Abstract
Optically active form of α-cyano-3-phenoxybenzyl (CPB) alcohol, building block of pyrethroid insecticides, was synthesized as its acetate by the combination of anion-exchange resin (D301)-catalyzed transcyanation between m-phenoxybenzaldehyde (m-PBA) and acetone cyanohydrin (AC), and lipase (from Alcaligenes sp.)-catalyzed enantioselective transesterification of the resulting cyanohydrin with vinyl acetate. Through optimizing technological conditions, the catalyzing efficiency was improved considerably compared to methods previously reported. Concentrations of CPB acetate were determined by gas chromatograph. The enantio excess (e.e.) values of CPB acetate were measured by NMR (nuclear magnetic resonance) method. Effects of solvents and temperatures on this reaction were studied. Cyclohexane was shown to be the best solvent among the three tested solvents. 55 °C was the optimal temperature for higher degree of conversion. External diffusion limitation was excluded by raising the rotational speed to 220 r/min. However, internal diffusion could not be ignored, since the catalyst (lipase) was an immobilized enzyme and its particle dimension was not made small enough. The reaction rate was substantially accelerated when the reactant (m-PBA) concentration was as high as 249 mmol/L, but decreased when the initial concentration of m-PBA reached to 277 mmol/L. It was also found that the catalyzing capability of recovered lipase was high enough to use several batches. Study of the mole ratio of AC to m-PBA showed that 2:1 was the best choice. The strategy of adding base catalyst D301 was found to be an important factor in improving the degree of conversion of the reaction from 20% to 80%. The highest degree of conversion of the reaction has reached up to 80%.
Keywords: Biosynthesis, α-cyano-3-phenoxybenzyl (CPB) acetate, Lipase, Optimization, Organic media
INTRODUCTION
Cyanohydrin compounds are useful synthetic intermediates in organic synthesis and are important starting materials for the synthesis of pharmaceuticals (Klibanov, 1990). Optically active cyanohydrin esters can be obtained by enzymatic transesterification of racemic cyanohydrins in organic solvent (Wang et al., 1989). (S)-α-cyano-3-phenoxybenzyl (CPB) alcohol is an important building block of pyrethroid insecticides and has received increasing interest for use in biological agrochemicals. So, results of study on the technological conditions for the synthesis of (S)-CPB will have practically significance.
Inagaki et al.(1991) first proposed a one-pot method to synthesize CPB acetate in an approach to the synthesis of (S)-CPB acetate through in situ racemization of corresponding cyanohydrin formed from m-phenoxybenzaldehyde (m-PBA) and acetone cyanohydrin (AC) by the combination of chemical and biochemical catalysts of anion-exchange resin and lipase. The whole reaction is depicted as Fig.1, in which R is m-phenoxybenzyl-.
Fig. 1.
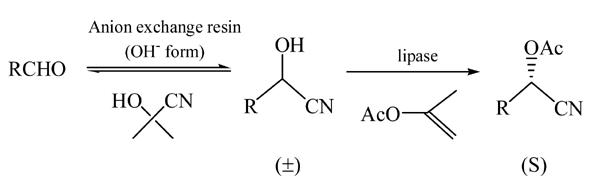
One-pot synthesis of CPB acetate
By using enol esters such as vinyl acetate, the reaction becomes irreversible with the yield of the resolution tending to reach 100%. Furthermore, the racemization of the product is greatly reduced when the hydroxyl group of cyanohydrin is protected by acylation. With quinidine used as a base catalyst to catalyze the reaction between m-PBA and AC, the yield of (S)-CPB acetate was only 20%, with the enantio excess (e.e.) being 83% (Inagaki et al., 1992a). Use of anion-exchange resin (AmberliteIRA-904) as a base catalyst for the same reaction greatly increased the yield of (S)-CPB acetate to 80%, while the e.e. is relatively unchanged (89%). Although the conversion of the reactant was as high as 80%, the reaction time was as long as 69.6 h (Inagaki et al., 1992b). Our group (Zhu et al., 1998) compared the different effects of different kinds of lipases, acylating agents (vinyl acetate and isopropenyl acetate) and resins (D301 and D296) on this reaction. Although the reaction time was as long as 24 h, the conversion degree of the reaction was smaller than 17%. Later, a more effective lipase catalyzing the transesterification was screened and the effects of solvent, temperature, reactant concentration and internal and external diffusion limitation on the lipase-catalyzed transesterification were studied (Zhang et al., 2002). To our knowledge, there are few published reports on the influence of technological conditions on the whole one-pot synthesis of (S)-CPB acetate in organic media. Optimization of technological conditions in the synthesis of (S)-CPB acetate is discussed in this paper.
