High-performance liquid chromatography has become the dominant method for the analytical and preparative separation of chiral pharmaceuticals.1-5 However, no current chiral stationary phase (CSP) uses zirconia or inorganic oxides other than silica as a substrate. Zirconia has many attractive properties for HPLC, including spherical particle shape and narrow size distribution. Additionally, it exhibits unsurpassed chemical and mechanical stability. Its surface chemistry is very different from silica gel due to the presence of a high population of strong Lewis acid (Zr+4) sites. If the presence of strong Lewis acid sites on the surface can be exploited, zirconia can provide a more robust and flexible platform for CSP design than silica gel.6-9 This is the core concept behind two Small Business Innovation Research (SBIR) grants awarded by NIH (Bethesda, MD) to fund research at ZirChrom Separations, Inc. (Anoka, MN), under the direction of Dr. Clayton V. McNeff.10,11 The synthetic chemistry aspect of the research is being carried out at the University of Minnesota (Minneapolis, MN) under the direction of Prof. Thomas R. Hoye. The use of zirconia as a stationary phase for HPLC was developed at the University of Minnesota by Prof. Peter W. Carr and is now exclusively licensed to ZirChrom Separations.
A novel approach to the production of chiral stationary phases on bare zirconia has been developed that may offer significant advantages over other currently used platforms. Zirconium atoms on the surface of zirconia (zirconium dioxide) particles act as strong Lewis acid sites that allow easy attachment of chiral stationary phases by a tethering group having strong electron donor chelating properties. Chiral stationary phases produced in this manner exhibit high stability under normal-phase HPLC operating conditions, but can be completely removed by washing under aqueous, high-pH conditions. Ultimately, chiral chelating reagents should become available to carry out reactions conveniently at or near room temperature and allow the chromatographer to use a single zirconia column to rapidly screen different chiral selector groups for enantiomer selectivity.
Experimental
A zirconia support particle, plus a family of 12 modifications for HPLC use, including polymer and carbon-coated reversed phases and several ion exchange phases, are manufactured by ZirChrom Separations. The base zirconia particles are made by aggregating 100-nm zirconia colloids into spherical particles having 250-Å pores and diameters from 3 to 25 μm. The 3-μm porous zirconia particles were used throughout this study.
Phase I feasibility studies for preparing zirconia CSPs initially involved a two-step approach: 1) attach an appropriate tethering group such as 3-aminopropylphosphonic acid to the zirconia surface through a Lewis acid-base reaction, and 2) covalently attach the desired CSP to the tethering group using amide bond formation chemistry.12 Pirkle-type CSPs were selected for initial experiments due to their ease of synthesis, wide scope of applicability, and large body of available silica-based separations data for comparison. An illustration of the two-step reaction scheme is shown in Figure 1, and a typical chemical reaction is shown in Figure 2 using pamadronic acid as a very strong tethering agent.
Figure 1.

Phase I reaction scheme. A reactive tethering group is attached first, and a chiral selector molecule is attached to the tethering group by an amide bond formation reaction.12
Figure 2.

Typical two-step chemical reaction involving the addition of a reactive chelator (pamidronic acid) followed by EEDQ amide bond formation with a chiral carboxylic acid reagent.12
Results and discussion
Durable, efficient CSP columns were successfully prepared in Phase I research by the two-step reaction scheme using the chiral selectors shown in Figure 3. The tethering group was allowed to react with the bare zirconia particles, and the chiral selector was covalently attached to the amino-modified zirconia using the N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) coupling reaction that is commonly employed for peptide synthesis.12 Columns produced in this manner were compared to silica columns having analogous chiral selectors and found to have similar separation characteristics for the targeted enantiomers. Most importantly, the chemisorbed chiral selectors on zirconia were found to be stable enough for extended routine use; however, they were completely removed by washing with a high pH (>pH 12) aqueous solution and could be easily regenerated. Results shown in Figures 4-6 for the zirconia-based CSP columns are comparable to the performance of silica-based columns having a similar chiral selector.
Figure 3.

Chiral selector molecules that were evaluated during Phase I research.
Figure 4.

Zirconia (R)-N-[1-(1-naphthyl)ethyl]succinamic acid CSP column [Zr-(R)-NESA] selectivity examples. Conditions: a) and b) 88.9/11/0.1 hexane/isopropanol/TFA (trifluoracetic acid), c) 75/15/9/1 hexane/methanol/isopropanol/TFA; 100 × 4.6 mm column; flow = 1.0 mL/min; 30°C.
Figure 6.

