Abstract
Multiple classes of precursor cells have been isolated and characterized from the developing spinal cord including multipotent neuroepithelial (NEP) stem cells and lineage-restricted precursors for neurons (NRPs) and glia (GRPs). We have compared the survival, differentiation and integration of multipotent NEP cells with lineage-restricted NRPs and GRPs using cells isolated from transgenic rats that express the human placental alkaline phosphatase gene. Our results demonstrate that grafted NEP cells survive poorly, with no cells observed 3 days after transplant in the adult hippocampus, striatum and spinal cord, indicating that most CNS regions are not compatible with transplants of multipotent cells derived from fetal CNS. By contrast, at 3 weeks and 5 weeks post-engraftment, lineage-restricted precursors showed selective migration along white-matter tracts and robust survival in all three CNS regions. The grafted precursors expressed the mature neuronal markers NeuN and MAP2, the astrocytic marker GFAP, the oligodendrocytic markers RIP, NG2 and Sox-10, and the synaptic marker synaptophysin. Similar behavior was observed when these precursors were transplanted into the injured spinal cord. Predifferentiated, multipotent NEP cells also survive and integrate, which indicates that lineage-restricted CNS precursors are well suited for transplantation into the adult CNS and provide a promising cellular replacement candidate.
Keywords: Stem cells, neurons, glial cells, neural progenitors, spinal cord injury
INTRODUCTION
Transplantation of neural stem cells (NSCs) and lineage-restricted precursors is a promising therapeutic strategy for the treatment of neurodegenerative disorders and CNS injuries (Fischer, 2000). The application of this therapeutic potential depends on a better understanding of the properties of defined populations of cells in vivo with respect to survival, migration, phenotypic fate and integration, irrespective of whether cells are used to deliver growth factors, mobilize endogenous stem cells or provide cellular replacement (Rao and Mayer Proschel, 2000).
Of the many cell types that have been transplanted (Rao and Mayer-Proschel, 2000) NSCs are theoretically the most logical candidates for cell replacement in the CNS. They are present in the CNS (Horner et al., 2000; Weiss et al., 1996), respond to injury in some regions by enhanced proliferation and differentiation (Johansson et al., 1999; Yamamoto et al., 2001), and possess the potential to generate all damaged cell types, including neurons, astrocytes and oligodendrocytes. Both immortalized (Vescovi and Snyder, 1999; Whittemore and Onifer, 2000) and non-immortalized (Cao et al., 2002a) NSCs have been used successfully to deliver trophic support, repair demyelination and provide neuronal replacement.
However, in most experiments cells have been transplanted after being maintained in culture as neurospheres (Reynolds and Weiss, 1996; Weiss et al., 1996; Svendsen et al., 1997; Ogawa et al., 2002; Pluchino et al., 2003). Generally, these contain only a small fraction of undifferentiated NSCs (~5%), with the majority of cells either differentiating lineage-restricted precursors or mature cell types (Tropepe et al., 1999; Suslov et al., 2002; Kim and Morshead, 2003; Seaberg and van der Kooy, 2003). Thus, it is unclear how a pure NSC population will behave in vivo in the adult CNS. Indeed, some groups have suggested that prolonged passaging of NSCs alters their behavior such that they are either transformed (Seaberg and van der Kooy, 2003) or biased towards a glial fate (Cao et al., 2002b) and might not reflect the properties of endogenous stem cells.
Further complicating the comparison and assessment of the behavior of stem cells in vivo are recent studies that show NSCs themselves are a heterogeneous population, with different classes of NSCs described (Pevny and Rao, 2003). Each class appears multipotent and has the ability to form neurospheres, but they differ in their initial state, bias in differentiation capacity and, possibly, degree of self-renewal. It is unclear which, if any, of these NSCs is a reasonable candidate for cell-replacement therapy. It is also unclear how to compare results from different laboratories, because often no distinctions are made between neurosphere-forming populations. Determining the optimal type(s) of cells for transplantation into the CNS, despite it obvious importance, therefore remains unanswered.
In addition to multipotent cells, several investigators have suggested that more restricted precursors could be used for transplantation (Cai and Rao, 2002). Stem cells generate mature CNS cell types via the intermediate production of lineage-restricted precursors, and several such cells with more limited differentiation potential have been identified (Rao, 1999). These include neuronal-restricted precursors (NRPs) and glial-restricted precursors (GRPs) (Mayer-Proschel et al., 1997; Rao and Mayer-Proschel, 1997). Previous work has shown that transplantation of either NRPs or GRPs separately into the CNS results in the robust survival of both cell types, and the production of neuronal and glial phenotypes, respectively (Yang et al., 2000; Herrera et al., 2001; Cao et al., 2002b; Han et al., 2002; Han et al., 2004). This work, as well as other studies that have transplanted several types of lineage-restricted precursors including oligodendrocyte progenitors (Roy et al., 1999; Zhang et al., 1999; Windrem et al., 2004), indicates that lineage-restricted cells are a promising source for transplantation into the CNS and may be a reasonable alternative to stem cells for cell replacement in the CNS.
The rationale is based on the observation that in most regions of the adult brain an appropriate stem cell niche does not exist, cues to direct stem-cell differentiation do not persist and endogenous stem cells do not appear to participate in repair in any reasonable number. In the spinal cord, for example, it has been shown that although stem cells exist (Weiss et al., 1996; Horner et al., 2000) and readily generate neurons in culture (Shihabuddin et al., 2000) transplantation of these cells does not promote neurogenesis in either intact or injured spinal cord (Shihabuddin et al., 2000; Cao et al., 2001). By contrast, transplantation of NRPs in identical transplant paradigms generates post-mitotic neurons that appear to integrate into the host environment (Cao et al., 2002b; Han et al., 2002).
To further compare NSCs and lineage-restricted precursors and to extend the observations to other regions of the brain, we have compared the ability of fetal NSCs to survive, integrate and differentiate with that of differentiated NSCs and mixed populations of lineage-restricted precursors (NRPs and GRPs) isolated from the same portion of the caudal neural tube. Fetal NSCs, termed neuroepithelial (NEP) stem cells (Kalyani et al., 1997), to distinguish them from later appearing neurosphere-forming NSCs and even later developing adult NSCs, were used in this study. NEP cells, like other NSCs, are multipotent and capable of self-renewal. Importantly for our purposes, NEP cells can be isolated as a near homogenous population by dissection at the appropriate developmental stage (Kalyani et al., 1997). Mixed populations of lineage-restricted NRPs and GRPs can be isolated from the caudal neural tube at embryonic day 13.5 (E13.5), a stage at which both populations of cells are present in a ratio of ~2:1 (Rao, 1999). Mixed populations of NRPs and GRPs were grafted because these transplants have the potential to produce all CNS cell types, which is an attractive property for many CNS pathologies.
