Abstract
Objective: To assess the effect of temporary occlusion of hepatic blood inflow on hepatic cancer treated with diode-laser induced thermocogation (LITT). Methods: The carcinoma Walker-256 was implanted in 40 SD rat livers. Twelve days later, the animals were randomly divided into 4 groups. Group A received LITT alone; group B received hepatic artery temporary occlusion during LITT; group C received portal vein temporary occlusion during LITT; group D received hepatic artery and portal vein temporary occlusion during LITT. Tumors were exposed to 810 nm diode-laser light at 0.95 watts for 10 min from a scanner tip applicator placed in the tumor. At the same time, the intrahepatic temperature distribution in rats with liver tumors was measured per 2 min during thermocoagulation. Tumor control was examined immediately 7 and 14 d after thermocoagulation. Results: There was significant difference of intrahepatic temperature distribution in rats with liver tumors among the 4 groups (P<0.05) except when group C samples were compared with group D samples at each time point, and group B samples were compared with group C samples at 120 s (P>0.05). Light microscopic examination of the histologic section samples revealed three separate zones: regular hyperthermic coagulation necrosis zone, transition zone and reference zone. Compared with the samples in group A and group B, group C and group D samples had more clear margin among the three zones. Conclusion: The hepatic blood inflow occlusion, especially portal vein hepatic blood inflow occlusion, or all hepatic blood inflow occlusion considerably increased the efficacy of LITT in the treatment of liver cancer.
Keywords: Hepatic carcinoma/thermotherapy, Laser/diode-laser, Hepatic blood inflow/occlusion
INTRODUCTION
In recent years, thermo-ablation of liver cancer has received increasing attention. Generally this can be achieved by radiofrequency (RF) or Nd:YAG laser (Tanabe et al., 2004; Nikfarjam and Christophi, 2003; Erce and Parks, 2003). Of great importance to the method’s success is the size of the induced coagulation necrosis; the lesion size largely depends on the temperature distribution in the target volume, which is determined partly by the blood perfusion in the target organ. In organs with high blood perfusion, such as the liver, a cooling effect results because the temperature difference between the hyerthermic area and blood. Germer’s study showed that hepatic artery embolism or laparoscopic hepatic artery and portal vein occlusion improved the effect of LITT of liver cancer (Germer et al., 1997; 1999). We investigated the effect of temporary interruption of hepatic artery, portal vein, and both vessels on hepatic cancer treatment with LITT system developed by ourselves in this study.
METHODS
Animals and tumor model
Forty SD rats without sex distinction weighing 180 g to 250 g (Zhejiang University Medical College experimental animals, China) were used in the study. The tumor was ascites carcinosarcoma root which had inoculations (obtained from Shanghai Biology and Industry laboratory, China) for 8 d. After dilution with the same quantity of normal saline, 0.5 ml tumor suspension hypodermic was injected into the groin of the SD rats and became entity tumor after 10 days’ growth. The entity tumor was excised from the rats and cut into about 1 mm3 pieces. For tumor implantation, the animals were laparotomized through a midline abdominal incision under aether anaesthsia. Tumor pieces were implanted under the capsule of the anterior surface of the left or right liver lobe of the SD rats. The puncture site was closed with acrylic glue to prevent the leakage of tumor tissue into the peritoneal cavity. Twelve days after being implanted, only the tumor with a diameter in 8 mm to 12 mm was used in this study.
Forty Walker-256 liver carcinoma rats were random divided into four groups. Group A received LITT alone, group B received LITT and hepatic artery temporary occlusion, group C received LITT and portal vein temporary occlusion, group D received LITT and hepatic artery and portal vein temporary occlusion.
Laser, laser application and temperature measurement
The LITT system was developed by us and obtained nation patent (ZL 02 2 17726.4). In our experiment, tumors were exposed to 810 nm diode-laser light at 0.95 watts for 10 min from a scanner fiber tip application. The length of therapeutic tip is 5 mm, which had a scanner tip application (outer diameter 0.8 mm and length 5.0 mm) at its distal end.
