergo a proteolytic cleavage at site 1 during their transit to the cell surface in mammals and reside on the surface as a heterodimer between the N- and C-terminal fragments. After activation by binding to transmembrane ligands of the Delta/Serrate/LAG-2 (DSL) family, proteolytic cleavage at site 2 in the extracellular domain results in ectodomain shedding. The remaining transmembrane portion of the receptor is then cleaved at site 3 in the transmembrane domain, which releases the intracellular domain. The released intracellular domain translocates to the nucleus where it forms a transcriptional activation complex with a sequence-specific DNA-binding protein LAG-1 (Suppressor of Hairless in Drosophila and CBF1/RBP-J in mammals) and other cofactors to promote transcription of target genes.
In Caenorhabditis elegans, three different classes of constitutively active forms of LIN-12 have been described. One class is encoded by hypermorphic alleles with missense mutations that alter the extracellular domain (Greenwald and Seydoux 1990). These alleles are termed lin-12(d), and recently, similar alleles have been found to be commonly associated with acute T lymphoblastic leukemia (Weng et al. 2004). The two other classes are engineered transgenic forms that have also been used extensively in Drosophila and mammalian studies. These truncated forms mimic the products formed from cleavage at site 2 (ectodomain shedding) or site 3 (transmembrane cleavage). In C. elegans, the site 2 cleavage mimic is called lin-12(ΔE) (Shaye and Greenwald 2005; described below) and the site 3 cleavage mimic is called lin-12(intra) (Struhl et al. 1993).
Constitutively active forms of LIN-12 affect many different cell fate decisions, leading to phenotypes that are amenable to genetic analysis. The most widely used basis for genetic screens has been a cell fate decision in the developing gonad, the anchor cell (AC)/ventral uterine (VU) precursor cell decision (Greenwald 1998). In wild-type hermaphrodites, two gonadal cells interact so that one becomes the AC and the other becomes the VU. lin-12 activity mediates this interaction, so that in animals homozygous for null alleles of lin-12 both cells become ACs and, conversely, in mutants with elevated lin-12 activity, both cells become VUs (Greenwald et al. 1983). The absence of an AC leads to the absence of a functional vulva and hence an egg-laying (Egl)-defective phenotype. The 0 AC-Egl phenotype of lin-12(d) mutants has been exploited in large-scale screens for suppressors, yielding intragenic revertants and extragenic suppressors defining seven suppressor/enhancer of lin-12 (sel) genes that modulate lin-12 activity (Greenwald et al. 1983; Ferguson and Horvitz 1985; Tax et al. 1997).
Tax et al. (1997) focused on extragenic suppressors and identified seven sel genes, five of which have been characterized to date. Three essential genes were identified by non-null alleles: lag-2, which encodes the DSL ligand that mediates the AC/VU decision (Tax et al. 1994); sup-17, which encodes an ADAM family protease that may cleave LIN-12 at site 2 in its extracellular domain and mediate ectodomain shedding (Tax et al. 1994, 1997; Wen et al. 1997); and sel-8, which encodes a component of the nuclear complex and appears to bridge LIN-12 and LAG-1 (Doyle et al. 2000; Petcherski and Kimble 2000). Tax et al. (1997) also recovered null alleles of sel-5, which encodes a cytoplasmic serine/threonine kinase that may influence LIN-12 trafficking (Fares and Greenwald 1999) and of sel-7, which encodes a novel nuclear protein (Chen et al. 2004). The screen performed by Tax et al. (1997) was not saturated and was understandably biased toward highly penetrant suppressors, which are easier to work with and to recognize among the background of intragenic revertants (see also results). The lack of saturation implied that there would be new genes to discover through this approach. In addition, the bias toward high penetrance and the requirement for robust egg laying would have precluded the identification of genes such as sel-12 presenilin, for which null alleles, because of functional redundancy and pleiotropic effects, do not restore normal egg laying at high penetrance.
To identify more factors that affect LIN-12/Notch signaling, we performed a screen for suppressors of the 0 AC-Egl defect of a lin-12(d) allele and characterized some of the low-penetrance suppressors that we obtained. We identified alleles of six apparent new sel genes, as well as intragenic revertants and alleles of previously identified sel genes. We cloned one of the new sel genes and found that it corresponds to bre-5, which codes for a β1,3 N-acetylglucosaminyltransferase and is orthologous to Drosophila brainiac (Goode et al. 1996b; Griffitts et al. 2001). We extend this observation to show that two other genes encoding glycosyltransferases thought to function in the synthesis of glycosphingolipids, structural components of lipid rafts, are positive regulators of LIN-12/Notch signaling and discuss how glycosphingolipids might affect LIN-12/Notch signaling.
MATERIALS AND METHODS
General C. elegans methods and strains:
Standard methods as described in Brenner (1974) were used for handling, maintenance, mutagenesis, and genetic analysis of C. elegans. Experiments were performed at 20° unless indicated otherwise. The wild-type parent for all strains was C. elegans var. Bristol N2 (Brenner 1974), except for mapping experiments using the Bergerac strains DP13 (Williams et al. 1992) and GS3063, in which lin-12(n302) had been placed into the Hawaiian strain CB4856 background by repeated backcrossing (data not shown).
The main alleles used in this study are: LG I—sup-17(n1258) (Tax et al. 1997), bre-4(ye27) (Marroquin et al. 2000); LG II—sel-4(n1259) (Tax et al. 1997); LG III—sel-8(sa54), sel-5(n1254) (Tax et al. 1997; Fares and Greenwald 1999; Doyle et al. 2000), lin-12(n302, n950, n941) (Greenwald et al. 1983; Greenwald and Seydoux 1990), lin-12(ar170) (Hubbard et al. 1996), glp-1(ar202) (Pepper et al. 2003), glp-1(e2141, e2142) (Priess et al. 1987), bre-3(ye26), bre-2(ye31) (Marroquin et al. 2000); LG IV—bre-1(ye4) (Marroquin et al. 2000), bre-5(ye17, ar560) (Marroquin et al. 2000; this study); LG X—sel-7(n1253) (Tax et al. 1997; Chen et al. 2004).
Additional information about the alleles listed above and about the markers used for mapping and for facilitating genetic analyses in this work can be found via Wormbase at http://www.wormbase.org. The transgene arIs53 [lin-12(ΔE)∷gfp] expresses a GFP-tagged version of LIN-12 lacking most of its extracellular domain under the sel-12 promoter (Shaye and Greenwald 2005).
Identification of extragenic suppressor mutations:
All suppressors discussed in this work were isolated after EMS mutagenesis of unc-36(e251) lin-12(n302) hermaphrodites. Three mutagenized L4 Po hermaphrodites were picked per 10-cm plate and allowed to self-fertilize for two generations. Animals carrying a mutation in a suppressor gene form a vulva and are egg laying competent, so any F2 eggs were transferred together to a new plate and adults were examined for egg-laying competence. A single Egl+ individual was used to found a revertant stock from a single Po plate to ensure independence of all mutations.
A major class of suppressors encompasses intragenic revertants in lin-12 that reduce or eliminate lin-12 activity (Greenwald et al. 1983; Tax et al. 1997). Since loss of lin-12 activity acts dominantly, we tested all suppressors for dominance as a triage step. sel; unc-36(e251) lin-12(n302) hermaphrodites were crossed with dpy-17(e164) lin-12(n302); him-5(e1467) males and 30 non-Unc F1's were scored for egg-laying ability. Suppressors that conferred egg-laying ability on >10% of heterozygous hermaphrodites in this test were discarded. As a reference point, ∼75% of hermaphrodites carrying lin-12(n302) in trans to a null allele are egg laying competent, and ∼62% of hermaphrodites carrying lin-12(n302) in trans to the hypomorphic allele lin-12(n676n930) are egg laying competent (Sundaram and Greenwald 1993a). Revertants that remained after this triage step were first tested for linkage to lin-12 and unlinked suppressors were subjected to sequence-tagged site mapping (Williams et al. 1992; data not shown).