We show that NEP cells do not survive in the intact adult striatum, hippocampus, and spinal cord, which indicates that most CNS regions do not support the survival of multipotent cells derived from fetal CNS. By contrast, mixed NRP/GRP grafts survive robustly, migrate selectively and extensively along white matter tracts, and differentiate into neurons, astrocytes and oligodendrocytes in the same three CNS regions. Furthermore, mixed NRP/GRP grafts survive and differentiate down all mature CNS neural lineages following transplantation into the injured spinal cord. This work indicates that transplantation of mixed populations of lineage-restricted precursors is a promising therapeutic strategy for CNS repair.
OBJECTIVE
The first objective of this study was to compare the in vivo fate of multipotent neural stem cells (NEP cells) with lineage-restricted precursor cells (NRPs and GRPs) following transplantation into the intact adult CNS, using cells derived from a transgenic donor rat (Kisseberth et al., 1999; Mujtaba et al., 2002) for reliable tracking. The second objective was to assess the most promising cell candidate for cellular replacement therapy following transplantation into the injured adult CNS.
METHODS
Cell isolation and culture
NEP cells
NEP cells were isolated from E10.5 transgenic Fisher 344 rats that express the marker gene, human placental alkaline phosphatase (AP). Briefly, trunk segments (caudal 10 somites) of embryos were isolated in a dish containing DMEM/F12 (Invitrogen). The neural tubes/somites were incubated in a collagenase Type I (10 mg ml−1, Worthington Biochemicals)/dispase II (20 ng ml−1, Roche Diagnostics)/HBSS (Cellgro) solution for 5 minutes at room temperature. Gentle trituration was employed to separate neural tubes from the somites. Tubes were dissociated using a 0.05% trypsin/EDTA (Invitrogen) solution for 5 minutes at 37°C, followed by plating in NEP basal medium [DMEM-F12, BSA (1 mg ml−1, Sigma), B27 supplement (Invitrogen), bFGF (20 ng ml−1, Peprotech), Pen-Strep (100 IU ml−1, Invitrogen), N2 (10 μl ml−1, Invitrogen)], supplemented with 10% CEE on human foreskin fibronectin-coated (250 μl ml−1, Fibrogenex) dishes.
Mixed NRPs and GRPs
Mixed NRPs/GRPs were isolated simultaneously from E13.5 transgenic Fischer 344 rats that express the AP marker gene. Briefly, embryos were isolated in a dish containing DMEM/F12. Trunk segments were incubated in a collagenase Type I (10 mg ml−1)/dispase II (20 ng ml−1)/HBSS solution for 8 minutes at room temperature to allow for peeling away of meninges from the cords. Cords were dissociated using a 0.05% trypsin/EDTA solution for 20 minutes at 37°C. Cells were then plated in NRP complete medium consisting of NEP basal medium, supplemented with 10 μl ml−1 bFGF and 10 μl ml−1 NT-3 (Peprotech) on dishes coated with poly-L-lysine (13.3 μl ml−1, Sigma) and laminin (20 μl ml−1, Invitrogen).
In vitro differentiation of NEP cells
NEP cells were cultured for three passages under non-differentiating conditions. Cells maintained their characteristic NEP-like morphology and did not express markers of either lineage-restricted precursors or mature cell types. NEP cells were then stimulated to differentiate towards the lineage-restricted precursor state. Cells were trypsinized and plated in NEP basal medium on dishes coated with fibronectin and laminin. After 7 days, cells were trypsinized and replated in NEP basal medium supplemented with retinoic acid (17.5 ng ml−1, Sigma) and NT-3 (10 ng ml−1) on dishes coated with poly-L-lysine and laminin.
Preparation of cells for grafting
NEP cells, differentiated NEP cells and NRPs/GRPs were dissociated from culture flasks using 0.05% trypsin/EDTA, washed and resuspended at a concentration of either 12 500 cells μl−1 in basal media or 150 000 cells μl−1 in an equal mixture of Vitrogen (a Type I Collagen matrix, Cohesion) and basal media for transplanting into the intact and injured CNS, respectively. Cells were placed on ice throughout the grafting session. At the completion of the grafting session, cell viability was assessed using the trypan blue assay. Viability was always >90%. Following embryonic dissection, NEP cells were cultured for 1–2 weeks, whereas NRPs/GRPs were cocultured for 1–10 days before transplantation.
Animal surgery/transplantation
Adult brain transplants
Adult, female Sprague-Dawley rats (~250 g) received i.p. injections of anaesthetic cocktail consisting of 0.7 mg kg−1 acepromazine maleate (Fermenta Animal Health), 95 mg kg−1 ketamine (Fort Dodge Animal Health) and 10 mg kg−1 xylazine (Bayer). Each animal received a single unilateral graft of 25 000 cells in 2 μl into either the hippocampal dentate gyrus or striatum. The coordinates used for grafting are: hippocampus, A/P) −3.5; L) ± 2.0; D/V) −3.5/−3.1; striatum, A/P) +0.6; L) ± 2.8; D/V) −4.8/−4.2. The tooth bar was set at −2.3, and all D/V coordinates were taken from the dura. Briefly, cells were delivered using a 10 μl Hamilton Gastight syringe (Hamilton) with a 33-gauge beveled needle. Cells were injected at both the deepest and shallowest D/V coordinates. The tip was held in place both before and after each of the two injections for 2 minutes. Cells were delivered at a rate of 0.5 μl minute−1. Animals were immunosuppressed by subcutaneous administration of 10 mg kg−1 cyclosporine A (Sandoz Pharmaceuticals) daily beginning three days before grafting and continued until sacrifice. Immunosuppression was utilized because of mismatch between Fischer-derived donor cells and Sprague-Dawley recipient animals.
Adult spinal cord transplants (intact)
Adult, female Sprague-Dawley rats (~250 g) received i.p. injections of anesthetic cocktail as above. The back musculature was excised and a laminectomy performed at the cervical 3 (C3)/C4 level. The dura was excised above the injection site using a 30-gauge needle. Each animal received a single unilateral graft of 25 000 cells in 2 μl into the right dorsal columns at the C4 level. Briefly, cells were delivered using a 10 μl Hamilton Gastight syringe with an attached borosillate glass tip (50–100 μm tip diameter). The injection pipette was secured to a manual micromanipulator (World Precision Instruments) attached to an 80° tilting base. The tip was lowered to 1 mm below the surface of the cord and was held in place for 2 minutes before and after cell injection. Cells were delivered under the control of a microsyringe pump controller (World Precision Instruments) at a rate of 1 μl minute−1. The dura was closed with 9–0 suture, muscle reapposed and skin closed with wound clips. Animals received Bupranorphin (Reckitt Benckiser) postoperatively. Animals were immuno-suppressed by subcutaneous administration of cyclosporine A (10 mg kg−1) daily beginning three days before grafting and continuously until sacrifice.