EXPERIMENTAL DETAILS
Chemicals and catalysts
Analytically pure grade vinyl acetate, diisopropyl ether, cyclohexane, n-hexadecane, m-PBA were all obtained from Shanghai Biochemical Reagent Corporation (China). Optically pure grade shift reagents Eu(hfc)3 and CDCl3 (containing 0.03% tetramethyl silicane (TMS)) were obtained from Sigma Corporation (USA).
CPB acetate used for coefficient calibration was prepared by the following protocol: Accurately weighed CPB alcohol (32%) 104.5 g, pyridine 26.3 g and cyclohexane 100 ml were added into a flask placed in a −5 °C ice bath and stirred. Weigh 17 g chloracetate and then put it into a titrating tube. The titration velocity of chloracetate should be appropriately adjusted and the titration reaction should be accompanied by stirring. Switch off the ice machine 1 h after completion of the reaction and switch off the stirrer machine 2 h after the ice bath machine is stopped. Filter the pyridine salt and distill the cyclohexane by vacuum distillation.
The lipase separated and purified from Alcaligenes sp. was immobilized and supplied by Meito Sangyo Corporation (Japan). The D301 which is a macroporous, weak base anion-exchange resin, was supplied by Hangzhou Zhengguang Chemical Factory (China).
Instruments
The instruments and apparatus used in the study are listed in Table 1.
Table 1.
Name, type and suppliers of the instruments used in this study
| Name | Type | Supplier |
| Isothermal shaker | THZ-82A | Shanghai Yuejing Medical Instruments Factory, China |
| Electronic balance | MD200-3 | Shanghai Balance Factory, China |
| Gas chromatograph | GC-9790 | Zhejiang Wenlin Analytical Instruments Factory, China |
| Chromatographic work station | N2000 | Institute of Intelligence & Information Engineering of Zhejiang University, China |
| NMR spectrometer | DMX500 | Bruker Spectrospin Corporation, Switzerland |
| Automatic polarimeter | WZZ-2 | Shanghai Physical Optics Instruments Factory, China |
Method to keep the water content stable
The immobilized lipase was put into a saturated LiCl solution container kept in a refrigerator at 4 °C for at least 48 h before the use so that the relative moisture was constant (11%). The reproducibility in regard to the reaction rate of lipase-catalyzed reaction was very high, so long as the experiments followed the procedure described by Halling (1994).
Analytical method
The samples were analyzed with gas chromatograph with 30 m long capillary (SE30), FID detector with temperature of 280 °C and nitrogen as the carrier gas. The oven temperature was programmed from 80 °C to 200 °C at rate of 20 °C/min and 200 °C to 270 °C at rate of 10 °C/min. The injector temperature was set to 320 °C. Internal standard method was chosen as quantitative analytical method and hexadecane was chosen as the internal standard compound. The calibration coefficient of product CPB acetate was determined to be 2.634. The repeatability was satisfactory.
Measurement of e.e. value of CPB acetate by NMR method
A sample (ca. 20 mg) was dissolved in a CDCl3-TMS (0.03%, V/V) solution (total 0.5 ml), into which Eu(hfc)3 (ca. 50 mg) was added. Two signals appeared, δ (chemical shift) 2.31 for (R)-isomer and δ 2.27 for (S)-isomer. Comparison of the integration of these two signals enabled the precise determination of the e.e.