Complementation tests with mutations in known sel genes:
Complementation tests were performed between new suppressor mutations and recessive alleles of known sel genes mapping on the same chromosome. unc-36(e251) lin-12(n302); sel(unknown) hermaphrodites were mated with lin-12(n302); sel(known); him-5(e1490) males, where “sel(known)” corresponds to sup-17(n1258), sel-4(n1259), sel-8(sa54), sel-5(n1254), or sel-7(n1253), and 30 non-Unc progeny were scored for egg-laying ability. Mutations were scored as failing to complement when the egg-laying ability of trans-heterozygous animals resembled that exhibited by animals homozygous for the canonical allele of the tested sel gene. Many suppressors that failed to complement a known sel gene were sequenced for the presence of a mutation within the coding region of that gene.
Ten alleles of sup-17 (ar528, ar537, ar540, ar542, ar543, ar546, ar552, ar553, ar569, and ar585), 1 putative allele of sel-4 (ar555), 13 new alleles of sel-5 (ar518, ar520, ar521, ar529, ar541, ar551, ar556, ar559, ar568, ar571, ar574, ar579, and ar588), and 6 alleles of sel-7 (ar516, ar517, ar523, ar539, ar558, and ar586) were identified by these tests. The sel-7 alleles are described in Chen et al. (2004).
ar519 mapped to chromosome IV and was found to be an allele of lag-1 on the basis of complementation tests, rescue experiments, and sequencing of the lag-1-coding region.
Atypical intragenic revertant alleles:
Other alleles linked to chromosome III either failed to complement both sel-5 and sel-8 alleles to some extent or complemented both sel-5 and sel-8 alleles. Three of these alleles were found to map to an ∼3-MU region between stP120 and stP127 that includes lin-12. We then sequenced the coding region of lin-12 in the two mutants that failed to complement both sel-5 and sel-8 alleles and in one mutant that complemented both sel-5 and sel-8 alleles and found missense changes in lin-12 (Table 1) in all of them. We therefore assume that a majority of mutations that were linked to lin-12 but were not sel-5 alleles encode second-site mutations in lin-12 itself and we did not analyze these further.
TABLE 1.
Suppressor alleles identified by Tax et al. (1997) and our screen
| Gene | Taxet al. (1997) | Our study | % suppresseda |
|---|---|---|---|
| lin-12b | 109 | 247 | ND |
| sup-17 | 5 | 10 | 52 (n = 353)c |
| lag-2 | 2 | 0d | 94 (n = 189)c |
| sel-6 | 2 | 0 | 90c |
| sel-5e | 2 | 13 | 51c |
| sel-4 | 1 | 1 | 31c |
| sel-8 | 1 | 0 | 25c |
| sel-7 | 1 | 6 | 53c |
| sel(ar526) | 0 | 1 | 10 (n = 68)f |
| lag-1g | 0 | 1 | 73 (n = 19)f |
| bre-5(ar560) | 0 | 1 | 4 (n = 193)f |
| sel(ar562) | 0 | 1 | 37 (n = 49)f |
| sel(ar584) | 0 | 1 | 17 (n = 51)f |
| sel(ar522, ar570) | 0 | 2 | 17 (n = 93)f |
| sel(ar578) | 0 | 1 | 5 (n = 40)f |
Suppression of the lin-12(n302) egg-laying defect by the most penetrant allele of each sel gene.
Sequence changes associated with new alleles of lin-12:ar563, E492K; ar564, R721Q; and ar575, K1127E.
Suppression of egg laying by the most penetrant allele. Data are from Tax et al. (1997).
lag-2 alleles recovered by Tax et al. (1997) were dominant and would have been eliminated by our triage step.
Sequence changes associated with new alleles of sel-5: ar568, Q551stop; ar571, g → a acceptor splice-site mutation; ar574, R91stop; ar579, 525-bp deletion within the coding region; and ar588, g → a acceptor splice-site mutation.
Animals were also homozygous for unc-36(e251).
lag-1(ar519) encodes the L302F change.
One mutation, ar562, failed to complement sel-8 but complemented sel-5. No sequence changes were present in the coding region of sel-8, which was also excluded as a candidate gene because ar562 maps to the right of unc-36. To confirm that ar562 was not one of the majority class of lin-12 intragenic mutation, we sequenced the coding region of lin-12 and found no changes. This mutant was also sequenced for changes in the coding region of bre-3, which can suppress the egg-laying defect of lin-12(n302)/+ animals (see below) and which maps to the right of lin-12 and close to it. No sequence changes were found.
Additional mapping of new sel genes:
sel(ar578) V homozygotes segregating from Bristol/DP13 heterozygotes were used to map sel(ar578) to the left of stP18 (data not shown). This position excludes sel(ar578) as a candidate sel-6 allele. Furthermore, there were no changes in the coding region of lag-2 or in the coding region of skp-1, the C. elegans homolog of the Notch interactor Skip (Zhou et al. 2000), which both map on chromosome V.
sel(ar570) IV homozygotes segregating from Bristol/GS3063 heterozygotes were used to map sel(ar570) to the right of SNP pkP4046 on clone Y105C5 on the right arm of chromosome IV (data not shown).
Identification of sel(ar560) as an allele of bre-5:
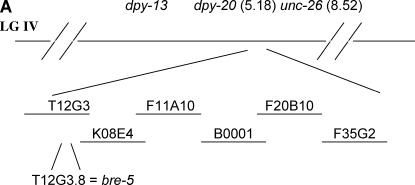
sel(ar560) IV homozygotes segregating from Bristol/GS3063 heterozygotes were used to map sel(ar560) to the right of the SNP pkP4079 on cosmid C28C12. We then constructed lin-12(n302); dpy-20(e1282) sel(ar560) and proceeded as described above. Further SNP mapping indicated that sel(ar560) mapped to the right of pkP4084 on cosmid M117. Finally, we constructed a lin-12(n302); dpy-20(e1282) sel(ar560) unc-26(e205) mapping strain. We mated these hermaphrodites with GS3063 males and picked non-Dpy, non-Unc F1's and from those obtained Dpy non-Unc recombinants and assessed the presence of sel(ar560), evidenced by segregating some non-Egl animals. Fourteen of 87 recombinants lost sel(ar560), while 73 of 87 recombinants kept it. SNP analysis of these recombinants delineated sel(ar560) between a new A → G SNP at position 17,458 of cosmid T12G3 and SNP F35G2.1 on cosmid F35G2. This region contains 30 predicted genes. A mutation in bre-5 was confirmed by sequencing the PCR product using one pair of primers: bre-5(CF) (5′-GGCTTAAGATCCACAAACACAG-3′) and bre-5(CR) (5′-GGAAGAATGCTTCTGGGAAG-3′).
bre mutant suppression of lin-12(n302)/+:
In the case of bre-1(ye4), bre-4(ye27), and bre-5(ye17), strains of unc-32(e189); bre hermaphrodites were mated with males of the genotype lin-12(n302); bre; him-5(e1490). Their non-Unc cross progeny were scored for the ability to lay eggs.
Complementation between bre-5(ar560) and bre-5(ye17) was assessed similarly: unc-32(e189); bre-5(ye17) hermaphrodites were mated with lin-12(n302); bre-5(ar560); him-5(e1490) males and their non-Unc progeny were scored for egg-laying ability.