Adult spinal cord transplants (injured)
Lateral funiculotomy injuries were created at the C4 spinal cord level. Adult female Sprague-Dawley rats (~250 g) received i.p. injections of anesthetic cocktail as above. The back musculature was excised and a laminectomy performed at the C3/C4 level. Dura was incised above the dorsal root entry zone. Microscissor cuts were created at the rostral and caudal extents of the injury. Aspiration was used to selectively ablate only the lateral white matter tracts and a minimal portion of the dorsal and ventral gray matter. However, the dorsal columns and central canal were unaffected. Once hemostasis was achieved, NRP/GRP cells in Vitrogen were implanted into the injury cavity. The cell/matrix mixture completely filled the cavities, and in each case ~500 000 cells were grafted into the injury site. The dura was closed with 9-0 suture, muscle was reapposed and the skin closed with wound clips. Animals received Bupranorphin and methylprednisolone (Pharmacia and Upjohn) postoperatively. Animals were immunosuppressed by subcutaneous administration of cyclosporine A (10 mg kg−1) daily beginning three days before grafting and continuously until sacrifice.
The care and treatment of animals in all procedures was conducted in strict accordance with the guidelines set by the NIH and Drexel University IACUC.
Tissue processing
Animals were sacrificed at 3 days, 1, 3 and 5 weeks following transplantation by transcardial perfusion with 0.3% saline, followed by ice-cold 4% paraformaldehyde (Fisher Scientific). Spinal cord and brain were removed from the animal, followed by cryprotection in 30% sucrose (Fisher Scientific)/0.1 M phosphate buffer at 4°C for 3 days. The tissue was embedded in Tissue-Tek (Fisher Scientific), fast frozen with dry ice, and stored at −80°C until processed. Spinal cord tissue blocks were cut in either the sagittal or transverse plane at 20 μm thickness, and brain tissue blocks were cut in either the coronal or sagittal plane at a thickness of 30–40 μm. Sections were collected on gelatin- and poly-L-lysine-coated glass slides and stored at −80°C until analyzed. Subsets of spinal cord and brain slices were collected in PBS for freefloating histochemistry.
AP histochemistry
Serial sections were analyzed by AP histochemistry to assess the presence, location, migration and morphology of graft-derived cells in the spinal cord, hippocampus and striatum. Sections were washed with PBS, heated at 60°C for 1 hour to inactivate endogenous enzyme activity, washed briefly in AP buffer (100 mM Tris, 100 mM NaCl, 50 mM MgCl2 at pH 9.5) and incubated at room temperature in the dark with AP-staining solution consisting of 1.0 mg ml−1 NBT (Sigma), 0.1 mg ml−1 BCIP (Sigma) and 5 mM levamisole (Sigma) in AP buffer for 1.5–2.0 hours. Slides were cover-slipped in hard-set Vectashield (Vector) and visualized with light microscopy.
Immunohistochemistry and phenotypic analysis
Tissue sections and cultured cells were washed in PBS, blocked in 10% goat serum (Invitrogen) for 1 hour at room temperature and incubated with primary antibody solution at 4°C overnight. Both monoclonal and polyclonal antibodies against human placental AP (1:200 dilution, Accurate) were used to identify graft-derived cells. Several primary antibodies were used to assess the phenotype of cells. Nestin (1:1000, monoclonal, Pharmingen) was used to identify undifferentiated neural precursor cells (NEP cells, NRPs and GRPs). NRPs were identified specifically using E-NCAM (1:200, Chemicon) and GRPs were identified using A2B5 (1:500, Chemicon). Neurons were identified using the antibodies Tuj1/BIII-tubulin (1:500, monoclonal, Babco); MAP2 (1:100, monoclonal, Chemicon); NeuN (1:100, monoclonal, Chemicon). Astrocytes were identified using GFAP (1:100, monoclonal, Chemicon). Oligodendrocyte precursors were identified using the antibodies directed against NG2 (1:250, polyclonal, Chemicon) and Sox-10 (1:200, polyclonal, Chemicon). Mature oligodendrocytes were identified with RIP (1:1000, monoclonal, Chemicon). Tissue was incubated for 2 hours at room temperature with goat anti-mouse and goat anti-rabbit secondary antibodies (1:200, Jackson) conjugated to either rhodamine or FITC. Tissue was counterstained with DAPI (1:1000, Sigma) to identify nuclei, and cover-slipped with anti-fade mounting media (Fluorosave, CN Biosciences). Slides were subsequently stored at −20°C. Images were acquired on either a Leica DMRBE fluorescence microscope (Leica Microsystems) using a Photometric Sensys KAF-1400 CCD camera (Roper Scientific) or a Leica TCS SP2 laser confocal microscope (Leica). Images were analyzed using either IP Lab (Scanalytics) or Leica confocal software version 2.0. Adobe Photoshop 7.0 was used to prepare figures.
RESULTS
In vitro characterization of cells
Cells isolated from AP rats were characterized in vitro before transplantation to determine the phenotypic composition of the grafted populations of cells. Before grafting, NEP cells were routinely cultured for 1–2 weeks following dissection of spinal cord from E10.5 rats. As reported previously (Kalyani et al., 1997), virtually all cells in the cultured NEP cell population displayed the characteristic flat neuroepithelial stem cell morphology (Fig. 1A). By contrast, NRPs and GRPs were cocultured for 1, 3 and 10 days following dissection of E13.5 spinal cord and showed the characteristic morphology of small, phase-bright, lineage-restricted precursors (Mayer-Proschel et al., 1997; Rao et al., 1998) throughout the mixed NRP/GRP cultures (Fig. 1G).
Fig. 1. In vitro characterization of grafted cells.

Before transplantation, NEP cells, differentiated NEP cells and mixed NRP/GRP cells were characterized to determine their morphological properties and antigenic profile. (A) NEP cells displayed a flat, neuroepithelial-like morphology and expressed the transgenic marker AP. (B) NEP cells also expressed nestin, the early neural marker. (C) When NEP cells were differentiated in culture before transplantation, the cells displayed the morphological characteristics of small, phase-bright, bipolar, lineage-restricted cells. (D) The majority of differentiated NEP-derived cells expressed nestin, and either of the cell surface ligands, E-NCAM (E) or A2B5 (F), which are markers of NRPs and GRPs, respectively. (G,H) The mixed NRP/GRP cultures consisted of only small, phase-bright, bipolar cells with all cells expressing nestin (H). (I,J) In addition, the mixed NRP/GRP cultures expressed either E-NCAM (I) or A2B5 (J). Table 1 shows the percentages of cells in the 3 types of culture that express nestin, E-NCAM and A2B5. Scale bars: 50 μm.
At the time of surgery, all cells in the NEP cell and mixed NRP/GRP cultures expressed the early neural marker, nestin (Fig. 1B,H). The NEP cell cultures were composed of a homogenous population of multipotent neural stem cells that expressed no markers of either mature cell types or lineage-restricted precursors (data not shown). Specifically, we did not detect expression of either E-NCAM or A2B5, which are markers of NRPs and GRPs, respectively. NEP cells also did not express markers of mature neurons (MAP2 and NeuN), astrocytes (GFAP) and oligodendrocytes (RIP, NG2 and Sox-10) (data not shown). Similarly, the mixed NRP/GRP cultures did not express markers of mature cell types (NeuN, GFAP, RIP, NG2 and Sox-10, data not shown). In contrast to NEP cells, nearly all cells in the mixed NRP/GRP cultures expressed either E-NCAM or A2B5 (Fig. 1I,J), confirming that they are composed exclusively of lineage-restricted precursors. Interestingly, the cell composition of the mixed NRP/GRP cultures changed during our culture process. At 24 hours post-dissection, the population consisted of a 1:1 ratio of NRPs:GRPs, and evolved into a ratio of ~1:5 following 1 week in culture (data not shown).
