Abstract
Voluntary wheel running of mice in pregnancy and lactation led to a twofold increase in hippocampal precursor-cell proliferation and in the number of Prox1-expressing lineage-determined cells at postnatal day 8 (P8). At P36, the number of newly generated granule cells approximately doubled, resulting in a 40% higher total number of granule cells in pups from running dams as compared with controls. Cell proliferation at embryonic day 15 (E15), in contrast, was decreased in the progeny of exercising mice, and the birth weight was reduced. At P49, body weight had normalized, and hippocampal neurogenesis was not different between the two groups. mRNA for FGF2 was expressed at higher levels at E15 and P8 in runner pups, whereas VEGF was increased only at E15. Insulin-like growth factor did not show differences at any time point. At P36, no differences for any of the factors were found. Our data indicate that maternal behavior and physical activity affects infantile growth-factor expression and can transiently stimulate postnatal hippocampal development in the offspring.
Keywords: exercise, hippocampus, mice
Hippocampal neurogenesis in mice continues throughout life, albeit at a low rate (1–3), and, even in 57- to 72-year-old humans, hippocampal neurogenesis has been found (4). The functional relevance of adult hippocampal neurogenesis is still unclear, but regulation of neuronal development in the adult hippocampus is influenced by stimuli that can be associated with hippocampal function (5–7). The generalizability of such findings, however, is not undisputed (8). Nevertheless, hypotheses have been proposed that link the generation of new neurons in the dentate gyrus with specific hippocampal tasks in the context of learning and memory (9, 10). Voluntary physical activity increased adult hippocampal neurogenesis in mice by exerting a dual effect on both the proliferation of precursor cells in the dentate gyrus and the recruitment of the newly generated cells into neuronal differentiation (11). The proproliferative effect primarily affected the population of transiently amplifying progenitor cells in the dentate gyrus (12). It is not clear how this effect is mediated, but several growth factors, hormones, and neurotransmitter systems have been discussed in this context (13–15).
Adult hippocampal neurogenesis is not a simple continuation of embryonic and early postnatal neurogenesis (16). Adult neurogenesis originates from a distinct precursor-cell population in the subgranular zone of the dentate gyrus, the tertiary germinative matrix, and shows a complex pattern of regulatory interdependencies. We are particularly interested in the regulation of neurogenesis that can be linked to physiological behavior such as physical and cognitive activity. Both types of activity, which are very dominant in adulthood, must play a quantitatively and qualitatively different role during embryonic and fetal neurogenesis, because in utero the individual is not yet capable of extensive independent activity. Consequently, relatively little is known about the activity-dependent regulation of embryonic and early postnatal hippocampal neurogenesis and its relationship to adult neurogenesis. In general, however, “activity” in a rather broad sense is considered to be beneficial for the brain and is, for example, associated with a reduced risk of neurodegenerative disorders in humans (17, 18). This finding has not yet been extended to early stages of brain development. We thus became interested in the effects of physical activity on the development of the hippocampus as one brain structure that showed suggestive activity-dependent cellular plasticity during aging (19). We designed the present experiment to investigate whether the effects of voluntary physical activity would be transmissible from exercising pregnant and lactating mice to their offspring.
Results
We gave pregnant mice unlimited voluntary access to a running wheel (Fig. 1A), beginning on embryonic day 1 (E1)/E2. Pregnant control mice lived in standard cages. Running activity was highest in early pregnancy and decreased to low levels postnatally. Dividing cells were labeled with proliferation marker BrdU. To assess cell proliferation in the developing dentate gyrus, six offspring from running (RUN) mice and eight offspring from control (CTR) mice received one single i.p. injection of BrdU (50 μg/kg of body weight) at postnatal day 7 (P7), and their brains were examined at P8 to assess cell proliferation in the dentate gyrus. Five RUN and six CTR pups were given one daily injection of BrdU over 3 days between P7 and P9 and were perfused at P36 to examine the surviving cells from early postnatal precursor-cell activity that differentiated into neurons.
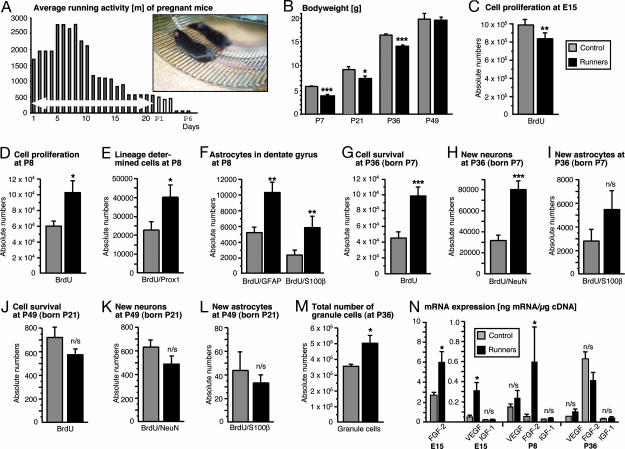
Fig. 1.