For bre-2(ye31), Egl lin-12(n302) bre-2(ye31) hermaphrodites were fed bacteria expressing an RNA-mediated interference (RNAi) construct against sel-7 to induce an AC, and non-Egl progeny of such worms were mated to bre-2(ye31) males. Cross progeny (verified by segregation of some non-Egl animals) were then scored for egg laying.
bre-5 interaction with other lin-12 and glp-1 alleles:
To assess the number of ACs, L3 larvae were scored by Nomarski optics and ACs were identified by morphology. For the Multivulva phenotype, L4 larvae were generally picked onto separate plates and checked for the number of pseudovulvae the next day. For germline proliferation, L1 larvae of hermaphrodites carrying glp-1(ar202) were transferred to individual plates at 25° and scored for progeny production. For embryonic lethality, individuals carrying glp-1(e2141) and glp-1(e2142) were picked as L4 larvae and transferred to fresh plates for 3 consecutive days and the eggs on plates were scored for hatching. This assay was conducted at 15°.
Complementation tests among mutations that map to chromosome IV:
Males of the genotype lin-12(n302); sel(ar522); him-5(e1490) were mated with non-Egl hermaphrodites homozygous for unc-36(e251) lin-12(n302); sel(ar570) or sel(ar560). F1 hermaphrodites were scored for egg laying. sel(ar522) failed to complement sel(ar570) but complemented sel(ar560).
sel(ar584) was tested for complementation with bre-5(ar560) and bre-5(ye17) as follows: unc-36(e251) lin-12(n302); sel(ar584) hermaphrodites were mated with bre-5(ar560) or bre-5(ye17) males (or N2 males as a negative control). Their non-Unc progeny were scored for the ability to lay eggs. The coding region of bre-5 was sequenced in the unc-36(e251) lin-12(n302); sel(ar584) background.
RNAi:
For RNAi feeding against bre-3, serine palmitoyltransferase subunit C23H3.4, and the putative glucosylceramide synthases F59G1.1, F20B4.6, and T06C12.10, we used bacterial strains from the C. elegans RNAi library (Kamath et al. 2003). L4 stage unc-32(e189)/lin-12(n302); him-5(e1490)/+ parents were placed onto lawns of such bacteria. Their non-Unc progeny were singly picked to separate plates, and their egg-laying ability and unc-32(e189) segregation were scored so that the effects of RNAi on unc-32(e189)/lin-12(n302) and lin-12(n302) worms could be separately analyzed. An empty feeding vector was used as a negative control.
Laser ablations of vulval precursor cells:
P3.p and P5.p-P8.p or P3.p-P7.p were killed with a laser microbeam in early L2 hermaphrodites before vulval precursor cell (VPC) fates were specified, as described in Levitan and Greenwald (1995), to isolate either P4.p or P8.p. Success of ablations was confirmed. A vulval fate was inferred if a VPC divided and an invagination was formed.
RESULTS
Identification of extragenic suppressors of the Egl defect of lin-12(n302) hermaphrodites:
Animals homozygous for the dominant hypermorphic lin-12(d) allele lin-12(n302) display a 0 AC-Egl defect but are otherwise normal and fertile. We screened 203,000 mutagenized haploid genomes by examining the F2 and F3 progeny of mutagenized unc-36(e251) lin-12(n302) hermaphrodites and identified 284 independent revertants of lin-12(n302) (see materials and methods). On the basis of the outcome of the previous screens, we anticipated that a large proportion of suppressors would result from intragenic lin-12 mutations, as loss or reduction of lin-12 activity behaves as a dominant suppressor of the hypermorphic lin-12(d) alleles (Greenwald et al. 1983; Sundaram and Greenwald 1993b; Tax et al. 1997). Therefore, we discarded all mutants that exceeded a threshold value of 10% semidominance, which is substantially lower than the dominance observed with lin-12 null intragenic revertants, the major class of revertant observed after mutagenesis of lin-12(d) alleles (Greenwald et al. 1983; see also materials and methods). A total of 74 revertants remained after this triage step.
Linkage analysis indicated that 50 of the revertant strains contained suppressor mutations that were tightly linked to lin-12, while 24 were unlinked. Our detailed analysis will be presented below and is summarized in Tables 1 and 2. Thirteen of 50 linked suppressors were found to be alleles of the known gene sel-5, and 36/50 appeared to be intragenic revertants; one linked suppressor appears likely to define a new sel gene. The 24 unlinked mutations were clearly extragenic suppressors, and these were mapped and assigned to complementation groups; 17/24 unlinked mutations appeared to be alleles of sup-17, sel-4, and sel-7, and 1/24 proved to be a non-null allele of lag-1. Six of 24 unlinked mutations defined five new potential sel genes. A total of six potential new sel genes were therefore identified in this screen.
Linked suppressors:
sel-5 and sel-8, as well as lin-12, map to chromosome III. We tested those mutations that showed linkage to lin-12 for complementation with canonical alleles of sel-5 and sel-8. Thirteen suppressors failed to complement sel-5(n1254) and complemented sel-8(sa54) and were therefore concluded to be alleles of the sel-5 gene. We have confirmed the presence of a mutation in the sel-5 coding region for five of them (Table 1).
The other alleles on chromosome III fall into two major categories with respect to sel-5 and sel-8 complementation, either complementing or, surprisingly, failing to complement reference mutations in both genes. We used STS mapping and discovered that the mutations in either category that were tested by this method map to a region that includes lin-12. We then sequenced the coding region of lin-12 in three mutants and saw specific missense changes (see Table 1). On the basis of the mapping and sequencing results with these alleles, we believe that the majority of other mutations showing linkage to lin-12 on chromosome III are likely to be atypical, very weakly semidominant second-site mutations in lin-12 itself that were not discarded after the triage step. Our observations suggest that the nonallelic noncomplementation observed between certain weak lin-12 alleles and sel-5 or sel-8 mutations can be explained by a synergistic interaction that lowers lin-12 activity sufficiently.
One mutation, sel(ar562), failed to complement sel-8(sa54) but sequence analysis of the sel-8 coding region failed to detect any alterations. Mapping data placing the sel(ar562) mutation to the right of the gene unc-36 indicated that sel(ar562) is not an allele of sel-8, which maps to the left of unc-36. We sequenced the lin-12 coding region of sel(ar562) and found no sequence alterations, suggesting that sel(ar562) is not one of the majority class of atypical lin-12 intragenic revertant recovered in our screen. These observations together suggest that sel(ar562) may define a novel sel gene on LG III, although we cannot exclude the possibility that it contains a mutation in a noncoding region that affects the expression of lin-12.
Unlinked suppressors:
To assign the mutations that did not show linkage to lin-12 to chromosomes, we used the STS mapping method (Williams et al. 1992) and tested the new mutations for complementation with the known recessive suppressors on their respective chromosomes, reasoning that these were likely candidates. Complementation tests suggest that we identified 10 new sup-17 alleles and 6 new sel-7 alleles. The new allele sel(ar555) appears likely to be an allele of sel-4, with the caveat that sel(ar555) acts semidominantly; sel(ar555) maps to the sel-4 region (data not shown), but as the molecular identity of sel-4 is not known, allelism could not be confirmed by sequencing.
Our genetic and molecular analysis indicates that one of the suppressors is a weak hypomorph of lag-1, which encodes a core component of the pathway that had not been previously recovered in suppressor screens (Table 1). The recovery of such an allele underscores the usefulness of this screen for identifying core components of the LIN-12 signaling pathway, even when their null phenotypes are lethal.
Seven mutations define six new sel genes:
Seven mutations do not appear to be alleles of known extragenic suppressors of lin-12 nor of lin-12 itself (Table 2). One of these is the linked mutation sel(ar562), described above.
TABLE 2.