The phenotypic characterization agrees with previous reports of the morphological properties and antigen expression by NEP cells, NRPs and GRPs (Kalyani, et al., 1997; Mayer-Proschel et al., 1997; Rao et al., 1998). The results demonstrate that NEP cells used for grafting are a homogenous population of neuroepithelial stem cells that are uncontaminated by differentiated precursors and mature neural cell types. Mixed lineage-restricted cells used for grafting consisted of different ratios of NRPs and GRPs, depending on the time spent in culture, and were devoid of multipotent stem cells and mature cell types.
Grafting NEP cells
Adult brain
Homogenous populations of NEP cells (25 000 cells per transplant) were grafted into both the intact adult hippocampal dentate gyrus and striatum. Serial sections were assessed by AP histochemistry to detect the presence of transgenically-labeled cells. NEP cells were present in both the hippocampus (Fig. 2A) and striatum (Fig. 3A) at 3 days post-transplantation. At this time the transplants appeared morphologically immature (Figs 2A inset and 3B). However, surviving cells were not observed at 7 days (Figs 2B, 3C), and at 3 and 5 weeks after grafting (data not shown). It is important to note that the loss of the AP signal is an unequivocal indication of cell loss because previous work has demonstrated that grafted APpositive cells can be detected up to 6 weeks after transplantaion without down-regulation of the transgene (Han et al., 2004).
Fig. 2. Grafting NEP cells and mixed NRPs/GRPs into the adult hippocampus.
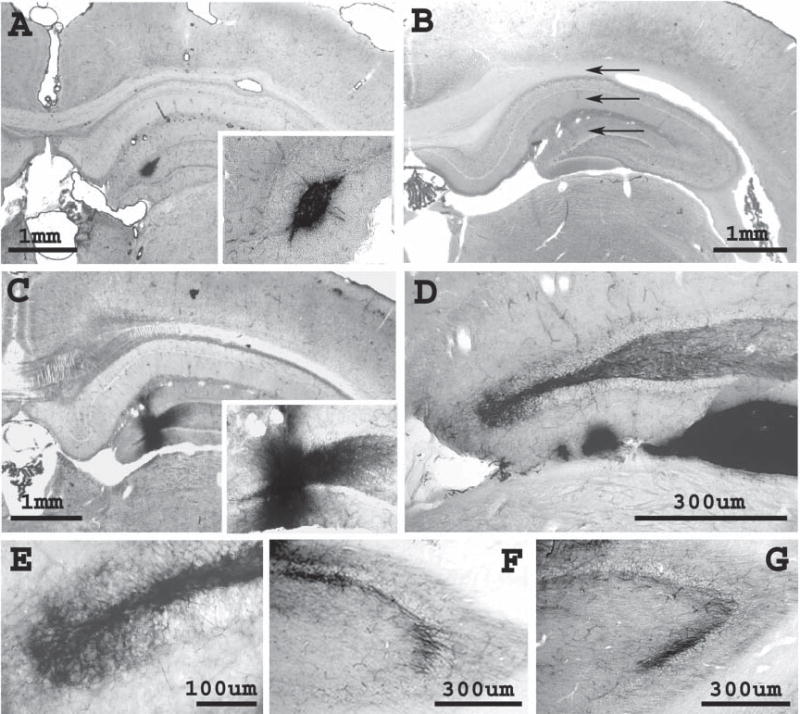
NEP cells and mixed populations of NRPs/GRPs were grafted into the intact hippocampus of adult rats under identical transplantation conditions, yielding distinct fates in vivo. AP histochemistry was used to detect the presence of transplanted cells. (A) Grafted NEP cells present in the dentate gyrus 3 days post-transplantation appeared morphologically immature (inset). (B) No surviving NEP cells were detected at 7 days (arrows denote remnants of injection tract). (C,D) In contrast to NEP cells, the survival of mixed NRP/GRP transplants could be detected 3 weeks (C) and 5 weeks (D) after engraftment. In addition, the NRP/GRP grafts appear morphologically mature (C, inset, D,E), and project fibers to surrounding hippocampal regions such as CA3 (F,G). Panels are coronal sections, with the dorsal aspect facing upwards.
Fig. 3. Grafting NEP cells and mixed NRPs/GRPs into the adult striatum.

NEP cells and mixed populations of NRPs/GRPs were grafted into the intact striatum of adult rats under identical transplantation conditions, yielding distinct fates in vivo. AP histochemistry was used to detect the presence of transplanted cells. Grafted NEP cells were present in the striatum at 3 days post-transplantation (A) and appeared morphologically immature (B). (C) However, no surviving NEP cells were detected at 7 days (arrows in denote remnants of injection tract). In contrast to NEP cells, survival of mixed NRP/GRP transplants could be detected 3 weeks and 5 weeks (D) after engraftment. NRP/GRP transplants appeared to integrate into the white-gray matter structure of the striatum (E), migrating long distances along white matter tracts, including the corpus callosum (F,G) and white matter bundles (H) within the striatum. (I,J) Grafted NRPs/GRPs showed mature morphologies with long process extension. Panels are coronal sections, with the dorsal aspect facing upwards, except (H), which is a sagittal section.
Adult spinal cord
Similar transplantation experiments showed that grafted NEP cells (25 000 cells per transplant) do not survive in the intact adult spinal cord. The cells were microinjected at the white matter–gray matter interface of the cervical spinal cord. Graft-derived cells were present 3 days after injection but appeared morphologically immature (Fig. 4A). No surviving cells were cell observed at 7 days (Fig. 4B), and at 3 and 5 weeks post-implantation (data not shown).
Fig. 4. Grafting NEP cells and mixed NRPs/GRPs into the adult spinal cord.