Running in pregnancy has multiple transient effects on the offspring. (A) Average running activity of mice during pregnancy. The average distance (in meters) decreased from 2,500 m/day during early pregnancy to <1,000 m after E10 and very low levels postpartum. (Inset) A pregnant running mouse. (B) Running during pregnancy led to a significant decrease in birth weight of the pups (in grams), measured at P7. In the course of the following weeks, the difference in body weights between CTR and RUN normalized and was absent at P49. (C) At E15, cell proliferation in the hippocampal anlage and the adjacent ventricular and SVZ (see Fig. 2 E and F) was reduced in RUN. (D) Proliferating cells, labeled at P7 with BrdU and examined at P8, increased by 71% in RUN as compared with CTR. (E) Transcription factor Prox1, which is specific to granule cells in the dentate gyrus, was used to further identify lineage-determination in newly generated cells. A significant increase was detected in RUN as compared with CTR. (F) Two different astrocytic markers, glial fibrillary acidic protein (GFAP) and S100β, showed a significant increase in the number of new, BrdU-labeled astrocytes at P8. The greater number of GFAP-positive cells is due to the fact that the putative stem cells of the adult dentate gyrus are GFAP-positive but S100β-negative. (G) At P49, 4 weeks after BrdU injection, the survival of newly generated cells was analyzed. There was a significant increase in BrdU-labeled cells in RUN compared with CTR. (H) Phenotypic analysis (see Fig. 3) of the BrdU-positive cells at P36 revealed an increase by 51% in the number of new neurons (BrdU/NeuN-double-positive) in RUN as compared with CTR. (I) In contrast, the number of newly generated astrocytes at P36 did not reach significance. (J) In animals injected with BrdU at P21, when postnatal hippocampal neurogenesis has largely ceased and the young mice have been weaned from their mother, the same number of surviving BrdU-labeled cells was detectable at P49, suggesting that neurogenesis had returned to control levels in RUN. (K and L) At P49, the number of BrdU/NeuN and BrdU/S100β double-positive cells that had originated from cell proliferation at P21 was not significantly different between RUN and CTR. (M) At P36, the stereologically determined total number of granule cells was significantly greater in RUN than in CTR. (N) The amount of mRNA as a measure of gene expression for three candidate mediators of activity-dependent effects on hippocampal neurogenesis was assessed by quantitative RT-PCR. At P8, FGF2, but not IGF1 and VEGF, was significantly increased in RUN. At E15, FGF2 and VEGF were increased; at P36, no significant differences in growth factor mRNA were detected. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; n/s, not significant
Exercise during pregnancy leads to a reduced birth weight in the offspring and a hypotrophic intrauterine development (20). At P7, RUN pups were significantly lighter than CTR (Fig. 1B; F1,12 = 135.12, P < 0.0001). The reduced birth weight normalized in the following weeks and was equal to CTR at P49. Maternal body weight at E15 was 34.0 ± 0 g in RUN (all numbers are mean ± SEM) and 38.7 ± 0.7 g in CTR, a nonsignificant difference (P = 0.73; after correction for litter size, P = 0.10). Given the fact that voluntary wheel running led to reduced body weight in previous studies (11), it is plausible that running mice in pregnancy do the same, even if it did not become obvious in this study.
To examine how maternal exercise might influence intrauterine neurogenesis, pregnant mice were injected with BrdU on E15. The brains of the embryos were examined on E16. In the area of the developing hippocampus and the entire adjacent ventricular zone, BrdU-labeled cells were quantified. There was a significant decrease in cell proliferation in RUN (Figs. 1C and 2E and F; F1,12 = 11.39; P < 0.01). It was not possible to separate counts from the ventricular and subventricular zones (SVZ) from the hippocampal anlage.
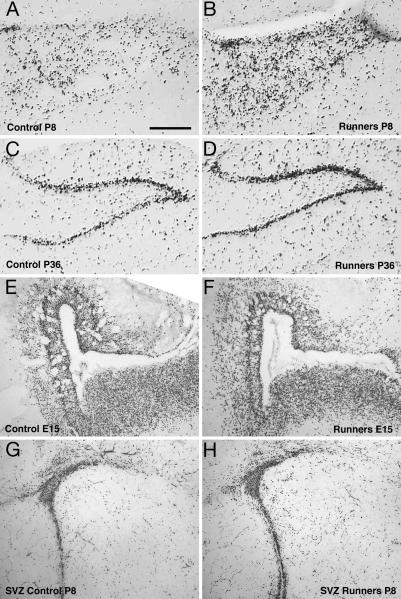
Fig. 2.