New extragenic suppressors of the lin-12(d) 0 AC-Egl defect
| Alleles of new sel genes | Relevant mapping and sequencing data | Penetrancea (% egg laying competent/total) |
|---|---|---|
| LG I | ||
| ar526 | Complements sup-17(n1258) | 10 (n = 68) |
| LG III | ||
| ar562 | Maps to the right of unc-36; no sequence changes in the lin-12 or bre-3 coding regions. | 37 (n = 49) |
| LG IV | ||
| ar560 | 4 (n = 193) | |
| ar584 | 17 (n = 51) | |
| ar522, ar570 | Single complementation group | 15 (n = 40), 17 (n = 93) |
| LG V | ||
| ar578 | No sequence changes in lag-2 and skp-1 coding regions; ar578 maps to the left of sel-6. | 5 (n = 40) |
Penetrance of the suppression of the egg-laying defect (after at least two backcrosses) was determined at 20° in animals homozygous for the indicated mutation and unc-36(e251) lin-12(n302) from Table 1.
We assigned the remaining six mutations to chromosomes by using STS mapping (see materials and methods). One mutation each mapped to chromosomes I and V, and four mutations mapped to chromosome IV. Inter se complementation tests among the mutations on chromosome IV showed that sel(ar570) and sel(ar522) form a single complementation group and that sel(ar584) and sel(ar560) are distinct complementation groups. We focused our analysis of sel(ar560), which mapped to the LG IV cluster.
sel(ar560) corresponds to bre-5, the C. elegans homolog of Drosophila brainiac:
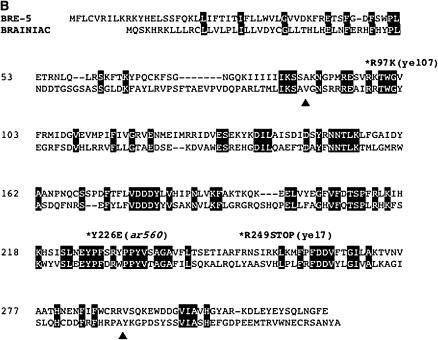
We mapped sel(ar560) to a small region containing 30 predicted genes (see materials and methods and Figure 1) and observed that one of the genes within this region is bre-5, which encodes a β1,3-GlcNAc-transferase and is orthologous to the Drosophila gene brainiac (Marroquin et al. 2000; Griffitts et al. 2001). The phenotype of brainiac null mutants and genetic interactions with Notch suggest that Brainiac modulates Notch signaling in Drosophila (Goode et al. 1992, 1996a). Indeed, sequence analysis of the coding region of bre-5 in the ar560 background revealed a missense mutation in a conserved residue of the glycosyltransferase domain (Figure 1). Alleles of bre-5 in C. elegans had previously been identified in a screen for animals resistant to a Bacillus thuringiensis (Bt) toxin, a process that has not been linked to LIN-12/Notch signaling. The bre-5(ye17) allele appears likely to be a strong loss of function or null for bre-5 function, as it encodes truncated mutant protein that has no enzymatic activity in vitro (Marroquin et al. 2000; Griffitts et al. 2003).
Figure 1.
Molecular cloning and DNA sequence analysis of bre-5(ar560). (A) Mapping and cloning of bre-5(ar560). Only the relevant genes and cosmids are shown. (B) Alignment of BRE-5 and D. melanogaster homolog Brainiac. Proteins were aligned using ClustalW and their alignments are superimposed here. Asterisks denote the amino acids mutated in bre-5 alleles ye107, ye17, and ar560. Shaded letters designate amino acid identity. Galactosyltransferase domain homology is in the region between the triangle symbols.
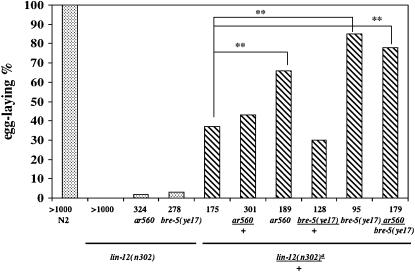
We used bre-5(ye17) to obtain further support for the conclusion that sel(ar560) is an allele of bre-5. First, we found that bre-5(ye17), like sel(ar560), is a low-penetrance but reproducible suppressor of lin-12(n302) (Figure 2). Second, bre-5(ye17), like sel(ar560), is a strong suppressor of lin-12(n302)/+ (Figure 2). Third, lin-12(n302)/+; sel(ar560)/bre-5(ye17) is strongly suppressed, indicating that sel(ar560) fails to complement bre-5(ye17) for suppression. We therefore will refer to sel(ar560) as bre-5(ar560) for the remainder of this work.
Figure 2.
sel(ar560) reduces the activity of a lin-12(d) allele and fails to complement bre-5(ye17). A chi-square test was performed to assess whether there is a significant difference between indicated strains. Asterisks denote differences significant at the 99% level. Numbers of animals scored are below corresponding bars. We note that, for lin-12(d) alleles, the ability to lay eggs correlates absolutely with the presence of an anchor cell; thus, the percentage of hermaphrodites that lay eggs is equivalent to the percentage of hermaphrodites that have an anchor cell (Greenwald et al. 1983). (a) Actual genotype on chromosome III: lin-12(n302)/unc-32(e189).
Characterization of bre-5 interactions with lin-12 and glp-1:
Neither of the bre-5 alleles shows any obvious phenotypes associated with reduction of lin-12 or glp-1 activity in a wild-type background. For example, the AC/VU decision proceeds normally in bre-5 mutant animals: 50/50 bre-5(ar560) and 74/74 bre-5(ye17) mutant animals have 1 AC. However, bre-5 appears to be a positive regulator of lin-12 activity in the AC/VU decision, as loss of bre-5 activity suppresses the 0 AC-Egl phenotype associated with elevating lin-12 activity. This suppression is the basis for concluding that bre-5 acts in the AC/VU decision, as we have not observed any synergy between bre-5 and null alleles of either sel-7 or sel-12 [83/83 bre-5(ye17); sel-7(n1253) and 80/80 bre-5(ye17); sel-12(ar171) double mutants have a single AC].
To test whether bre-5 positively regulates lin-12 activity in other cell fate decisions, we looked for suppression of the ectopic vulval induction caused by lin-12(n950) and did not observe any effect. However, bre-5 is able to suppress ectopic vulval induction caused by lin-12(ΔE), the engineered transgenic form of LIN-12 missing most of the extracellular domain that mimics the product formed from cleavage at site 2 (Table 3), suggesting that bre-5 also positively regulates LIN-12 activity in vulval precursor cell specification.
TABLE 3.
bre-5 affects lin-12 activity in VPC specification
| Genotype | No. of Muva animals/total (%) | Average no. of pseudovulvae |
|---|---|---|
| lin-12(n950) | 77/77 (100) | 4.8 +/− 0.1 |
| lin-12(n950); bre-5(ar560) | 62/62 (100) | 4.6 +/− 0.1 |
| lin-12(n950); bre-5(ye17) | 93/93 (100) | 4.7 +/− 0.1 |
| lin-12(ΔE)∷gfp | 115/116 (99) | 2.7 +/− 0.1 |
| bre-5(ar560); lin-12(ΔE)∷gfp | 95/96 (99) | 1.7 +/− 0.1 |
| bre-5(ye17); lin-12(ΔE)∷gfp | 52/94 (55) | 0.7 +/− 0.1 |
| lin-12(0); lin-12(ΔE)∷gfpb | 44/92 (48) | 0.7 +/− 0.1 |
| lin-12(0); bre-5(ye17); lin-12(ΔE)∷gfpb | 36/113 (32) | 0.5 +/− 0.1 |
These strains were grown and scored in parallel. All animals containing arIs53[lin-12(ΔE)∷gfp] were able to lay eggs.
Muv is defined as the presence of one or more pseudovulva.
lin-12(0) = unc-36(e251) lin-12(n941).