Zirconia (S)-DNB-L-leucine CSP column [Zr-(S)-Leu] selectivity examples. Conditions: 99/1 hexane/isopropanol (all runs); other conditions as in Figure 4.
Selectivity is compared in Figure 7 for 12 probe solute enantiomers on zirconia (S)-dinitrobenzoyl-L-leucine CSP and zirconia (S)-dinitrobenzoyl-L-phenylglycine CSP. As expected, changing the chiral selector had a significant effect on the resolution of enantiomers. This ability to change chiral selectors on the same column can reduce the influence of other column factors and allow the focus to be placed on choosing the best chiral selector during method development.
Figure 7.

Selectivity comparison between zirconia (S)-DNB-L-leucine CSP and zirconia (S)-DNB-L-phenylglycine CSP columns [Zr-(S)-Leu and Zr-(S)-PG]; conditions as in Figure 6.
Data on the stability and reproducibility for the zirconia (S)-DNB-L-leucine CSP column used in Figure 7 are shown in Figures 8 and 9, respectively. A two-step reaction scheme was employed using three different types of tethering agents. Figure 8 confirms that the choice of tethering groups has a great effect on zirconia CSP column stability. Aminopropylphosphonic acid (APPA) was the most stable among these three types with wash solvents that would be used in analytical mobile phases. Lot reproducibility studies shown in Figure 9 confirmed that a chiral selector can be attached reproducibly to the zirconia surface using the APPA tethering group in a two-step reaction scheme. Data that are not shown indicate that the choice of tethering group can also have a minor effect on chiral selectivity.
Figure 8.

Stability comparison of zirconia (S)-DNB-L-leucine CSP column [Zr-(S)-Leu] prepared with different Lewis base tethering groups in a two-step process. APPA= aminopropylphosphonic acid; DHNP= dihydroxynorephedrine; ASPA = aspartic acid.
Figure 9.

QC tests for two different batches of zirconia with APPA-tethered (S)-DNB-L-leucine and nine columns packed with these two batches; probe solutes were (R/S)-naphthyll-eucine ethyl ester; retention factor is shown for R-naphthyll-eucine ethyl ester.
Figure 10 illustrates the single-step reaction scheme that is targeted for commercial applications. A separation on a CSP column prepared with N-(4-nitrobenzoyl)-L-glutamic acid using a single-step reaction process is shown in Figure 11; the column was stripped and regenerated to demonstrate reproducibility of the simple, one-step loading process. After stripping, the bare zirconia column did not exhibit any retention or selectivity for the chiral analytes. Work is continuing as part of the NIH Phase II SBIR Grant to develop a wider range of chiral selector reagents with integral tethering groups that permit single-step loading under mild conditions. Phase II research has also concentrated on phosphonic acid and certain di-phosphonic acids (see pamidronic acid in Figure 2) as ideal tethering groups for preparing very robust commercial products that are easy to coat and stable under a variety of useful mobile phase conditions.
Figure 10.

Phase II reaction scheme. A chiral reagent containing an integral tethering group is attached to bare zirconia in a simple, one-step process.
Figure 11.
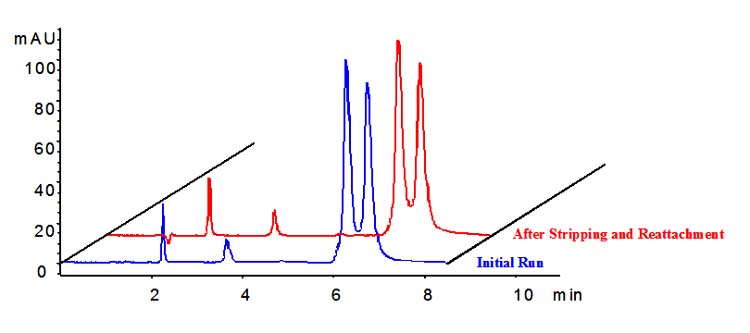
Comparison between the separation of (R/S)-2,2,2-trifluoro-1-(9-anthryl)ethanol before and after stripping and reattaching an N-(4-nitrobenzoyl)-L-glutamic acid (CSP) reagent according to the Phase II scheme shown in Figure 10. Conditions: 99/1 hexane/isopropanol; other conditions as in Figure 4.
Conclusion
A promising new route to preparing chiral stationary phases using a zirconia substrate has been developed. Chiral selectors that can be easily removed and replaced by simple, low-temperature reactions are targeted for commercialization. They will allow users to rapidly evaluate the suitability of different chiral selectors for resolving enantiomers. A single, highly stable zirconia column plus a kit of pure CSP coating reagents can eliminate the need to store and maintain many expensive CSP columns, and can also ensure that fresh columns with maximum selectivity are always available for method development. Five products with different chiral selectors include: (S)-DNB-L-leucine (π electron acceptor), (S)-DNB-L-phenylglycine (π electron acceptor), (R)-DNB-L-phenylglycine (π electron acceptor), (R)-N-[1-(1-naphthyl)ethyl]succinamic acid (π electron donor), and (S)-N-[1-(1-naphthyl)ethyl]succinamic acid (π electron donor). Chiral selectors with multiple chiral centers, featuring both π donor and acceptor groups, are also targeted for development, as are chiral selectors based on polysaccharides.
Figure 5.