Proliferating cells in the dentate gyrus of RUN and CTR pups at P8 and P36. The representative micrographs show an anti-BrdU staining in the granule-cell layer of the dentate gyrus. (Scale bar, 250 μm.) (A and B) At P8, proliferating BrdU-labeled cells were dispersed over the granule-cell layer in RUN (n = 6) and CTR (n = 8). RUN had 71% more BrdU-labeled cells than CTR (P = 0.01). (C and D) At P36, most of the BrdU-labeled cells were detected in the characteristic cell band of the granule-cell layer. Also, at this time point, the total number of granule cells of RUN (n = 5) was 140% of CTR (n = 6), P = 0.001. (E and F) At E15, in RUN, a reduced number of proliferating (i.e., BrdU-labeled) cells in the hippocampal anlage and the adjacent ventricular and SVZ was found. This difference is not obvious in the photomicrograph but was statistically significant upon quantification (see Fig. 1C). (G and H) At P8, no obvious difference (as in the hippocampus, see A and B) in the number of BrdU-labeled cells was found in the SVZ. However, the experiment was not designed to study the SVZ quantitatively, and no strong conclusions should be drawn from the impression gained from these images.
At P8, proliferating cells were dispersed over the granule-cell layer of the dentate gyrus (Fig. 2 A and B). RUN had 71% more BrdU-labeled cells than CTR (Fig. 1D), indicating an increased proliferative activity (F1,12 = 9.28; P = 0.01). This effect might be specific to the hippocampus, because no obvious difference was found in the SVZ, another brain area with adult neurogenesis (Fig. 2 G and H). In the adult, physical activity increases neurogenesis in the hippocampus but not in the SVZ and olfactory bulb (21).
In the hippocampus, transcription factor Prox1 is specific for granule cells (22) and is expressed early in the course of granule-cell development. One day after the incorporation of BrdU, in RUN, 39.23 ± 1.78% and, in CTR, 35.73 ± 3.65% of the BrdU-labeled cells expressed Prox1 (F1,10 = 0.74; P = 0.41), relating to a significant increase in absolute numbers of BrdUrd-labeled Prox1-expressing cells (F1,10 = 6.04; P = 0.03; Figs. 1E and 3A).
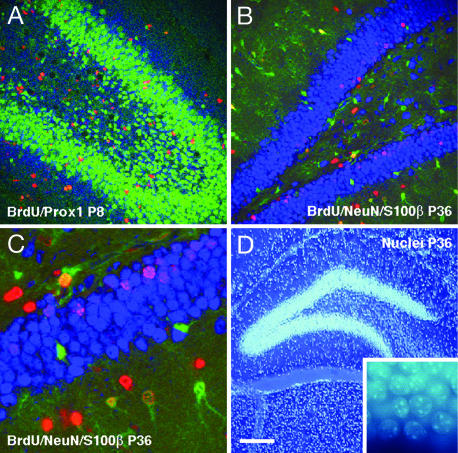
Fig. 3.
Confocal microscopic and DNA stain bisbenzemide analysis of granule cells and astrocytes in the dentate gyrus (DG) of pups from running mothers (RUN). Within each panel, the antigens investigated are listed in the order red, blue, and green. (A) Distribution of granule cells in the dentate gyrus at P8 was identified by their expression of the neuronal transcription factor Prox1. Newly generated granule cells were identified by immunoreactivity for proliferation marker BrdU (red) and Prox1 (blue). One day after BrdU application, the number of Prox1-expressing newly generated granule cells increased significantly in RUN compared with CTR. (B and C) At P36, survival of newly generated cells was quantified by their incorporation of BrdU (red). Neurons and astrocytes were characterized by their expression of NeuN (blue) or S100β (green). The survival rate of new neurons and astrocytes was significantly increased in RUN compared with controls. (D) Total granule-cell numbers were determined by using stereology (optical fractionator) based on DNA stain bisbenzemide (Hoechst 33258; see inset for higher magnification). Increased neurogenesis in the DG in RUN resulted in an absolute increase in the total number of granule cells at P36. [Scale bar in D for all panels; 100 μm (A and B), 30 μm (C), and 200 μm (D).]
There were 6,157 ± 1,367 new S100β-positive astrocytes in the RUN, as compared with 2,050 ± 485 in CTR at age P8 (Fig. 1F; F1,9 = 9.31; P = 0.01). In contrast, in RUN, there were 10,217 ± 3,039 new, GFAP-labeled cells compared with 5,159 ± 1,525 cells in CTR (Fig. 1F; F1,10 = 13.27; P < 0.01).