We also investigated whether bre-5 can affect the signaling of the LIN-12 homolog, GLP-1. We combined bre-5(ye17) with the partial loss-of-function alleles glp-1(e2141) or glp-1(e2142) (Priess et al. 1987). We saw no effect on total brood size or maternal-effect lethality (data not shown). In addition, bre-5(ye17) did not suppress the temperature-sensitive sterility caused by the constitutive glp-1 allele ar202 (Pepper et al. 2003): 119/119 glp-1(ar202); bre-5(ye17) were sterile at 25°, similar to glp-1(ar202) control animals (115/115 animals were sterile at 25°). Also, bre-5 mutant larvae do not exhibit embryonic lethality or the characteristic Lag phenotype associated with abrogating both LIN-12 and GLP-1 signaling (Lambie and Kimble 1991): 398/398 eggs laid by bre-5(ar560) animals hatched, while 158/160 eggs laid by bre-5(ye17) animals hatched. All of those larvae grew to adulthood and were able to lay eggs.
Suppression of elevated lin-12 activity by reducing the activity of other genes important for glycosphingolipid synthesis:
There are five bre genes that are necessary for Bt toxin susceptibility. Four of them—bre-2, bre-3, bre-4, and bre-5—encode glycosyltransferases that are believed to act in a single pathway (Griffitts et al. 2003, 2005). Drosophila egghead (egh), whose mutant phenotype is very similar to that of brn, is homologous to bre-3, so it appears that this pathway has been conserved between Drosophila melanogaster and C. elegans (Goode et al. 1996a; Griffitts et al. 2003). Drosophila BRN and EGH have been shown to catalyze successive steps in the synthesis of glycosphingolipids (Muller et al. 2002; Schwientek et al. 2002; Wandall et al. 2003, 2005).
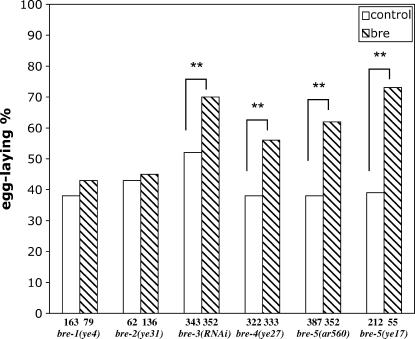
These observations prompted us to test whether loss of bre gene activity suppresses the 0 AC-Egl phenotype associated with lin-12(n302) (data not shown) or lin-12(n302)/+ (Figure 3) and the Muv phenotype associated with lin-12(ΔE) (Table 4). We used mutant alleles of bre-1, bre-2, and bre-4 and bre-3(RNAi) to circumvent the difficulty of constructing a double mutant with the tightly linked lin-12 gene.
Figure 3.
Suppression of the 0 AC Egl defect of lin-12(n302)/+ animals by bre-4 and bre-5 mutations and bre-3 RNAi. lin-12(n302)/unc-32(e189); bre hermaphrodites were compared to control lin-12(n302)/unc-32(e189) hermaphrodites. For bre-2, both strains contained unc-32(+) in lieu of unc-32(e189). lin-12(n302)/unc-32(e189); bre hermaphrodites were compared to control lin-12(n302)/unc-32(e189) hermaphrodites. For bre-2, both strains contained + in lieu of unc-32(e189). We note that the higher percentage of egg-laying-competent hermaphrodites observed for lin-12(n302)/unc-32(e189) grown on “feeding vector” bacteria as opposed to OP50 may be attributed to the RNAi conditions (I. Katic, unpublished observations). A chi-square test was performed to assess whether there is a significant difference between bre mutants and a negative control in the percentage of egg-laying animals. Asterisks indicate differences significant at the 99% level. Numbers of animals scored are below corresponding bars.
TABLE 4.
lin-12 activity in VPC specification is affected by certain other bre genes
| Genotype | Muva/n (%) | Average no. of pseudovulvae |
|---|---|---|
| lin-12(ΔE)∷gfp | 164/164 (100) | 2.4 +/− 0.1 |
| bre-1(ye4); lin-12(ΔE)∷gfp | 98/100 (98) | 2.2 +/− 0.1 |
| bre-2(ye31); lin-12(ΔE)∷gfp | 132/134 (99) | 2.3 +/− 0.1 |
| bre-3(ye26); lin-12(ΔE)∷gfp | 93/115 (81) | 1.5 +/− 0.1 |
| bre-4(ye27); lin-12(ΔE)∷gfp | 72/92 (78) | 1.3 +/− 0.1 |
These strains were grown and scored in parallel. All animals containing lin-12(ΔE)∷gfp were able to lay eggs.
Muv is defined as the presence of one or more pseudovulva.
bre-4(ye27) and bre-3(RNAi) or bre-3(ye26) significantly suppressed the 0 AC-Egl phenotype of lin-12(n302)/+ and the Muv phenotype associated with lin-12(ΔE), suggesting that bre-3 and bre-4, like bre-5, are also positive regulators of lin-12 activity. bre-2(ye31) did not suppress the 0 AC-Egl phenotype of lin-12(n302)/+ or the Muv phenotype associated with lin-12(ΔE). As bre-2(ye31) is a missense mutation, it may not reduce bre-2 activity sufficiently to see suppression. bre-1(ye4) also does not suppress lin-12(n302)/+. The bre-1 gene has not been molecularly characterized, so this allele may be a hypomorph; however, it has a different Bt toxin resistance phenotype and appears sicker than the others (Marroquin et al. 2000) so the absence of suppression may be further evidence that it acts by a mechanism different from other bre genes.
Since bre-5 and other cloned bre genes affect glycosphingolipid synthesis (Kawar et al. 2002; Griffitts et al. 2005), we tested some other enzymes known or hypothesized to be involved in glycosphingolipid synthesis for influence on lin-12 activity. Serine palmitoyltransferases catalyze the first step in the biosynthesis of sphingolipids by condensation of serine and palmitoyl CoA (Hanada 2003). RNAi against the C. elegans ortholog of the LCB1 subunit of serine palmitoyltransferase, C23H3.4, causes lin-12(n302)/+ worms to arrest prior to adulthood, so its effect on egg laying could not be assessed. Glucosylceramide synthases are enzymes that add UDP-glucose to ceramide in the first step of glycosphingolipid synthetic pathway (Leipelt et al. 2001). RNAi against two genes encoding proteins that exhibit glucosylceramide synthase activity in vitro (F59G1.1 and F20B4.6) and one predicted (T06C12.10) glucosylceramide synthase does not compromise viability. lin-12(n302)/+ hermaphrodites subjected to RNAi against each of these genes singly survived to adulthood, but their egg-laying ability was unchanged as compared to an empty-vector control (data not shown). BRE-3 and BRE-5 catalyze biosynthetic steps subsequent to the one catalyzed by glucosylceramide synthases, so this lack of effect might be due to functional redundancy between different glucosylceramide synthases.
Genetic evidence that bre-5 acts prior to LIN-12 activation by ligand-induced ectodomain shedding:
lin-12(ΔE), as a putative S2 cleavage mimic, would be expected to be constitutively active; ablation experiments described in the next section support this inference. However, we identified a surprising genetic property of lin-12(ΔE): it depends on the presence of lin-12(+) for full activity; i.e., lin-12(ΔE) has a more highly penetrant Muv phenotype in a lin-12(+) background than in a lin-12(0) background (Table 3, lines 4 and 7). As a short extracellular domain is believed to be sufficient to mark a transmembrane protein as a substrate for presenilin-mediated transmembrane cleavage (Struhl and Adachi 2000), our observation suggests that the LIN-12(+) protein may be playing a role in the trafficking, processing, or stability of LIN-12(ΔE). To our knowledge, the possibility that equivalent truncated forms in other systems also depend on endogenous wild-type activity has not been examined.