General methods for kinetic study
The reaction was carried out in a batch reactor (55 ml vial). The reactants (AC, m-PBA, vinyl acetate) and the lipase were weighed accurately. The reaction was started by the addition of the resin D301. In the reaction course, the reactor was placed in a shaker whose rotational speed was controllable and temperature was kept constant. A 0.05 μl of the well-stirred reaction mixture was taken at intervals. The concentrations of CPB acetates were measured by gas chromatograph. Water activity of the solution was kept at 0.01 with definite amount of molecular sieve (Valivety et al., 1992).
RESULTS AND DISCUSSIONS
Choice of solvents
The enantioselectivity of the enzyme-catalyzed reaction and the reaction rate in nonaqueous media greatly depended on the solvents (Carrea and Riva, 2000; Klibanov, 2001).
Three solvents were compared in this reaction system. Under the experimental condition (45 °C,220 r/min, the lipase 2.5 mg/ml, D301 5 mg/ml, initial m-PBA concentration 70 mmol/L, initial AC concentration 258 mmol/L), progress curves for the three solvents (vinyl acetate, diisopropyl ether, cyclohexane) were measured. In a preliminary experiment, these three solvents had almost the same solubility of substrates, m-PBA and AC. The effect of the three solvents on enzyme deactivation could be ignored in the reaction time period. The results are shown in Fig.2.
Fig. 2.
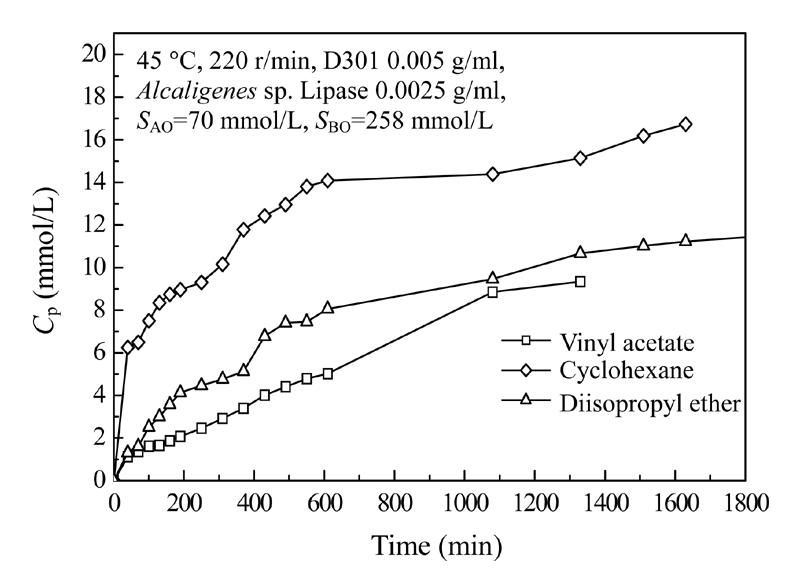
Effect of solvents on the one-pot reaction
C p: Concentration of product; S A0: Initial concentration of reactant m-PBA; S B0: Initial concentration of reactant cyanohydrin
Fig.2 showed that cyclohexane is the first choice to obtain more product in the shortest time, and that diisopropyl ether could make do with the second best. This test just showed the importance of variety of solvent in affecting the degree of conversion of this reaction system. More work should be done to search for the better solvent.
Effect of temperature
It was widely believed that enzymes exhibit their highest stereoselectivity at low temperatures (Phillips, 1996). This belief was supported by some experimental observations. In our temperature test, the one-pot reaction was performed under the following conditions: 220 r/min, initial m-PBA concentration 70 mmol/L, initial AC concentration 276 mmol/L, the lipase 2.5 mg/ml, D301 5 mg/ml, vinyl acetate acting as both acyl donor and solvent. In this reaction system, there existed two catalysts–D301 and lipase. The temperature effect on the system must have resulted from the comprehensive influence of the two catalysts. The progress curves of the reaction at different temperatures are shown in Fig.3 and the initial rates, degree of conversion and e.e. values were given in Table 2.
Fig. 3.

Effect of temperature on the one-pot reaction
Table 2.