Over the following 4 weeks, new neurons generated from cell divisions at P7 to P9 developed into mature neurons, as assessed by immunoreactivity for NeuN, and were examined at P36. In RUN, 115% of the number of BrdU-labeled cells present in CTR were present at 4 weeks after BrdU (Fig. 1G). Of these cells, 81 ± 7.3% were NeuN-positive in RUN and 68.33 ± 4.9% in CTR, relating to a 151% increase in BrdU-positive/NeuN-positive cells in RUN as compared with CTR. In absolute terms, there were 79,896 ± 7,529 new, NeuN-positive cells in RUN compared with 31,807 ± 4,619 in CTR (Fig. 1H; F1,9 = 32.00; P < 0.001). There were 2,781 ± 955 new, S100β-labeled astrocytes in CTR as compared with 5,459 ± 1,547 in RUN; this apparent difference was not significant (Fig. 1I; P = 0.16).
We repeated this part of the study in an additional independent second series with 11 RUN2 and 5 CTR2. In the second series, the number of new neurons (BrdU-positive and NeuN-positive) at P36 was 50,027 ± 5,154 in RUN2 (n = 5) compared with 31,951 ± 5,450 in CTR2 (n = 5) (F1,8 = 5.81; P = 0.04).
In the first set of animals, we studied the effects on hippocampal neurogenesis in the offspring after direct contact with the mother had been terminated at P21. BrdU was injected at P21 to P23. At P49, 4 weeks after BrdU, there was no significant difference in hippocampal neurogenesis between CTR and RUN (Fig. 1 J–L). This finding applied to both the number of surviving BrdU-positive cells (F1,17 = 1.34; P = 0.26) and to the number of new neurons (BrdU/NeuN; F1,17 = 1.55; P = 0.23). This result suggests that, after direct contact with the mothers ceased, hippocampal neurogenesis returned to control levels. This finding relates to our previous report that environmental enrichment between P6 and P21 also had no lasting effects on adult hippocampal neurogenesis (23).
At P36, we also determined the total number of granule cells by stereological methods (optical fractionator). The total number of granule cells was 504,480 ± 53,760 in RUN as compared with 353,600 ± 13,280 in CTR (Fig. 1M; F1,9 = 8.85; P = 0.02). Thus, increased hippocampal neurogenesis in pups from running mothers resulted in an absolute increase in the total number of granule cells at P36.
Among the growth factors that have been discussed as key mediators of activity-dependent effects on hippocampal neurogenesis, experimental evidence exists for insulin-like growth factor (IGF)-1, VEGF, or FGF2 (13, 14, 24). We thus measured mRNA expression for these three factors in brain homogenate of CTR and RUN pups (Fig. 1N). At E15, we found an up-regulation of FGF2 (Z = −2.31; P = 0.02) and VEGF (Z = −2.31, P = 0.02) in RUN but not of IGF; Z = − 0.29; P = 0.77). At P8, we saw a significant up-regulation of FGF2 (Z = −2.02; P = 0.04) but not of the other two factors (VEGF: Z = −0.58, P = 0.56; IGF: Z = 0.00, P > 0.99). At P36, we detected no significant differences in mRNA expression (VEGF: Z = −1.73, P = 0.08; FGF2: Z = −1.73, P = 0.08; IGF: Z = −0.86, P = 0.39).
Discussion
In this study, we show that maternal voluntary physical activity during pregnancy and lactation has profound effects on intrauterine and postnatal development of the hippocampal dentate gyrus. An exercise-induced decrease in cell genesis was followed by a postnatal increase in neurogenesis, leading to a 40% net increase in the total number of granule cells. The postnatal induction of hippocampal neurogenesis occurred at a time when the actual maternal physical activity was relatively low. Our result could thus be interpreted as enhanced neurogenesis after slowed development in utero. Such delayed effect on hippocampal plasticity is conceivable in the light of many reports demonstrating long-lasting effects of maternal behavior on brain development, some of which resulted in measurable cognitive differences in old age (25, 26).
Neurogenesis after weaning showed similar normal levels in both experimental groups. In earlier experiments studying the effects of environmental enrichment on adult neurogenesis, we had found that, besides an acute effect on the progeny of dividing neural precursor cells, living in a challenging environment might also have a trophic effect on the population of precursor cells itself (19, 27). It is thus possible that the early postnatal manipulation might have more long-lasting effects on neurogenesis than became obvious in this study. Future experiments will have to test whether maternal activity, indeed, leads to a higher number of local precursor cells, as the increase in GFAP-positive, S100β-negative cells, and BrdU-labeled cells expressing Prox1 seems to suggest (28). In any case, there was a net effect on the total number of granule cells. The estimated granule-cell number in CTR of our present study is in the range of published data (29, 30), but neither the methods nor the age investigated match exactly. However, Amrein et al. (31) obtained the total granule-cell count in a hybrid cross of four commonly used laboratory strains (including C57BL/6) and, with ≈0.5 million granule cells, found a number that was closer to the number in wild mouse strains than in normal laboratory strains. It is tempting to speculate that the natural behavior of increased maternal physical activity as compared with the rather sedentary life in standard laboratory cages brought back granule cell numbers into a more normal range, but this remains to be shown positively.