The dependence of lin-12(ΔE) on lin-12(+) for its constitutive activity enabled us to ask whether bre-5 suppresses the activity of the site 2 cleavage mimic or the intact LIN-12(+) form by testing the ability of bre-5(ye17) to suppress lin-12(ΔE) in the presence or the absence of lin-12(+). Suppression was observed in the presence of lin-12(+), but was not observed in the absence of lin-12(+), even though the presence of lin-12(+) makes lin-12(ΔE) “stronger” (Table 3). This observation indicates that bre-5 reduces the activity of lin-12(+), rather than the constitutive activity of lin-12(ΔE), and therefore suggests a role for bre-5 prior to ligand-induced ectodomain shedding. Ablation experiments described in the next section support this interpretation.
We note that hermaphrodites carrying transgenes that express LIN-12(ΔE) execute a normal AC/VU decision, so we could not assess the effect of bre-5 on lin-12 activity in that context. There are recurring problems with transgene expression in the AC/VU pair, so we believe that transgene expression, rather than an additional unusual property of lin-12(ΔE), accounts for the lack of a mutant phenotype in the AC/VU decision.
VPC isolation experiments also suggest that bre-5 acts prior to LIN-12 activation by ligand-induced ectodomain shedding. We performed cell ablation experiments in which all VPCs except P4.p or P8.p were killed by a laser microbeam in hermaphrodites carrying the lin-12(ΔE) transgene in a lin-12(+) background [referred to here as lin-12(+); lin-12(ΔE) for convenience]. The fate of the isolated VPC should reflect its intrinsic level of lin-12 activity, as the source of any potential lateral signal has been eliminated. In wild-type hermaphrodites, an isolated P4.p or P8.p generally adopts a nonvulval fate (Sulston and White 1980; Sternberg and Horvitz 1986). In contrast, in lin-12(+); lin-12(ΔE) hermaphrodites, an isolated P4.p or P8.p often adopts a vulval fate (Table 5, lines 1 and 5), indicating that it has elevated intrinsic lin-12 activity. Similarly, an isolated lin-12(+); bre-5(ye17); lin-12(ΔE) VPC also appears to have elevated intrinsic activity, as it always adopts a vulval fate (Table 5, lines 3 and 7). If loss of bre-5 were to affect the trafficking, processing, or stability of LIN-12(+) cell-autonomously, then we would have expected the intrinsic activity of lin-12(ΔE) in an isolated VPC to be reduced in the bre-5(ye17) background, and a nonvulval fate adopted, since the activity of lin-12(ΔE) depends on lin-12(+). The observation that intrinsic activity of lin-12(ΔE) is higher after the ablation is consistent with a nonautonomous function of bre-5. Alternatively, bre-5 function may be cell-autonomous to allow LIN-12(+) to be activated by ligand, if such activation is necessary for LIN-12(+) to potentiate lin-12(ΔE) activity.
TABLE 5.
VPC isolation experiments
| Genotypea | VPC | Vulval fate | Nonvulval fate |
|---|---|---|---|
| lin-12(ΔE)∷gfp | P4.p (isolated) | 6 | 0 |
| lin-12(ΔE)∷gfp | P4.p (unoperated) | 7 | 0 |
| bre-5(ye17); lin-12(ΔE)∷gfp | P4.p (isolated) | 11 | 0 |
| bre-5(ye17); lin-12(ΔE)∷gfp | P4.p (mock operated)b | 4 | 12 |
| lin-12(ΔE)∷gfp | P8.p (isolated) | 12 | 0 |
| lin-12(ΔE)∷gfp | P8.p (unoperated) | 7 | 0 |
| bre-5(ye17); lin-12(ΔE)∷gfp | P8.p (isolated) | 9 | 0 |
| bre-5(ye17); lin-12(ΔE)∷gfp | P8.p (mock operated)b | 6 | 10 |
These animals are also lin-12(+). Each unoperated animal of either genotype has a normal anchor cell and forms a functional vulva.
Mock-operated animals were treated in exactly the same way as laser operated ones, but no VPCs were ablated. Each VPC was scored as adopting either a vulval or a nonvulval fate; all pseudovulvae that were observed had the characteristic morphology associated with the 2° fate.
DISCUSSION
We reverted the 0 AC-Egl phenotype caused by a hypermorphic lin-12(d) allele and identified six new potential positive regulators of lin-12 activity. We molecularly characterized one of the new loci and found that it corresponded to bre-5, which encodes an enzyme of the glycosphingolipid biosynthetic pathway. We discuss first some general issues raised by this suppressor screen and then we focus on possible roles for glycosphingolipids in LIN-12/Notch signaling.
General issues raised by the suppressor screen:
Suppression of the 0 AC-Egl phenotype caused by lin-12(d) mutations has proven to be a powerful way to identify positive regulators of lin-12 activity, including core components and modulators. The characterization of sup-17 (Wen et al. 1997), lag-2 (originally known as sel-3; Tax et al. 1994), and sel-8 (Doyle et al. 2000; Petcherski and Kimble 2000) indicated that these genes encode core components of the LIN-12/Notch pathway.
Screens for suppressors of the 0 AC-Egl phenotype caused by lin-12(d) mutations have been carried out on a large scale, with >350,000 mutagenized haploid genomes scored and 14 extragenic suppressor loci identified (Tax et al. 1997; this work). Despite the large scale, the screen is far from saturation: about half of all genes identified in these screens are defined by single alleles. Furthermore, there is a remarkable lack of overlap between the set of genes defined by Tax et al. (1997) and by us (see Table 1).
Why has saturation been so difficult to achieve? We believe there are several contributing factors. One is that revertants must be viable and fertile to be recovered. Thus, only non-null alleles of genes required for lin-12 and/or glp-1 activity, or of genes having other pleiotropies, would be recovered. This problem is exemplified by the recovery of only single alleles of sel-8 (Tax et al. 1997; Doyle et al. 2000) and lag-1 (this work) in suppressor screens. Nevertheless, the suppressor screen is able to detect such genes: although lag-1 was also identified on the basis of phenotypic criteria, sel-8 was not (Lambie and Kimble 1991). Another factor is that the large number of intragenic revertants means that there is a large background that is tedious to sort through; the triage step that we designed to minimize the recovery of intragenic revertants, on the basis of past experience, was only partially effective.
Some of the technical issues that have limited conventional genetic suppressor screens may in principle be circumvented by RNAi, as genes essential for embryonic development may be identified by “feeding” L1 larvae (Timmons et al. 2001), and the variability inherent in reducing gene activity by RNAi may offer a wider range of reduced lin-12 activity and would bypass the problem of lin-12 intragenic revertants. The existence of libraries containing feeding constructs corresponding to a large percentage of C. elegans genes makes this approach practical (Kamath et al. 2003). However, pilot experiments have suggested that there is a high rate of false positives when RNAi is used in a 0 AC-Egl suppressor screen; so in this case, it is probably not a useful adjunct to the conventional suppressor screen (I. Katic, unpublished observations).
Glycosphingolipids and LIN-12/Notch signaling:
We have found that bre-3, bre-4, and bre-5, genes encoding three enzymes involved in the glycosphingolipid biosynthetic pathway, are positive regulators of lin-12 function. The Drosophila homologs of two of these genes, egghead (bre-3) and brainiac (bre-5), have also been studied in relation to Notch signaling. We discuss here the findings in C. elegans and Drosophila and potential ways in which glycosphingolipids may influence LIN-12/Notch activity.
Goode et al. (1996a) studied the roles of brn and egh during Drosophila oogenesis. They reported that brn and egh appear to be essential for the organization, but not the specification, of stalk and polar cells, whereas Notch is involved in specification of a stalk/polar cell fate decision as well as the polarity of these cell types. Thus, egh and brn do not appear to be involved in a Notch-mediated lateral interaction during oogenesis, but instead appear to play a role in the development and maintenance of epithelial cells. On the basis of their observations, they proposed that brn and egh regulate follicular morphogenesis by mediating germline-follicle cell adhesion.