The animals were placed in a recumbent position and fixed after aether anaesthsia. Hepatic artery, portal vein and hepatoduodenal ligament of the animals was dissected in B, C and D groups by surgical technique. The left or right liver lober with tumor was exposed to incision. Two pinpricks were punctured by 18G (gauge) PTC (percutaneous transhepatic catheter) needle in the center of the liver cancer and in the location 3 mm to the center. The whole laser applicator was inserted into the pinprick of the tumor center. Hepatic artery, the portal vein and hepatoduodenal ligament of the rats were occluded and the application time was 10 min. For temperature measurement during laser application, a nickelchrome-nickel thermocouple 300 µm in diameter was placed into the other pinprick located 3 mm from the center. The geometric relation of temperature probe and the applicator was maintained with an external fixation device through which the probe and the applicator were inserted. Baseline temperature was recorded for 2 min before treatment. During application, the intrahepatic temperature was measured at 120 s intervals.
Specimens collection
Four, three, three animals in each group were killed at 0 h, 7 d and 14 d after treatment respectively. The tumor tissue and the surrounding liver tissue were chipped into many slices in vertical direction of the optic fiber. The specimens were fixed in 10% formalin for 24 h then embedded in paraffin and stained with H&E. Light microscopy was used to examine the pathological changes of tissue.
Statistical analysis
The mean temperature±SEM were calculated for each group. The differences between the temperatures at different time point in the same group were determined using one way analysis of variance and SAS software. The temperatures differences between different groups were analyzed using q test.
RESULTS
Temperature measurements
All rats survived after operation. The temperature of liver tissue in four groups before therapy was (34.00±0.00) °C. The temperatures of liver tissue 3 mm to the tumor center at different time points after therapy are shown in Table 1 and Fig.1. The results showed that there were significant differences of the tissues temperatures between different time points in each group and that there were significant differences of the tissues temperatures between the same time point of different groups but not between B and C groups at 120 s or between C and D groups at each time point.
Table 1.
Temperature distribution vs time*
| Time (s) | Temperature (°C) (χ¯±s) |
|||
| A (n=10) | B (n=10) | C (n=10) | D (n=10) | |
| 120 | 49.10±7.72 | 55.80±5.07+ | 62.10±6.90+ | 63.80±8.27+ |
| 240 | 52.30±7.29 | 60.60±6.31 | 69.80±7.80+ | 71.10±8.16+ |
| 360 | 49.90±5.88 | 60.40±4.90 | 69.70±9.33+ | 69.10±9.93+ |
| 480 | 50.50±5.10 | 60.90±6.94 | 71.40±9.26+ | 71.10±10.05+ |
| 600 | 51.60±5.87 | 60.50±5.34 | 69.20±10.44+ | 68.70±11.47+ |
Compared with each other at the same time point
P>0.05, the others P<0.05
Fig. 1.