The initial rates (V 0) and e.e. values and degree of conversion at 1330 min for the one-pot reaction at various temperatures
| T (°C) | V0×105 mol/(L·s) | e.e. (%) | Degree of conversion at 1330 min (%) |
| 35 | 0.031 | 88.2 | 7.4 |
| 40 | 0.036 | 82.5 | 13.4 |
| 45 | 0.039 | 65.0 | 14.2 |
| 50 | 0.046 | 50.0 | 24.6 |
| 55 | 0.098 | 40.2 | 37.5 |
Fig.3 and Table 2 showed that 55 °C was the optimal temperature for higher degree of conversion. However, the e.e. values of the product decreased gradually with increased reaction temperature. This showed that the enantioselectivity of the lipase decreased with increased temperature, and e.e. value was found to be in negative correlation to degree of conversion in this reaction system.
Effect of internal and external diffusion
Due to the insolubility of enzyme and D301 in organic media, external diffusion limitation must exist in this reaction system. Furthermore, since the Alcaligenes lipase was an immobilized enzyme, the reactants must first diffuse from the surface of the enzyme to the inner parts of the enzyme and they could then be transformed to products. Therefore, internal diffusion limitation is also existent. Effects of rotational speed and enzyme concentrations on the reaction when the reactant concentration was high enough were studied to reveal the effects of the external and internal diffusion limitations. The effect of rotational speed on the reaction was shown in Fig.4 and Table 3.
Fig. 4.

Effect of external diffusion on the one-pot reaction
Table 3.
Effect of rotational speed on the initial rates of one-pot reaction (45 °C, S A0=70 mmol/L, Alcaligenes sp. lipase 2.5 mg/ml)
| Rotational speed (r/min) | Initial rate [mmol/(L·min)] |
| 110 | 0.011 |
| 155 | 0.015 |
| 190 | 0.019 |
| 220 | 0.024 |
| 240 | 0.024 |
| 260 | 0.024 |
Fig.4 showed that the reaction rate increased at rotational speed of 110 r/min to 220 r/min and was almost unchanged at rotational speed higher than 220 r/min (the three curves covered each other). Thus, the conclusion that the external diffusion could be excluded when the rotational speed was higher than 220 r/min could be reasonably drawn.
Under the experimental conditions (45 °C, 220 r/min, S A0=70 mmol/L, S B0=276 mmol/L), the progress curves of the reaction in different enzyme concentrations were measured (Fig.5). The reaction rate increased with increased enzyme concentration (Fig.5). The initial rates of each progress curve were calculated and a V 0 (initial reaction rate, mol/(L·s)) vs E 0 (initial enzyme concentration, mol/L) curve was drawn (Fig.6).
Fig. 5.

Effect of enzyme concentration on the initial rates of the one-pot reaction
Fig. 6.

Relationship between E 0 and the initial rates of the one-pot reaction
Fig.6 showed that V 0 is not linearly correlated with E 0. This suggested that the internal diffusion limitation exist in this system (Bedell et al., 1998).
Effect of reactant concentrations
Under the reaction condition (45 °C, 220 r/min, D301 5 mg/ml, lipase 25 mg/ml, vinyl acetate as acyl donor and solvent), the effect of m-PBA concentration (AC concentration was designed to be double of m-PBA) on the one-pot reaction system had been studied. Each progress curve at different m-PBA concentrations was shown in Fig.7.
Fig. 7.

Effect of m-PBA concentrations on the one-pot reaction
Fig.7 showed that the reaction rate increased when the initial m-PBA concentration increased from 70 mmol/L to 249 mmol/L but decreased when the m-PBA concentration reached to 277 mmol/L. This implied that substrate inhibition may exist in this reaction system though reduced reaction rate at high substrate concentrations in immobilized enzyme system may also be due to a reaction limited process. The initial rate, degree of conversion and e.e. values were given in Table 4. Degree of conversion at 1840 min decreased when initial m-PBA concentration reached 277 mmol/L. However, change of e.e. had no regularity with the increasing of m-PBA concentration. This reactant concentration test is important and useful for further kinetic study of this reaction.
Table 4.