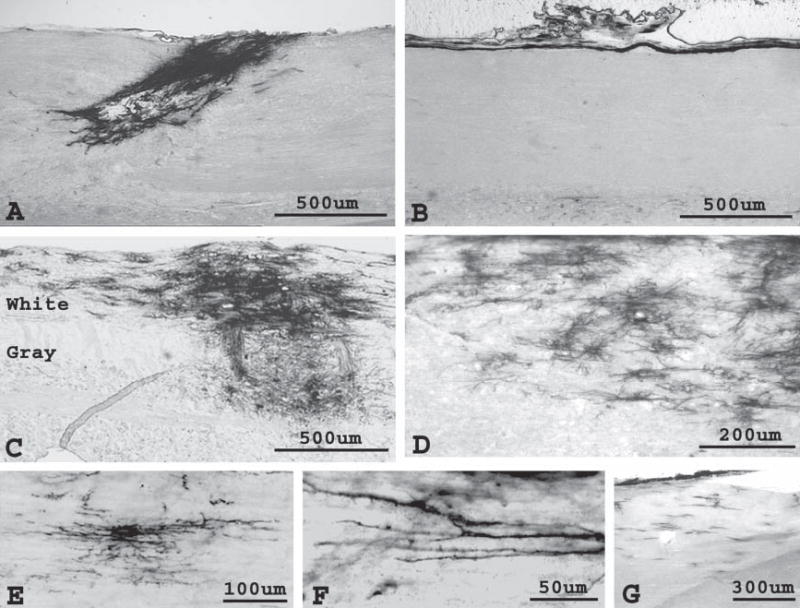
NEP cells and mixed populations of NRPs/GRPs were grafted into the intact spinal cord of adult rats under identical transplantation conditions, yielding distinct fates in vivo. AP histochemistry was used to detect transplanted cells. (A) Grafted NEP cells were present in the spinal cord 3 days post-transplantation and appeared morphologically immature. (B) Survival of NEP cells was not detected at 7 days. In contrast to NEP cells, survival of mixed NRP/GRP transplants could be detected in both the gray and white matter 3 weeks (C) and 5 weeks after engraftment. Migration occurs preferentially along white matter tracts (C), with cells migrating up to 15 mm (G) by 3 weeks post-transplantation. (D–F) Cells acquired several mature morphologies. Panels are sagittal sections, with the dorsal aspect facing upwards.
Grafting predifferentiated NEP cells
To further explore and validate the in vivo properties of NEP cells with respect to their poor survival we predifferentiated NEP cells in vitro from homogenous multipotent NSCs into more mature precursor cell phenotypes. The morphology of the NEP-derived cells mirrored that of the mixed NRP/GRP cultures. Cells were small, phase-bright and bipolar (Fig. 1C). The majority of NEP-derived cells still expressed nestin (Fig. 1D); however, they also expressed either of the lineage-restricted markers, E-NCAM (Fig. 1E) and A2B5 (Fig. 1F). In contrast to the original NEP cells from which they were derived, differentiated NEP cells (25 000 cells per transplant) survived transplantation into the intact adult hippocampus (Fig. 5A,B) and striatum (Fig. 5C,D), indicating that cells that have reached the lineage-restricted precursor stage survive in the adult CNS.
Fig. 5. Grafting pre-differentiated NEP cells into the adult hippocampus and striatum.
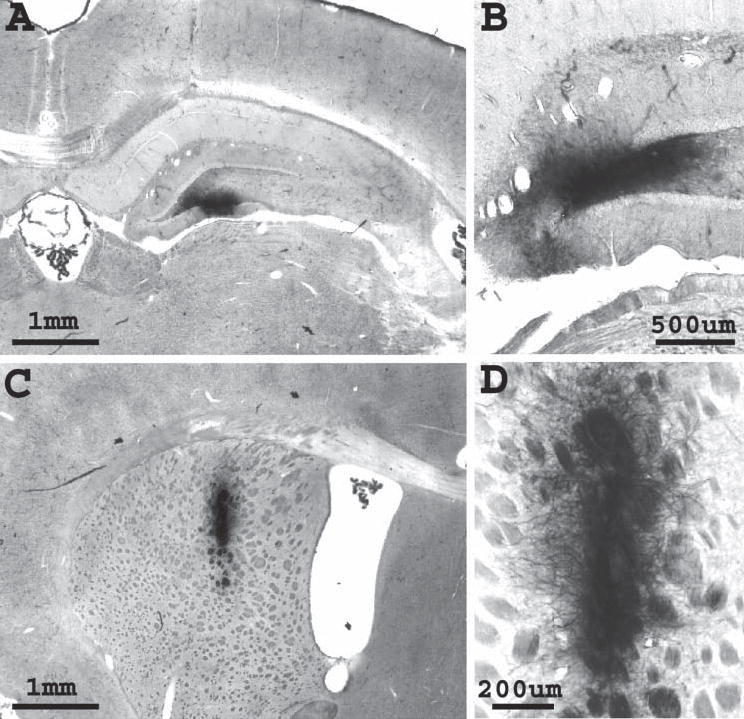
NEP cells were pre-differentiated in vitro before transplantation into the intact hippocampus and striatum of adult rats. AP histochemistry was used to detect graft-derived cells. Unlike undifferentiated NEP cells, differentiated NEP cells survived for 3 weeks (A,C) and 5 weeks following transplantation into the hippocampus (A) and striatum (C). (B,D) Grafts appeared morphologically mature. Panels are coronal sections, with the dorsal aspect facing upwards.
Morphological analysis of NRP/GRP grafts
Adult brain
Under identical transplantation conditions to NEP cell grafts, mixed NRP/GRP grafts (25 000 cells per transplant) survived robustly in the intact adult hippocampal dentate gyrus and striatum. Graft-derived cells were present at 3 and 5 weeks post-transplantation in both brain regions (Figs 2C–G and 3D–J). Cells appeared to integrate into the host white matter–gray matter architecture of the striatum (Fig. 3E). In addition, transplanted cells migrated selectively along white matter tracts of the brain, including the corpus callosum (Fig. 3F–G) and white matter bundles (Fig. 3H) in the striatum. By 5 weeks post-transplantation cells migrated up to 15 mm from the grafting site. Graft-derived cells appeared morphologically mature in both the hippocampus and striatum. In the hippocampus, transplanted cells extended processes to neighboring hippocampal regions such as CA3 (Fig. 2D,F,G). Within the striatal parenchyma, grafted cells extended neurites (Fig. 3I) and differentiated into cells with highly mature morphologies (Fig. 3J).
Adult spinal cord
Similar to the robust properties exhibited by mixed, lineage-restricted precursors in the adult brain, NRP/GRP grafts (25 000 cells per transplant) survived well in the intact adult spinal cord. Cells were transplanted at the interface between the dorsal column white matter and underlying gray matter under the same conditions as NEP cell grafts into the spinal cord. Cells survived in both white and gray matter, but demonstrated selective migration along white matter tracts, similar to results observed in the brain (Fig. 4C,G). Graft-derived cells appeared morphologically mature by 3 weeks post-implantation, displaying complex cellular morphologies including process extension (Fig. 4D–F). Graft-derived cells migrated up to 15 mm by 3 weeks post-transplantation, with cells at the greatest distances displaying the least mature morphologies (Fig. 4G).
Phenotypic analysis of NRP/GRP grafts
Neuronal cells
We assessed the phenotypic fate of mixed NRP/GRP grafts following transplantation into the adult CNS. By 3 weeks post-transplantation, large numbers of graft-derived cells differentiated into mature neurons in both the white and gray matter of the intact adult hippocampus, striatum, and spinal cord. Neuronal differentiation was assessed by colocalization of the AP transgene with either NeuN (Fig. 6A–E) or MAP2 (Fig. 6F–J) staining. Most graft-derived neurons were found close to the injection site, in accordance with previous work demonstrating the limited migratory capacity of NRPs compared to GRPs (Han et al., 2002; Han et al., 2004). In addition, graft-derived cells expressed the synaptic marker, synaptophysin (Fig. 6K–O). Grafts of mixed cells maintained in culture for 10 days contained fewer cells that differentiated into neurons following transplantation than cells cultured for either 1 or 3 days (data not shown), possibly because of the altered NRP:GRP ratio observed in culture.