Our data show that effects of physical activity on neurogenesis can be passed from the mother to their progeny. However, the effect did not consist of a straight transfer across the placental or mammary barrier. Rather, the effect in the pups differed considerably from the effects of voluntary physical activity on adult hippocampal neurogenesis. The effect was biphasic: A decrease in neurogenesis was followed by an even stronger, but transient, increase. This consequence was not necessarily due to the transplacental action of one circulating factor or a combination of several circulating factors, such as IGF1, VEGF, or FGF2 (13, 14, 24), which have been reported to show rather acute effects, consistent with the acute stimulation of cell proliferation by running. The temporal course was also distinct from the finding of a pregnancy-induced increase in maternal hippocampal neurogenesis, which was found to be mediated by prolactin (15). All of these mechanisms might be relevant, but the temporal pattern in our present study suggests contributions of additional factors.
On the basis of mRNA expression in the brain, we found that, at P8, FGF2, but not IGF1 and VEGF, was significantly up-regulated in RUN compared with CTR, which is in line with reports that FGF2 is necessary for the postnatal development of the dentate gyrus (32). At E15, both FGF2 and VEGF mRNA were up-regulated, which was in contrast to the observed decrease in cell proliferation. Consistent with our other results, we did not detect differences in growth-factor expression at P36. The finding that FGF2 expression correlated with cell proliferation at P8 but not at E15 fits with the hypothesis of DiCicco-Bloom and colleagues (32) that FGF2 “plays a developmental stage-specific role in regulating neurogenesis during the perinatal period of hippocampal development.” The dissociation of mRNA expression and cell genesis at E15 deserves further investigation.
In this context, it would be interesting to know whether there is a quantitative correlation between the amount of running activity and the size of the effects on hippocampal neurogenesis. Such a study, however, would require a very high N to achieve the necessary statistical power. This question was, thus, not within the scope of the present investigation.
The availability of a running wheel might have changed maternal behavior in more than one way and beyond the mere increase in running activity. The strongest effect on neurogenesis was found at a time when actual running activity of the mothers was low. But other maternal behaviors, e.g., licking and grooming, which might have strong and lasting effects on brain development, behavior, and on the hippocampus (33, 34), were not assessed in our study. It was not within the scope of our study to separate the direct from indirect effects. However, our data at E16 indicate that the effect cannot be due only to altered maternal behavior toward their pups, because this becomes effective only postnatally. We propose, rather, that the transient increase in postnatal neurogenesis is a rebound phenomenon after mildly hypotrophic intrauterine development.
Delayed effects of pre- or perinatal manipulations on hippocampal neurogenesis have been reported in other contexts as well. Kainic-acid-induced seizures, for example, which robustly induce adult hippocampal neurogenesis in adults (35, 36), caused an initial depression of early postnatal hippocampal cell proliferation, followed by an overshooting increase later (37). A similar pattern was found for prenatal protein malnutrition (38) and for and global neonatal asphyxia (39). In none of these studies was the total number of granule cells determined, so that the net effects are not known. Our experimental paradigm differs from these situations, in that we studied the consequences of a physiological behavior. Under feral conditions, maternal physical activity is to be expected. Nevertheless, running activity during pregnancy and lactation might have exerted stress on the pups in utero. However, strong prenatal stress had long-lasting negative effects on hippocampal neurogenesis (40, 41), and no rebound in neurogenesis has been reported so far. In any case, the role of stress (and stress hormones) on the regulation of hippocampal neurogenesis is complex and far from understood (3, 42–44). An association of suppressed hippocampal neurogenesis with increased glucocorticoid levels after early postnatal seizures has been proposed (45). Forced physical activity in adulthood, however, still had an up-regulating effect on hippocampal neurogenesis (46), and physical activity is generally associated with increased corticosterone levels (47). Consequently, the specific role of glucocorticoids in neurogenic effects such as those described here remains to be elucidated. In addition, it will be interesting to study neurogenesis in pups from mothers habituated to increased physical activity and in an experimental design in which progeny of exercising mothers is fostered by sedentary dams and vice versa.