Effect of initial m-PBA concentrations on the one-pot reaction
| SA0 (mmol/L) | Initial rate [mmol/(L·min)] | Degree of conversions at 1840 min (%) | e.e. (%) |
| 70 | 0.028 | 23.2 | 63.3 |
| 126 | 0.032 | 22.3 | 73.3 |
| 189 | 0.051 | 24.1 | 55.6 |
| 224 | 0.088 | 22.3 | 82.5 |
| 249 | 0.143 | 22.5 | – |
| 277 | 0.116 | 17.8 | – |
Comparison of unused lipase and reused lipase
Lipase is a relatively expensive catalyst. One of the advantages of synthesis in organic solvent is the convenient recovery of catalyst. In order to check the inactivation rate of lipase in this one-pot reaction system, the catalytic ability of unused lipase and reused lipase was compared (Fig.8). It was shown that the catalytic efficiency of new lipase was slightly better than that of the reused lipase. Lipase in organic media could be used for at least more than once or even several times.
Fig. 8.

Comparison of new lipase and reused lipase for the one-pot reaction
Optimization of the mole ratio of the two reactants:AC and m-PBA
Since there were two reactants, AC and m-PBA in this one-pot reaction system, the mole ratio of the two reactants might be important for obtaining high degree of conversion. In order to determine such effect, we chose five ratios, 0.5:1, 1:1, 1.5:1, 2:1, 3:1 for testing. The reaction degrees of conversions of the five ratios are shown in Table 5. Ratio 2:1 (AC:m-PBA) is shown to be the best choice.
Table 5.
Effect of different ratio of AC to m-PBA on the conversion of the one-pot reaction (45 °C, 220 r/min, D301 6.7 mg/ml, lipase 0.01 g/ml)
| Mole ratio of AC to m-PBA | Conversions at 1500 min (%) |
| 0.5:1 | 2.5 |
| 1:1 | 4.8 |
| 1.5:1 | 10.3 |
| 2:1 | 21.5 |
| 3:1 | 19.1 |
Therefore, it is important to choose an appropriate mole ratio of the two reactants in industrial production in order to get higher conversion.
Effect of addition strategy of catalyst D301
Under reaction condition (45 °C, 220 r/min, lipase 20 mg, vinyl acetate as solvent 30 ml, S A0=120 mmol/L, S B0=240 mmol/L), 0.7 g D301 was added into the system at the beginning of the reaction. The degree of conversion of m-PBA was only 20% at 2500 min. Later, the addition strategy of D301 was changed to adding D301 0.3 g at the beginning of the reaction and then adding D301 0.2 g every 12 h for two times. Surprisingly, the degree of conversion reached 66% at 1500 min, and even as high as 80% at 2500 min (Fig.9). We repeated the experiment again, and found that the result was almost the same. The e.e. value of the product measured by NMR was 64.5%. This showed that the base catalyst D301 was easily inactivated in organic solvent, and the addition strategy of D301 is important for getting higher degree of conversion. This result is useful in industrial application.
Fig. 9.