In studying modulators of lin-12 activity, we have found that bre-3, bre-5, and bre-4, another gene involved in glycosphingolipid biosynthesis, influence two lin-12-mediated cell fate decisions. One, VPC specification, involves cell-cell interactions between polarized epithelial cells. The other, the AC/VU decision, does not; instead, it involves two mesodermally derived cells that do not have the characteristics of polarized epithelial cells. These observations contrast with those of Goode et al. (1996a) and suggest a broader role for glycosphingolipids in influencing LIN-12/Notch activity in conventional signaling. However, we did not find a role for bre genes in other lin-12- or glp-1-mediated decisions. Negative results do not necessarily prove that the bre genes do not contribute to other decisions, but they do raise the possibility that glycosphingolipids are involved only in a subset of lin-12/Notch-mediated decisions.
Glycosphingolipids are components of lipid rafts, which are thought to partition proteins into specific membrane microdomains and to provide platforms for association between certain kinds of proteins (Simons and Toomre 2000). Signaling molecules such as G proteins, Ras, and receptor tyrosine kinases have been found to be associated with lipid rafts (Waugh et al. 1999; Simons and Toomre 2000). It is not known whether rafts can include LIN-12/Notch proteins or their DSL family ligands, but it has been hypothesized that brn might affect Notch signaling through its effects on raft composition (Schwientek et al. 2002). Alternatively, a glycosphingolipid may act directly to modify LIN-12 or another factor in the LIN-12 signaling pathway, analogously to EGF receptor binding to a ganglioside (Miljan et al. 2002).
In assessing potential roles for bre-5/brainiac and bre-3/egghead in LIN-12/Notch signaling, it is important to account for the evidence that these genes function cell-nonautonomously. Goode et al. (1996a) have shown that while Notch appears to be necessary in the somatic follicular cells, egh and brn are required in the germline. We have shown that, in lin-12(ΔE); bre-5(ye17) animals, an isolated VPC has an elevated activity associated with lin-12(ΔE) and adopts a vulval fate. Thus, the function of bre-5/brainiac is more consistent with a role for glycosphingolipids in the signaling side of the LIN-12-mediated cell-cell interactions that specify cell fate. In view of this result, it does not appear likely that the effect of bre-5 on lin-12 activity is mediated through the γ-secretase complex, which has been shown to associate with lipid rafts in cell culture (Vetrivel et al. 2004; Urano et al. 2005), as γ-secretase activity is cell-autonomous for LIN-12/Notch signaling (Levitan and Greenwald 1995).
The activity of LIN-12(ΔE), the putative site 2 mimic, is higher in the presence of LIN-12(+), suggesting that it needs LIN-12(+) for its trafficking, processing, or stability. As bre-5 also requires lin-12(+) activity to suppress lin-12(ΔE), we infer that bre-5 affects a process that acts on or requires the extracellular domain of LIN-12.
The results of VPC isolation experiments, together with the inferred action of bre-5 prior to ectodomain shedding, are consistent with a role in signaling by DSL ligands. In Drosophila, endocytosis of DSL proteins is required for their signaling activity (Wang and Struhl 2004) and there may be a role for endocytosis of DSL proteins in certain situations in C. elegans as well (Tian et al. 2004). Although lin-12(d) alleles are constitutively active in the absence of ligand (Greenwald and Seydoux 1990), they remain sensitive to ligand, as can be seen when their activity or expression is low (Sundaram and Greenwald 1993b; C. Wen and I. Greenwald, unpublished observations). Furthermore, there are mutant ligand alleles that reduce lin-12 activity, although it is not known whether their function is cell-autonomous or cell-nonautonomous (Tax et al. 1994); nevertheless, in principle, it is possible that certain ligands would be able to engage the receptor in a nonproductive way. Thus, one model is that glycosphingolipids influence ligand conformation or activity, so that in the absence of the bre genes, the ligands are “worse” at engaging the receptor in a productive, signal-transducing event. Alternatively, glycosphingolipids may regulate the activity of a factor that modifies the extracellular milieu so as to influence the proper folding and activity of the extracellular portion of LIN-12, perhaps influencing its receptivity to ligand or influencing the productivity of the ligand-receptor interaction in some other way.
Phenotypic similarities and genetic interactions point to the importance of glycosphingolipids for proper LIN-12/Notch signaling in D. melanogaster and C. elegans (Goode et al. 1996a; this study). In C. elegans, the lack of overt phenotypic abnormalities and the low level of suppression of the lin-12(n302) egg-laying defect suggest that the contribution of bre gene function to LIN-12/Notch activity is modest. Even in Drosophila, where egh and brn mutations cause lethality, many Notch signaling processes do not appear to be affected (Goode et al. 1992, 1996a). Perhaps other mechanisms are redundant with glycosphingolipid function with respect to LIN-12/Notch signaling, or the small contribution is significant under conditions in nature.
It remains to be established whether glycosphingolipids affect Notch signaling in vertebrates, but there is no reason to believe that this is exclusively an invertebrate phenomenon. While the structures of glycosphingolipids are markedly different in invertebrates and vertebrates, most biosynthetic steps are catalyzed by homologous glycosyltransferases, and a mammalian glycosphingolipid precursor with a core structure different from those found in Drosophila is indeed functional in Drosophila (Wandall et al. 2005). Thus, these complex molecules appear to be functionally conserved through evolution, making their potential conservation in LIN-12/Notch signaling more plausible.
Acknowledgments
We gratefully acknowledge Jiabin Chen and Abigail Druck-Shudofsky for their contributions to this work. We also thank Richard Ruiz for excellent technical assistance, Sophie Jarriault, Natalie de Souza, and Oliver Hobert for comments on the manuscript, and past and present members of the Greenwald laboratory for useful discussions. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. This work was supported by NIH grants NS35556 and CA095389 (to I.G.). I.K. is currently a Postdoctoral Associate, and I.G. is an Investigator, of the Howard Hughes Medical Institute.
References
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., X. Li and I. Greenwald, 2004. sel-7, a positive regulator of lin-12 activity, encodes a novel nuclear protein in Caenorhabditis elegans. Genetics 166: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, T. G., C. Wen and I. Greenwald, 2000. SEL-8, a nuclear protein required for LIN-12 and GLP-1 signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 97: 7877–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares, H., and I. Greenwald, 1999. SEL-5, a serine/threonine kinase that facilitates lin-12 activity in Caenorhabditis elegans. Genetics 153: 1641–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, E. L., and H. R. Horvitz, 1985. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics 110: 17–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, S., D. Wright and A. P. Mahowald, 1992. The neurogenic locus brainiac cooperates with the Drosophila EGF receptor to establish the ovarian follicle and to determine its dorsal-ventral polarity. Development 116: 177–192. [DOI] [PubMed] [Google Scholar]
- Goode, S., M. Melnick, T. B. Chou and N. Perrimon, 1996. a The neurogenic genes egghead and brainiac define a novel signaling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development 122: 3863–3879. [DOI] [PubMed] [Google Scholar]
- Goode, S., M. Morgan, Y. P. Liang and A. P. Mahowald, 1996. b Brainiac encodes a novel, putative secreted protein that cooperates with Grk TGF alpha in the genesis of the follicular epithelium. Dev. Biol. 178: 35–50. [DOI] [PubMed] [Google Scholar]
- Greenwald, I., 1998. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 12: 1751–1762. [DOI] [PubMed] [Google Scholar]
- Greenwald, I., 2005. LIN-12/Notch signaling in C. elegans, in WormBook, edited by The C. elegans Research Community (doi/10.1895/wormbook.1.10.1; http://www.wormbook.org).