Fig. 6. Mixed NRP/GRP grafts differentiate into neurons and associate with synapses in the adult CNS.

In the intact, adult CNS (hippocampus, striatum and spinal cord) mixed NRP/GRP grafts differentiated into mature neurons. Double immunofluorescence staining for alkaline phosphatase (A,F, green) and either NeuN (B, red) or MAP2 (G, red) shows neuronal differentiation of graft-derived cells in the spinal cord (C,H, overlay). In all three CNS regions, grafted NRPs/GRPs expressed synaptic markers. Double immunofluorescence staining for AP (K, green) and synaptophysin (L, red) indicates the possible association of grafted cells with synapse formation in the spinal cord (M, overlay). Confocal sections demonstrate the co-localization of AP with NeuN (D,E), MAP2 (I,J) and synaptophysin (N,O) following engraftment into the spinal cord. Arrows and arrowheads denote double-labeled cells. Nuclei were counterstained with DAPI (blue). Panels are sagittal sections. Scale bars: 100 μm.
Glial cells
Mixed NRP/GRP grafts differentiated into astrocytes and oligodendrocytes following grafting into the intact adult hippocampus, striatum and spinal cord. The astrocytic phenotype was assessed by colocalization of AP and GFAP staining (Fig. 7A–E) and graft-derived oligodendrocytes were identified by colocalization of staining for AP and RIP (mature oligodendrocytes, Fig. 7F–J), NG2 (oligodendrocyte precursors, Fig. 7K–L), and Sox-10 (oligodendrocyte precursors, Fig. 7M–O). Unlike graft-derived neurons, graft-derived glial cells were found at substantial distances from the injection site, in addition to near the site of engraftment. Cells that migrated the greatest distances from the transplant site along white matter tracts were often immature cell types that had not fully differentiated. These cells possessed more immature morphologies compared to cells closer to the injection site (Fig. 4G). In addition, graft-derived cells that had migrated further expressed markers such as NG2 (Fig. 7K–L) and Sox-10 (Fig. 7M–O) but not RIP, indicating that they are committed to an oligodendrocyte lineage but have not yet fully differentiated.
Fig. 7. Mixed NRP/GRP grafts differentiate into astrocytes and oligodendrocytes in the adult CNS.

In the intact, adult CNS (spinal cord, hippocampus and striatum), mixed NRP/GRP grafts differentiated into various glial cell types. Double immunofluorescence staining for AP (A, green) and GFAP (B, red) shows astroglial differentiation of graft-derived cells in the striatum (C, overlay). In these three CNS regions, grafted NRPs/GRPs also differentiated into oligodendrocytes. Double immunofluorescence staining for AP (F, green) and RIP (G, red) shows graft-derived oligodendrocytes in the striatum (H, overlay). Staining for AP (K,M, green) and either NG2 (L, red) or Sox10 (M, red) identifies graft-derived oligodendrocyte precursors that have migrated from the sites of transplantation in the spinal cord and corpus callosum, respectively. Confocal sections demonstrate co-localization of AP with GFAP (D,E), RIP (I,J) and Sox10 (N,O) following engraftment (arrows denote double-labeled cells). Nuclei were counterstained with DAPI (blue). Panels are coronal sections, except K and L, which are sagittal sections. Scale bars: 100 μm.
Grafting of NRPs/GRPs into the injured adult spinal cord
Morphological analysis
The promising properties exhibited by the NRP/GRP grafts in the intact adult CNS indicate the potential usefulness of these cells for transplantation into the injured CNS. We therefore examined the survival and migration of NRPs/GRPs following transplantation into a hemisection model of spinal cord injury; a lateral funiculotomy lesion at the C4 level. The mixed, lineage-restricted precursors, suspended in Vitrogen, were grafted into the lesion cavity at the time of injury (500 000 cells per transplant). The grafts survived well within the injury cavity for up to 5 weeks post-engraftment (Fig. 8A), filling the entire cavity. We predict that survival will continue for longer periods of time. In addition, robust, long-distance migration of up to 20 mm was observed (Fig. 8A). Graft-derived cells in the injury site and the host spinal cord adopted mature morphologies (Fig. 8A inset).
Fig. 8. Mixed NRP/GRP grafts survive, migrate, differentiate into neurons and associate with synapses in the injured spinal cord.

Mixed NRPs/GRPs were transplanted into the injured cervical spinal cord acutely following lateral funiculotomy injury. AP histochemistry was used to detect the presence of graft-derived cells. Grafted cells survived in the injury cavity for 3 weeks (A) and 5 weeks following transplantation, filling the entire injury cavity. In addition, grafted cells migrated out of the injury site into the host CNS for up to 20 mm by 3 weeks post-engraftment. Both migrating cells (A, inset) and those in the injury site developed mature morphologies. In the injured spinal cord mixed NRP/GRP grafts differentiated into neurons. Double immunofluorescence staining for AP (B,G, green) and either NeuN (C, red) or MAP2 (H, red) shows neuronal differentiation of graft-derived cells (D,I, overlays). NRP/GRP grafts expressed synaptic markers. Double immunofluorescence staining for AP (L, green) and synaptophysin (M, red) indicates the possible association of grafted cells with synapse formation in the injured spinal cord (N, overlay). Confocal sections demonstrate co-localization of NeuN (E,F), MAP2 (J,K) and synaptophysin (O,P) with AP (arrows denote double-labeled cells). Nuclei were counterstained with DAPI (blue). Panels are transverse sections, except A, which are sagittal sections. Scale bars: 50 μm except A.
Phenotypic analysis
Similar to transplantation in the intact CNS, the NRP/GRP grafts differentiated into neurons, astrocytes and oligodendrocytes in the injury site itself. Furthermore, a substantial proportion of graft-derived cells differentiated down these three lineages by 3 weeks post-transplantation. Neuronal differentiation was assessed by co-localization of the AP transgene with either NeuN (Fig. 8B–F) or MAP2 (Fig. 8G–K) staining. Identification of astrocytic differentiation employed overlap of AP and GFAP staining (Fig. 9A–E), whereas oligodendrocytic phenotypes were identified by co-localization of AP with RIP (Fig. 9F–J), Sox-10 (Fig. 9K–L) and NG2 (Fig. 9M–N). In addition to neuronal differentiation, graft-derived cells expressed the synaptic marker, synaptophysin (Fig. 8L–P).
Fig. 9. Mixed NRP/GRP grafts differentiate into astrocytes and oligodendrocytes in injured spinal cord.