The activity-induced differences in morphology of the hippocampus that we found might be suggestive of cognitive correlates, although, in the context of neurogenesis, “more” is not necessarily “better.” The total granule-cell number is a poor predictor of hippocampal performance (48) and so is the rate of adult neurogenesis (49, 50). However, the amount of adult hippocampal neurogenesis explains part of the variance in the performance during the acquisition of a hippocampus-dependent learning task (48, 51).
From our findings in rodents, one must not extrapolate to the situation in humans. Most studies on physical activity during human pregnancy were concerned with the potential maternal benefits or risks (52, 53). However, one study suggested that children from exercising mothers did better on standardized intelligence tests at the age of five (54, 55). Our data encourage further investigations in this direction, but, at present, functional benefits are not proven. Because many aspects of fetal brain development are influenced by maternal behavior, an influence is conceivable on the maturation of the hippocampal precursor-cell populations and, thus, on the potential contribution of these cells to hippocampal function (9, 56, 57). With this study, we intended to describe a biological principle. We neither suggest a simple extrapolation to the human situation nor give concrete advice to pregnant women.
Methods
Animals and Housing Conditions.
Three sets of female C57BL/6 mice (n = 16 each) were mated with C57BL/6 males. The morphological results of this study are based on set 1. Set 2 was used to confirm the core finding. Set 3 was used for mRNA expression analysis. The three experiments were independent and done consecutively. After mating, the females were randomly assigned to the experimental groups and individually housed in standard laboratory cages (CTR) or the identical cages equipped with an electronically monitored running wheel (RUN). In RUN conditions, the running wheel was available beginning on E1/E2. In RUN, three, two, and two mice developed pregnancies, with a total of 18, 14, and 20 progeny, respectively. In CTR, three, two, and two mice developed a pregnancy, with a total of 18, 6, and 17 progeny, respectively. Litter size was 7–10 in all cases. Proliferating cells were labeled at P7 with a single injection of BrdU; 50 μg/g of body weight in 0.9% sterile saline, 10 μg/ml; Sigma) to assess cell proliferation (examination at P8) or three single injections of BrdU, P7–P9, with examination of cell survival and differentiation at P36.
To investigate the effects of maternal running on intrauterine development, additional pregnant mice were injected with BrdU at E15, and 8 exercising and 30 control pups were examined at E16. For measuring hippocampal neurogenesis after weaning, when direct contact with the mother had been terminated, 14 RUN pups and 5 CTR pups were injected with BrdU on P21 and examined 4 weeks later (P49).
All animals received water and food ad libitum. All applicable regulations of animal welfare were followed.
Immunohistochemistry.
At E16 and P8, brains were fixed in 4% paraformaldehyde by immersion and, at P36 and P49, by perfusion. All immunohistochemical analyses were performed as described in ref. 58. BrdU was visualized with a monoclonal rat-anti-BrdU antibody (Biozol) at 1:500 after pretreatment of the tissue with 1 N hydrochloric acid for 15 min at 37°C, followed by 5 min in borate buffer. The numbers of BrdU-positive cells were determined in light-microscopic specimens, visualized with the peroxidase method (Vectastain ABC Elite; Vector Laboratories). Phenotypes were analyzed in triple-stained immunofluorescent labelings and confocal microscopic analysis. Analyses at E16 were done on mounted and postfixed cryosections of 15-μm thickness. All other studies were done on free-floating 40-μm sections as described in ref. 58. The antibody against Prox1 was used at 1:5,000 (Chemicon).
Stereology.
To determine the number of BrdU-labeled cells in the dentate gyrus and the total granule-cell count, the optical-fractionator method was used as implemented in the semiautomatic stereology system stereoinvestigator 5.4.3 (MicroBrightField, Magdeburg, Germany). Actual section thickness was measured, and appropriate guard zones at the top and the bottom of the section were defined to avoid oversampling. Measurements were made in a systematic series of 8–10 coronal sections, 240 μm apart, with a random starting point and spanning the entire dentate gyrus in its rostrocaudal extension. Total granule-cell numbers were determined in sections treated with DNA-stain bisbenzemide (Hoechst 33258; Sigma; 50 ng/ml Tris-buffered saline for 15 min). All analyses were done blinded regarding the experimental groups. The procedure followed the directions given by the software. The size of the counting frame was 15 × 15 μm, the thickness of the tissue averaged 20 μm, the height of the disector cube was 10 μm, and the area associated with each x–y movement was 10,000 μm2.
Quantitative RT-PCR.