Effect of mobile phase changes on zirconia (R)-N-[1-(1-naphthyl)ethyl]succinamic acid CSP column [Zr-(R)-NESA]. Conditions: a) 89/11 hexane/isopropanol, b) 95/2/3 hexane/methanol/isopropanol, c) 87/10/3 hexane/methanol/isopropanol; other conditions as in Figure 4.
Footnotes
This work was supported by Phase I and Phase II Small Business Innovation Research (SBIR) grants from NIH (Bethesda, MD). Patents are pending.
Contributor Information
Richard A. Henry, Consultant, ZirChrom Separations, Inc., 617 Pierce St., Anoka, MN 55303, U.S.A.; tel.: 886-782-2531; fax: 763-421-2319; e-mail: rhenry@zirchrom.com.
Clayton V. McNeff, CEO, ZirChrom Separations, Inc., 617 Pierce St., Anoka, MN 55303, U.S.A.; tel.: 886-782-2531; fax: 763-421-2319
Bingwen Yan, Production Manager, ZirChrom Separations, Inc., 617 Pierce St., Anoka, MN 55303, U.S.A.; tel.: 886-782-2531; fax: 763-421-2319.
Thomas R. Hoye, Professor, University of Minnesota, Department of Chemistry, Minneapolis, MN.
References
- Beesley TE, Scott RPW. Chiral chromatography. John Wiley & Sons; New York: 1998. [Google Scholar]
- Boehm RE, Martire DE, Armstrong DW. Theoretical considerations concerning the separation of enantiomeric solutes by liquid chromatography. Anal Chem. 1988;60:522–8. doi: 10.1021/ac00157a006. [DOI] [PubMed] [Google Scholar]
- Subramanian PW, Carr CV, McNeff CV. Use of spray-dried zirconia microspheres in the separation of immunoglobulins from cell culture supernatant. J Chromatogr. 2000;890(1):15–23. doi: 10.1016/s0021-9673(00)00289-2. [DOI] [PubMed] [Google Scholar]
- Schleimer M, Pirkle WH, Schurig V. Enantiomer separation by high performance liquid chromatography on polysiloxane-based chiral stationary phases. J Chromatogr A. 1994;679:23–34. [Google Scholar]
- Pirkle WH, Readnour RS. The influence of end-capping on the enantioselectivity of a chiral phase. Chromatographia. 1991;31:129–32. [Google Scholar]
- Cecelia B, Castells CB, Carr PW. Cellulose tris(3,5-dimethylphenylcarbamate) coated zirconia as a chiral stationary phase for HPLC. Anal Chem. 1999;71:3013–21. doi: 10.1021/ac990021f. [DOI] [PubMed] [Google Scholar]
- Castells CB, Carr PW. A study of thermodynamics and influence of temperature of chiral high-performance liquid chromatographic separations using cellulose tris(3,5-dimethylphenylcarbamate) coated zirconia stationary phases. Chromatographia. 2000;52(910):535–42. [Google Scholar]
- Park JH, Ryu JK, Park JK, McNeff CV, Carr PW. Separation of enantiomers on bovine serum albumin coated zirconia in reversed-phase liquid chromatography. Chromatographia. 2001;53(78):405–8. [Google Scholar]
- Park SY, Park JK, Park JH, McNeff CV, Carr PW. Separation of racemic 2,4-dinitrophenyl amino acids on carboxymethyl-β-cyclodextrin coated zirconia in RPLC. Microcol J. 2001;70:179–85. [Google Scholar]
- Phase I SBIR Grant (NIH R43 HL070334-01)
- Phase II SBIR Grant (NIH R44 HL070334-02A2).
- Yang A, Gehring A, Li T. J Chromatogr A. 2000;878:165–70. doi: 10.1016/s0021-9673(00)00332-0. [DOI] [PubMed] [Google Scholar]