- Greenwald, I., and G. Seydoux, 1990. Analysis of gain-of-function mutations of the lin-12 gene of Caenorhabditis elegans. Nature 346: 197–199. [DOI] [PubMed] [Google Scholar]
- Greenwald, I. S., P. W. Sternberg and H. R. Horvitz, 1983. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell 34: 435–444. [DOI] [PubMed] [Google Scholar]
- Griffitts, J. S., J. L. Whitacre, D. E. Stevens and R. V. Aroian, 2001. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293: 860–864. [DOI] [PubMed] [Google Scholar]
- Griffitts, J. S., D. L. Huffman, J. L. Whitacre, B. D. Barrows, L. D. Marroquin et al., 2003. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxin-host interactions. J. Biol. Chem. 278: 45594–45602. [DOI] [PubMed] [Google Scholar]
- Griffitts, J. S., S. M. Haslam, T. Yang, S. F. Garczynski, B. Mulloy et al., 2005. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 307: 922–925. [DOI] [PubMed] [Google Scholar]
- Hanada, K., 2003. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632: 16–30. [DOI] [PubMed] [Google Scholar]
- Hubbard, E. J., Q. Dong and I. Greenwald, 1996. Evidence for physical and functional association between EMB-5 and LIN-12 in Caenorhabditis elegans. Science 273: 112–115. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kawar, Z. S., I. Van Die and R. D. Cummings, 2002. Molecular cloning and enzymatic characterization of a UDP-GalNAc:GlcNAc(beta)-R beta1,4-N-acetylgalactosaminyltransferase from Caenorhabditis elegans. J. Biol. Chem. 277: 34924–34932. [DOI] [PubMed] [Google Scholar]
- Lambie, E. J., and J. Kimble, 1991. Two homologous regulatory genes, lin-12 and glp-1, have overlapping functions. Development 112: 231–240. [DOI] [PubMed] [Google Scholar]
- Leipelt, M., D. Warnecke, U. Zahringer, C. Ott, F. Muller et al., 2001. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J. Biol. Chem. 276: 33621–33629. [DOI] [PubMed] [Google Scholar]
- Levitan, D., and I. Greenwald, 1995. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature 377: 351–354. [DOI] [PubMed] [Google Scholar]
- Marroquin, L. D., D. Elyassnia, J. S. Griffitts, J. S. Feitelson and R. V. Aroian, 2000. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics 155: 1693–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljan, E. A., E. J. Meuillet, B. Mania-Farnell, D. George, H. Yamamoto et al., 2002. Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J. Biol. Chem. 277: 10108–10113. [DOI] [PubMed] [Google Scholar]
- Muller, R., F. Altmann, D. Zhou and T. Hennet, 2002. The Drosophila melanogaster brainiac protein is a glycolipid-specific beta 1,3N-acetylglucosaminyltransferase. J. Biol. Chem. 277: 32417–32420. [DOI] [PubMed] [Google Scholar]
- Pepper, A. S., D. J. Killian and E. J. Hubbard, 2003. Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics 163: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcherski, A. G., and J. Kimble, 2000. LAG-3 is a putative transcriptional activator in the C. elegans Notch pathway. Nature 405: 364–368. [DOI] [PubMed] [Google Scholar]
- Priess, J. R., H. Schnabel and R. Schnabel, 1987. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell 51: 601–611. [DOI] [PubMed] [Google Scholar]
- Schwientek, T., B. Keck, S. B. Levery, M. A. Jensen, J. W. Pedersen et al., 2002. The Drosophila gene brainiac encodes a glycosyltransferase putatively involved in glycosphingolipid synthesis. J. Biol. Chem. 277: 32421–32429. [DOI] [PubMed] [Google Scholar]
- Shaye, D. D., and I. Greenwald, 2005. LIN-12/Notch trafficking and regulation of DSL ligand activity during vulval induction in Caenorhabditis elegans. Development 132: 5081–5092. [DOI] [PubMed] [Google Scholar]
- Simons, K., and D. Toomre, 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39 (erratum: Nat. Rev. Mol. Cell Biol. 2: 216). [DOI] [PubMed] [Google Scholar]
- Sternberg, P. W., and H. R. Horvitz, 1986. Pattern formation during vulval development in C. elegans. Cell 44: 761–772. [DOI] [PubMed] [Google Scholar]
- Struhl, G., and A. Adachi, 2000. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol. Cell 6: 625–636. [DOI] [PubMed] [Google Scholar]
- Struhl, G., K. Fitzgerald and I. Greenwald, 1993. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74: 331–345. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., and J. G. White, 1980. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev. Biol. 78: 577–597. [DOI] [PubMed] [Google Scholar]
- Sundaram, M., and I. Greenwald, 1993. a Genetic and phenotypic studies of hypomorphic lin-12 mutants in Caenorhabditis elegans. Genetics 135: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram, M., and I. Greenwald, 1993. b Suppressors of a lin-12 hypomorph define genes that interact with both lin-12 and glp-1 in Caenorhabditis elegans. Genetics 135: 765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax, F. E., J. J. Yeargers and J. H. Thomas, 1994. Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature 368: 150–154. [DOI] [PubMed] [Google Scholar]
- Tax, F. E., J. H. Thomas, E. L. Ferguson and H. R. Horvitz, 1997. Identification and characterization of genes that interact with lin-12 in Caenorhabditis elegans. Genetics 147: 1675–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, X., D. Hansen, T. Schedl and J. B. Skeath, 2004. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development 131: 5807–5815. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Urano, Y., I. Hayashi, N. Isoo, P. C. Reid, Y. Shibasaki et al., 2005. Association of active gamma-secretase complex with lipid rafts. J. Lipid Res. 46: 904–912. [DOI] [PubMed] [Google Scholar]
- Vetrivel, K. S., H. Cheng, W. Lin, T. Sakurai, T. Li et al., 2004. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 279: 44945–44954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandall, H. H., J. W. Pedersen, C. Park, S. B. Levery, S. Pizette et al., 2003. Drosophila egghead encodes a beta 1,4-mannosyltransferase predicted to form the immediate precursor glycosphingolipid substrate for brainiac. J. Biol. Chem. 278: 1411–1414. [DOI] [PubMed] [Google Scholar]
- Wandall, H. H., S. Pizette, J. W. Pedersen, H. Eichert, S. B. Levery et al., 2005. Egghead and brainiac are essential for glycosphingolipid biosynthesis in vivo. J. Biol. Chem. 280: 4858–4863. [DOI] [PubMed] [Google Scholar]
- Wang, W., and G. Struhl, 2004. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131: 5367–5380. [DOI] [PubMed] [Google Scholar]
- Waugh, M. G., D. Lawson and J. J. Hsuan, 1999. Epidermal growth factor receptor activation is localized within low-buoyant density, non-caveolar membrane domains. Biochem. J. 337: 591–597. [PMC free article] [PubMed] [Google Scholar]
- Wen, C., M. M. Metzstein and I. Greenwald, 1997. SUP-17, a Caenorhabditis elegans ADAM protein related to Drosophila KUZBANIAN, and its role in LIN-12/NOTCH signalling. Development 124: 4759–4767. [DOI] [PubMed] [Google Scholar]
- Weng, A. P., A. A. Ferrando, W. Lee, J. P. t. Morris, L. B. Silverman et al., 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306: 269–271. [DOI] [PubMed] [Google Scholar]
- Williams, B. D., B. Schrank, C. Huynh, R. Shownkeen and R. H. Waterston, 1992. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131: 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S., M. Fujimuro, J. J. Hsieh, L. Chen, A. Miyamoto et al., 2000. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC to facilitate NotchIC function. Mol. Cell. Biol. 20: 2400–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]