Quantitative RT-PCR was done as described by Morsczeck et al. (59). Four female animals each from sets 1 (P8) and 3 (E15 and P36) were randomly chosen for this experiment. RNA extraction from one brain hemisphere was performed with QIAzol lysis reagent and RNeasy lipid tissue mini kit (Qiagen) according to the manufacturer’s instructions. For each RNA sample (1 μg/μl), three independent RT reactions were performed by using the dNTPs-mix, oligo(dT)12–18 primer and SuperScript II RNase H− reverse transcriptase (Invitrogen), followed by incubation with RNase H (Invitrogen) for 20 min at 37°C. cDNA from each RT batch was adjusted to equal amounts.
For the three molecules of interest, FGF-2, IGF-1, and VEGF, we generated an external standard by using a PCR product of ≈500 bp, which included the target fragment. Primers were generated with primer3 software (60) (see Table 1, which is published as supporting information on the PNAS web site). To generate standards from whole cDNA, we performed a PCR (High-fidelity Super Mix; Invitrogen) and ran the product on a 1.5% agarose gel. From fragments of ≈500 bp, we eluted the cDNA (E.Z.N.A. gel extraction kit; Peqlab Biotechnologie, Erlangen, Germany), measured the concentration, and performed a quantitative PCR run with different dilutions of the standard cDNA and the inner primer pair on an Opticon II thermal cycler (MJ Research). We used SYBR green (Quantitect SYBR green PCR kit; Qiagen). A melting-curve analysis verified the specificity of the reaction. The PCR protocol was 3 min at 95°C, 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C for 40 cycles of steps 2–4, followed by a melting curve (55–90°C).
From each RNA sample, three RT reactions were performed, in which all three molecules were measured by quantitative PCR. Means from the replicates were used for further statistical analysis by a Mann–Whitney U test.
Supplementary Material
Acknowledgments
We thank Ruth Zarmstorff and Irene Thun for technical support. This work was supported by Volkswagen-Stiftung.
Abbreviations
- CTR
control
- En
embryonic day n
- IGF
insulin-like growth factor
- Pn
postnatal day n
- RUN
running
- SVZ
subventricular zone.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kuhn H. G., Dickinson-Anson H., Gage F. H. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron H. A., McKay R. D. Nat. Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 3.Montaron M. F., Petry K. G., Rodriguez J. J., Marinelli M., Aurousseau C., Rougon G., Le Moal M., Abrous D. N. Eur. J. Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson P. S., Perfilieva E., Björk-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Leuner B., Mendolia-Loffredo S., Kozorovitskiy Y., Samburg D., Gould E., Shors T. J. J. Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 7.Snyder J. S., Hong N. S., McDonald R. J., Wojtowicz J. M. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Ehninger D., Kempermann G. Genes Brain Behav. 2006;5:29–39. doi: 10.1111/j.1601-183X.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Deisseroth K., Singla S., Toda H., Monje M., Palmer T. D., Malenka R. C. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 10.Kempermann G. J. Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Praag H., Kempermann G., Gage F. H. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 12.Kronenberg G., Reuter K., Steiner B., Brandt M. D., Jessberger S., Yamaguchi M., Kempermann G. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- 13.Trejo J. L., Carro E., Torres-Aleman I. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C. J., Palmer T. D. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 15.Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C., Cross J. C., Weiss S. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 16.Altman J., Bayer S. A. J. Comp. Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 17.Wilson R. S., Mendes De Leon C. F., Barnes L. L., Schneider J. A., Bienias J. L., Evans D. A., Bennett D. A. J. Am. Med. Assoc. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 18.Colcombe S. J., Kramer A. F., Erickson K. I., Scalf P., McAuley E., Cohen N. J., Webb A., Jerome G. J., Marquez D. X., Elavsky S. Proc. Natl. Acad. Sci. USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempermann G., Gast D., Gage F. H. Ann. Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 20.Pinto M. L., Shetty P. S. Br. J. Nutr. 1995;73:645–653. doi: 10.1079/bjn19950070. [DOI] [PubMed] [Google Scholar]