Effect of the addition mode of D301 on the one-pot reaction (add D301 0.2 g every 12 h)
CONCLUSION
The one-pot reaction catalyzed by the combination of anion-exchange resin D301 with lipase to synthesize CPB acetate in organic media was studied. By using lipase from Alcaligenes sp., the catalyzing efficiency was improved considerably compared to methods previously reported. Effects of solvents and temperatures on this reaction were studied. External diffusion limitation could be prevented by raising the rotational speed to 220 r/min. In contrast, internal diffusion could not be ignored, since the diameter of the immobilized lipase was not made small enough. The reaction rate was substantially accelerated with the increase of the reactant (m-PBA) concentration to 249 mmol/L, but decreased when the initial concentration of m-PBA was higher than 277 mmol/L. It was also found that the catalyzing capability of recovered lipase was high enough for use in several batches. Finally, the mole ratio of AC to m-PBA and addition strategy of base catalyst D301 to improve the conversion of substrates were studied. By using adequate amount of lipase (0.66 mg/L), adequate amount of D301 (23.33 mg/L) and appropriate addition strategy, the conversion of this one-pot reaction could be as high as 80% even when the temperature was 45 °C, and initial concentration of m-CPB was only 120 mmol/L. The e.e. values of the product decreased gradually when the reaction temperature was raised. This showed that the enantioselectivity of the lipase decreased when the temperature increased. However, change of e.e. had no regularity with the increasing of m-PBA concentration. The highest product e.e. value we achieved was 88.2%.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 20336010) and the National Basic Research Program (973) of China (No. 2003CB716008)
References
- 1.Bedell BA, Mozhaev VV, Clark DS, Dordick JS. Testing for diffusion limitations in salt-activated enzyme catalysts operating in organic solvents. Biotech & Bioeng. 1998;58(6):654–657. [PubMed] [Google Scholar]
- 2.Carrea G, Riva S. Properties and synthetic applications of enzymes in organic solvents. Angew Chem Int Ed. 2000;39:2226–2254. [PubMed] [Google Scholar]
- 3.Halling PJ. Thermodynamic predictions for biocatalysis in nonconventional media: theory, tests, and recommendations for experimental design and analysis. Enzyme Microb Technol. 1994;16:178–206. doi: 10.1016/0141-0229(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki M, Hiratake J, Nishioka T, Oda J. Lipase-catalyzed kinetic resolution with in situ racemization: one-pot synthesis of optically active cyanohydrin acetates from aldehydes. J Am Chem Soc. 1991;113:9360–9361. [Google Scholar]
- 5.Inagaki M, Hatanaka A, Mimura M, Hiratake J, Nishioka T, Oda J. One-pot synthesis of optically active cyanohydrin acetates from aldehydes via quinidine-catalyzed transhydrocyanation coupled with lipase-catalyzed kinetic resolution in organic solvent. Bull Chem Soc Jpn. 1992;65:111–120. [Google Scholar]
- 6.Inagaki M, Hiratake J, Nishioka T, Oda J. One-pot synthesis of optically active cyanohydrin acetates from aldehydes via lipase-catalyzed kinetic resolution coupled with in situ formation and racemization of cyanohydrins. J Org Chem. 1992;57:5643–5649. [Google Scholar]
- 7.Klibanov AM. Asymmetric transformation catalyzed by enzymes in organic solvents. Acc Chem Res. 1990;23:114–120. [Google Scholar]
- 8.Klibanov AM. Improving enzymes by using them in organic solvent. Nature. 2001;409:241–246. doi: 10.1038/35051719. [DOI] [PubMed] [Google Scholar]
- 9.Phillips RS. Temperature modulation of the stereochemistry of enzymatic catalysis: prospects for exploitation. TIBTECH. 1996;14:13–16. [Google Scholar]
- 10.Valivety RH, Halling PJ, Macrae AR. Reaction rate with suspended lipase catalyst shows similar dependence on water activity in different organic solvents. Biochim Biophys Acta. 1992;1118:218–222. doi: 10.1016/0167-4838(92)90278-l. [DOI] [PubMed] [Google Scholar]
- 11.Wang YF, Chen ST, Liu KKC, Wong CH. Lipase-catalyzed irreversible transesterification using enol esters: resolution of cyanohydrins and syntheses of ethyl (R)-2-hydroxy-4-phenylbutyrate and (S)-propranolol. Tetrahedr Lett. 1989;30:1917–1920. [Google Scholar]
- 12.Zhang TZ, Yang LR, Zhu ZQ, Wu JP. The kinetic study on lipase-catalyzed transesterification of α-cyano-3-phenoxybenzyl alcohol in organic media. J Mol Catal B: Enzymatic. 2002;18:315–323. [Google Scholar]
- 13.Zhu Y, Yang LR, Zhu ZQ, Yao S, Cen P. Lipase-catalyzed enantioselective transesterification of cyanohydrins for the synthesis of (S)-α-cyano-3-phenoxybenzyl acetate. Annals N Y Acad of Sci. 1998;864:646–648. doi: 10.1111/j.1749-6632.1998.tb10397.x. [DOI] [PubMed] [Google Scholar]