Double immunofluorescence staining for AP (A, green) and GFAP (B, red) demonstrates astroglial differentiation of graft-derived cells (C, overlay). NRP/GRPs grafts also differentiate into oligodendrocytes in the injured spinal cord. Double immunofluorescence staining for AP (F, green) and RIP (G, red) shows graft-derived oligodendrocytes in the injury site (H, overlay). Staining for AP (K,M, green) and either Sox-10 (L, red) or NG2 (N, red) identifies graft-derived oligodendrocyte precursors that have migrated out of the injury site into the host spinal cord. Confocal sections demonstrate co-localization of AP with GFAP (D,E), RIP (I,J), Sox10 (K,L) and NG2 (M,N) following engraftment (arrows denote double-labeled cells). Nuclei were counterstained with DAPI (blue). Panels are transverse sections, except K–N, which are sagittal sections. Scale bars: 50 μm.
CONCLUSIONS
Fetal multipotent neural stem cells (NEP cells) do not survive in the adult hippocampus, striatum and spinal cord.
By contrast, lineage-restricted neural precursors (NRPs and GRPs) survive well in both the intact and injured CNS.
Lineage-restricted neural precursors (NRPs and GRPs) migrate selectively along white-matter tracts, integrate and differentiate into all mature CNS cell types in both the intact and injured CNS.
Multipotent neural stem cells (NEP cells) that are pre-differentiated towards the lineage-restricted-precursor stage prior to transplantation also survive and integrate in the adult CNS.
DISCUSSION
In this study we analyzed the behavior of defined populations of fetal stem cells and lineage-restricted cells. Because these cells were isolated from AP transgenic rats, we could identify them unambiguously and follow their behavior following transplantation. We demonstrate that cells transplanted in identical paradigms behave differently. Our results indicate that most CNS regions do not support the survival of multipotent NSCs derived from the fetal CNS but can direct the survival, integration and differentiation of more mature lineage-restricted precursors.
We confirmed that failure of the NSCs to survive was not due to a problem with the process of implantation because we showed deposition and survival of cells for at least 3 days in animals sacrificed shortly after transplant, and demonstrated that cells left over from implantation survive and proliferate in culture. Our results are supported by multiple grafting paradigms in several brain and spinal cord regions, and by using a reliable transgenic AP reporter under the control of the ubiquitous Rosa 26 promoter to track the cells (Kisseberth et al., 1999; Mujtaba et al., 2002). The validity of our results is also supported by showing that when multipotent NSCs were predifferentiated into lineage-restricted precursors before grafting, their fate changed dramatically and the cells survived for long periods of time. Furthermore, when NRP and GRP cells were grafted and analyzed under the same conditions as NSCs, they showed robust survival and differentiation, indicating that it is unlikely that positional cues are sufficiently different to prevent either integration or survival of cells derived from the caudal neural tube.
Our previous studies (Han et al., 2002; Han et al., 2004) and present results are consistent with our hypothesis that the inability of NEP cells to survive and integrate following transplantation is likely to be caused by a failure of the host to provide an appropriate environment for NSCs rather than a failure to support the survival of either lineage-restricted cells or neurons and glia (Cai and Rao, 2002; Ginis and Rao, 2003). Based on these results, we reason that the appropriate niche for grafted fetal NSCs only occurs in a few restricted regions of the adult CNS where there is neurogenesis from stem cells and the environment is similar to that of the developing CNS. Therefore, our results could be extrapolated to other regions of the CNS where there is either limited or no evidence for large populations of stem cells. Indeed, even when NEP cells were transplanted into the hippocampus, they did not survive and integrate into the host environment, consistent with previous observations that indicate that the cells in the hippocampus are neuronal progenitors rather than NSCs (Seaberg and van der Kooy, 2002).
These results also shed light on the interpretation of the literature dealing with fetal spinal cord tissue transplants. Fetal tissue, prepared from E11–14 spinal cord, were among the first types of transplants grafted into the injured spinal cord. They showed consistent, robust survival and differentiation into neurons and glia. Some studies reported that transplants undergo attrition during the first week following engraftment into the injured spinal cord, followed by a subsequent dramatic expansion which might originate from a small number of surviving cells (Theele et al., 1996). Consistent with our studies it is likely that the NRPs and GRPs present in these grafts survived and differentiated, but the NSCs died within a few days.
Our results are in apparent contrast to numerous reports of robust survival and integration after neurosphere transplantation (Cao et al., 2002). Neurospheres do not contain a pure population of stem cells; therefore, it is plausible that the cells that survive after transplantion do not originate from the NSCs, rather they are from the more differentiated cell types. Previously published data showing relatively rapid differentiation in the graft core and an absence of large amounts of cell death support this conclusion. It is also possible that the in vivo fate of NEP cells grown as adherent monolayers differs from the fate of multipotent cells present in neurospheres. The presence of more mature cell types in the neurosphere-generated grafts may protect stem cells in the adult CNS by creating a supportive microenvironment that allows for NSC survival. Although we believe that our results are clear and important in re-evaluating other studies, it is possible that some multipotent NSCs can survive in the adult CNS, particularly in combination with strategies such as predifferentiation and the inclusion of appropriate survival factors. To our knowledge, no experiments that examine integration and long-term survival with a pure population of NSCs have been performed. We should note that mice in which green fluorescent protein (GFP) expression is under the control of a Sox-2 promoter have recently been generated (Hayashi et al., 2002; Graham et al., 2003; D’Amour and Gage, 2003; Pevny and Rao, 2003), thereby allowing the harvest of a relatively pure population of NSCs for transplantation.
Our results do not directly address the issue of whether adult NSCs fare better than fetal NSCs when transplanted into the adult CNS. Multiple populations of adult stem or stem-like cells have been described, including astrocytic, radial glial, ependymal and SVZ-derived stem cell populations (Goldman, 2003; Johansson, 2003). Our results and those of others show that the properties of adult NSCs are different from those of fetal NSCs, particularly in their growth factor requirements (Kalyani and Rao, 1998). Therefore, it is possible that adult stem cells may fare better than fetal-derived populations, but this remains to be tested. Again, the ability to harvest stem cells from Sox-2–GFP transgenic mice makes this hypothesis testable.
The robust survival of mixed NRP/GRP grafts is not surprising because these individual cell types have been grafted successfully into the adult CNS (Herrera et al., 2001; Cao et al., 2002b; Han et al., 2002; Han et al., 2004). As expected, mixed precursor grafts demonstrate the combined effect of individual transplantation of NRPs and GRPs. Transplants generated neurons, astrocytes and oligodendrocytes in each of the three regions of engraftment. These properties are important for treating CNS injury because a successful repair strategy is likely to supply and replace either some or all three CNS cell types. The mixed precursor transplantation protocol is also attractive with respect to effective therapeutic mechanisms that can utilize the differential migration of GRPs relative to NRPs. Thus, NRPs, which mainly remain at the grafting site, have the potential to form relays for reconstructing circuits and creating a permissive environment at the injury site. Migrating GRPs can guide regenerating fibers beyond the site of injury and participate in the remyelination process. Indeed, our experiments show that the mixed precursor grafts generated neurons with apparent synaptic connections in the injury site. By contrast, previous studies that graft only NRPs showed limited survival and a lack of neuronal differentiation in the spinal cord injury site (Cao et al., 2002b). Therefore, we reason that the combined presence of both NRPs and GRPs might allow the survival and neuronal differentiation of NRPs by providing glial support and establishing a permissive environment during the acute stages of the injury. However, it is important to note that differences in the types of injury and times of engraftment are also likely to affect the fate of the grafted precursors.