- 21.Brown J., Cooper-Kuhn C. M., Kempermann G., Van Praag H., Winkler J., Gage F. H., Kuhn H. G. Eur. J. Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 22.Pleasure S. J., Collins A. E., Lowenstein D. H. J. Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohl Z., Kuhn H. G., Cooper-Kuhn C. M., Winkler J., Aigner L., Kempermann G. Genes Brain Behav. 2002;1:46–54. doi: 10.1046/j.1601-1848.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Pinilla F., Dao L., So V. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 25.Meaney M. J., Aitken D. H., van Berkel C., Bhatnagar S., Sapolsky R. M. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 26.Vallée M., Maccari S., Dellu F., Simon H., Le Moal M., Mayo W. Eur. J. Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 27.Kempermann G., Gage F. H. Hippocampus. 1999;9:321–332. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Kempermann G., Jessberger S., Steiner B., Kronenberg G. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Abusaad I., MacKay D., Zhao J., Stanford P., Collier D. A., Everall I. P. J. Comp. Neurol. 1999;408:560–566. doi: 10.1002/(sici)1096-9861(19990614)408:4<560::aid-cne9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Lu L., Airey D. C., Williams R. W. J. Neurosci. 2001;21:3503–3514. doi: 10.1523/JNEUROSCI.21-10-03503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amrein I., Slomianka L., Lipp H. P. Eur. J. Neurosci. 2004;20:3342–3350. doi: 10.1111/j.1460-9568.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y., Black I. B., DiCicco-Bloom E. Eur. J. Neurosci. 2002;15:3–12. doi: 10.1046/j.0953-816x.2001.01832.x. [DOI] [PubMed] [Google Scholar]
- 33.Bredy T. W., Grant R. J., Champagne D. L., Meaney M. J. Eur. J. Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu D., Diorio J., Day J. C., Francis D. D., Meaney M. J. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 35.Parent J. M., Yu T. W., Leibowitz R. T., Geschwind D. H., Sloviter R. S., Lowenstein D. H. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bengzon J., Kokaia Z., Elmér E., Nanobashvili A., Kokaia M., Lindvall O. Proc. Natl. Acad. Sci. USA. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong H., Csernansky C. A., Goico B., Csernansky J. G. J. Neurosci. 2003;23:1742–1749. doi: 10.1523/JNEUROSCI.23-05-01742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King R. S., DeBassio W. A., Kemper T. L., Rosene D. L., Tonkiss J., Galler J. R., Blatt G. J. Brain Res. Dev. Brain Res. 2004;150:9–15. doi: 10.1016/j.devbrainres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Scheepens A., Wassink G., Piersma M. J., Van de Berg W. D., Blanco C. E. Brain Res. Dev. Brain Res. 2003;142:67–76. doi: 10.1016/s0165-3806(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 40.Lemaire V., Koehl M., Le Moal M., Abrous D. N. Proc. Natl. Acad. Sci. USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coe C. L., Kramer M., Czeh B., Gould E., Reeves A. J., Kirschbaum C., Fuchs E. Biol. Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 42.Cameron H. A., Gould E. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 43.Gould E., McEwen B. S., Tanapat P., Galea L. A. M., Fuchs E. J. Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heine V. M., Maslam S., Zareno J., Joels M., Lucassen P. J. Eur. J. Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu H., Kaur J., Dashtipour K., Kinyamu R., Ribak C. E., Friedman L. K. Exp. Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y. P., Kim H., Shin M. S., Chang H. K., Jang M. H., Shin M. C., Lee S. J., Lee H. H., Yoon J. H., Jeong I. G., et al. Neurosci. Lett. 2004;355:152–154. doi: 10.1016/j.neulet.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Sellers T. L., Jaussi A. W., Yang H. T., Heninger R. W., Winder W. W. J. Appl. Physiol. 1988;65:173–178. doi: 10.1152/jappl.1988.65.1.173. [DOI] [PubMed] [Google Scholar]
- 48.Kempermann G., Gage F. H. Eur. J. Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 49.Bizon J. L., Gallagher M. Sci. Aging Knowledge Environ. 2005;7:re2. doi: 10.1126/sageke.2005.7.re2. [DOI] [PubMed] [Google Scholar]
- 50.Merrill D. A., Karim R., Darraq M., Chiba A. A., Tuszynski M. H. J. Comp. Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- 51.Drapeau E., Mayo W., Aurousseau C., Le Moal M., Piazza P. V., Abrous D. N. Proc. Natl. Acad. Sci. USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Committee on Obstetric Practice. Am. Coll. Obstr. Gynecol. Int. J. Gynaecol. Obstet. 2002;77:79–81. [Google Scholar]
- 53.Sport. Med. Assoc. J. Sci. Med. Sport. 2002;5:11–19. [Google Scholar]
- 54.Clapp J. F., III J. Pediatr. 1996;129:856–863. doi: 10.1016/s0022-3476(96)70029-x. [DOI] [PubMed] [Google Scholar]
- 55.Clapp J. F., III Clin. Sport. Med. 2000;19:273–286. doi: 10.1016/s0278-5919(05)70203-9. [DOI] [PubMed] [Google Scholar]
- 56.Kempermann G., Wiskott L., Gage F. H. Curr. Opin. Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., et al. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 58.Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F. H. Development (Cambridge, U.K.) 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 59.Morsczeck C., Langendorfer D., Schierholz J. M. J. Biochem. Biophys. Methods. 2004;59:217–227. doi: 10.1016/j.jbbm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Rozen S., Skaletsky H. In: Bioinformatics Methods and Protocols: Methods in Molecular Biology. Krawetz S., Misener S., editors. Toronto: Humana; 2000. pp. 365–386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.