In practical terms, under the right culture conditions, mixed NRP/GRP grafts can generate different ratios of precursor cells and obviate the need for sorting specific populations of cells. NRPs and GRPs can be individually isolated from mixed precursor populations using fluorescence-activated cell sorting or immunopanning based on the differential expression of the cell-surface ligands, E-NCAM and A2B5, respectively, but this process often results in low cell yields (Mayer-Proschel et al., 1997; Rao and Mayer-Proschel, 1997). Our studies also underscore the dynamic composition of the mixed NRP/GRP cultures, which depends on conditions that are preferential to a particular population of cells by either selective survival or proliferation. The initial composition of mixed NRP/GRP cultures is ~1:1 NRPs:GRPs 24 hours following dissociation and plating. After 10 days in culture, the population shifts to ~1:5 NRPs:GRPs. This in vitro pattern of increasing GRPs and decreasing NRPs mirrors the pattern of spinal cord development observed in vivo (Rao, 1999). Intriguingly, these proportions manifest themselves in the patterns of graft-derived differentiated cell types seen following transplantation, with the numbers of neurons generated in vivo declining with older cultures. Understanding the ratio of precursor types in the population might be useful therapeutically. Depending on the type of injury and the relative need for replacement of several mature cell types, appropriate mixes of lineage-restricted precursors can be utilized for transplantation.
In summary, by grafting well-defined populations of multipotent NEP cells and lineage-restricted precursors derived from AP transgenic rats into the adult CNS we directly and unambiguously compared the fate of various classes of neural precursors with respect to survival, migration, phenotypic changes and integration. Our results indicate that, despite their multipotent properties, NSCs derived from the fetal CNS might not be an optimal cell source for grafting into the CNS. By contrast, transplanting mixed populations of lineage-restricted precursors is a promising therapeutic strategy for CNS injury that should achieve excellent survival, useful migration patterns and multiple therapeutic approaches. Although much remains to be understood about how cells interact with the environment, our results show that our current ability to identify, isolate, culture and expand the cell types present in the embryonic spinal cord allows us to design optimal grafting protocols, carefully analyze their fate and, possibly, improve efficacy with respect to recovery. In this defined model system, in which the optimal cell sources and the desired functional properties for transplantation and repair of the injured CNS can be tested, the optimum ratios of NRPs and GRPs, as well as alternate delivery methods, can be assessed.
Acknowledgments
We would like to acknowledge all members of our laboratories for stimulating discussions, particularly Dr Barry Himes and Theresa Connors for their technical expertise. A.C.L. thanks Dr Birgit Neuhuber for discussion and constructive criticism, the Neuroscience graduate program for its support and John, Paul, George and Richard for their incalculable assistance. J.C. was supported by an IRTA fellowship from the NIA. M.S.R. was supported by the NIH, NIA, CNS Foundation and Packard Center. M.S.R. acknowledges the contributions of Dr. S. Rao that made this project possible. I.F. was supported by NIH grants NS24707 and NS 37515.
References
- Cai J, Rao MS. Stem cell and precursor cell therapy. Neuromolecular Medicine. 2002;2:233–249. doi: 10.1385/NMM:2:3:233. [DOI] [PubMed] [Google Scholar]
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. Journal of Neuroscience Research. 2002a;68:501–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Experimental Neurology. 2002b;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Experimental Neurology. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Gage FH. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America 100 Suppl. 2003;1:11866–11872. doi: 10.1073/pnas.1834200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I. Candidate cells for transplantation into the injured CNS. Progress in Brain Research. 2000;128:253–257. doi: 10.1016/S0079-6123(00)28022-9. [DOI] [PubMed] [Google Scholar]
- Ginis I, Rao MS. Toward cell replacement therapy: promises and caveats. Experimental Neurology. 2003;184:61–77. doi: 10.1016/s0014-4886(03)00256-5. [DOI] [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends in Neurosciences. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Experimental Neurology. 2002;177:360–375. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mechanisms of Development. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Herrera J, Yang H, Zhang SC, Proschel C, Tresco P, Duncan ID, et al. Embryonic-derived glial-restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Experimental Neurology. 2001;171:11–21. doi: 10.1006/exnr.2001.7729. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. The Journal of Neuroscience. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB. Mechanism of stem cells in the central nervous system. Journal of Cellular Physiology. 2003;196:409–418. doi: 10.1002/jcp.10293. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kalyani A, Hobson K, Rao MS. Neuroepithelial stem cells from the embryonic spinal cord: isolation, characterization, and clonal analysis. Developmental Biology. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- Kalyani AJ, Rao MS. Cell lineage in the developing neural tube. Biochemistry and Cell Biology. 1998;76:1051–1068. [PubMed] [Google Scholar]
- Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. The Journal of Neuroscience. 2003;23:10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Developmental Biology. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Han SS, Fischer I, Sandgren EP, Rao MS. Stable expression of the alkaline phosphatase marker gene by neural cells in culture and after transplantation into the CNS using cells derived from a transgenic rat. Experimental Neurology. 2002;174:48–57. doi: 10.1006/exnr.2001.7847. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. Journal of Neuroscience Research. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- Pevny L, Rao MS. The stem-cell menagerie. Trends in Neurosciences. 2003;26:351–359. doi: 10.1016/S0166-2236(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Rao MS. Multipotent and restricted precursors in the central nervous system. The Anatomical Record. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Developmental Biology. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Precursor cells for transplantation. Progress in Brain Research. 2000;128:273–292. doi: 10.1016/S0079-6123(00)28025-4. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Developmental Biology. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. The Journal of Neuroscience. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. The Journal of Neuroscience. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends in Neurosciences. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. The Journal of Neuroscience. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Experimental Neurology. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- Theele DP, Schrimsher GW, Reier PJ. Comparison of the growth and fate of fetal spinal iso- and allografts in the adult rat injured spinal cord. Experimental Neurology. 1996;142:128–143. doi: 10.1006/exnr.1996.0184. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Developmental Biology. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Snyder EY. Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo. Brain Pathology. 1999;9:569–598. doi: 10.1111/j.1750-3639.1999.tb00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. The Journal of Neuroscience. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore SR, Onifer SM. Immortalized neural cell lines for CNS transplantation. Progress in Brain Research. 2000;127:49–65. doi: 10.1016/s0079-6123(00)27005-2. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, 2nd, et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature Medicine. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Experimental Neurology. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- Yang H, Mujtaba T, Venkatraman G, Wu YY, Rao MS, Luskin MB. Region-specific differentiation of neural tube-derived neuronal restricted progenitor cells after heterotopic transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13366–13371. doi: 10.1073/pnas.97.24.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, Ge B, Duncan ID. Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4089–4094. doi: 10.1073/pnas.96.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]