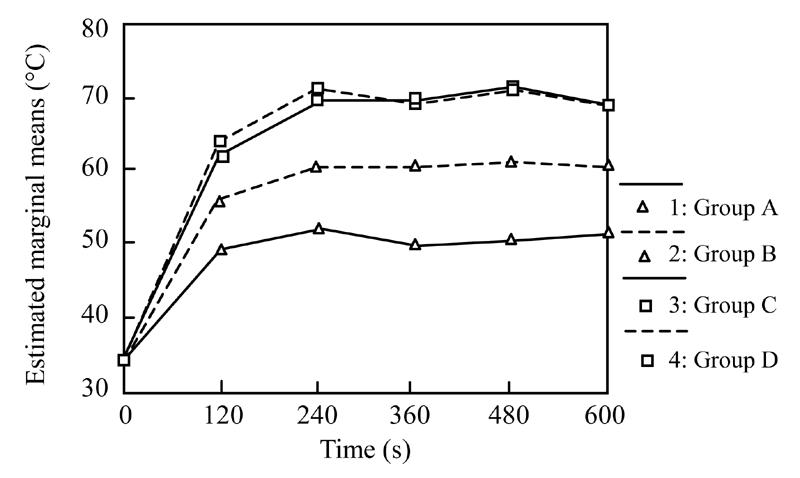
The temperature course
Histology
Four, three, three rats of each group were killed 0 h, 7 d and 14 d after treatment respectively. In all tissue samples of the 4 groups, the central zone was coagulative necrosis tissue, and peripheric zone was necrosis-tumor tissues border. Hemorrhages and hyperemins zones and peripheric normal tissues control zones existed in partial samples. Central zone: tumor cells displayed clear shrinkage and partial loss of cell contact. The cell nuclei were karyopyknosis (The rupture of cell nucleus in which the chromatin disintegrates into formless granules excluded from the cell) and karyorrhexis (A thickening, especially degeneration of a cell in which the nucleus shrinks in size and the chromatin condenses to a solid mass or masses without definite form). Tumoric organic-like structure were partially observed on the central zone. Marginal zone: all of the tumor cells were eosinophilic. The cell nuclei were characterized by karyopyknosis and karyorrhexis. The dividing line of the three zones in groups C and D was clearer than that of group B. Seven days after treatment, we observed the eosinophilic cytoplasma of whole tumor cells of the liquefaction necrosis zone, inflammatory cell infiltration and cancer cell alternate infiltration transition zone and liver cell zone. Fourteen days after treatment, we observed that the liquefaction necrosis zone reduced, cancer cell of A and B groups invaded the central and fibre envelope that developed being outside the transition zone of C and D groups whose border in the three zones was still clear.
DISCUSSION
Studies showed that the effect of thermocoagulation therapy was influenced by the tumor environment. For example, the tumor cells with low pH were sensitive to high temperature, the quantity of heat was easily absorbed by blood when tumor with abundant blood was treated with thermotherapy. Liver is an organ with abundant blood inflow of about 1000 ml/min to 1800 ml/min or 1500 ml/min in average. Twenty-five percents to 30% blood came from hepatic artery and 70% to 75% of that came from the portal vein. So, we concluded that hepatic blood is the critical histologic and biologic factor of thermotherapy. With the development of DSM and laparoscopy technology, a less invasive alternative for temporary interruption of hepatic artery and portal vein was applied in clinical diagnosis and therapy. Thus, it is necessary to assess the effect of temporary interruption of hepatic blood inflow on hepatic cancer treated with LITT.
Tissue temperature is the decisive factor of biologic reaction. The study of tumor thermo-biology show that tumor cells will be killed at temperature of 42 °C to 45 °C and that the heating of biologic tissue with laser light is decisively influenced by the temperature distribution in the target volume. The time course of temperature distribution is determined by local heat generation and by the simultaneous conduction of heat energy (Li and Hu, 1995; Zhang and Jiang, 1999; Heisterkamp et al., 1999). The results of our research showed that there was significant differences of intrahepatic temperature distribution in rats with liver tumors among the 4 groups (P<0.05) except when group C samples were compared with group D samples at each time point, and group B samples were compared with group C samples at 120 s (P>0.05). This demonstrated that the effect of LITT was improved significantly by interrupted hepatic artery temporary occlusion, portal vein temporary occlusion or hepatic artery and portal vein temporary occlusion, but there was no difference between portal vein temporary occlusion alone and hepatic artery and portal vein temporary occlusion. Some reports showed that the diameter of coagulative necrosis liver cancer tissue treated with LITT and hepatic artery and portal vein temporary occlusion was increased by 47% (Heisterkamp et al., 1997). Matthewson’s report showed that liver tissue or liver cancer close to blood vessel was still alive after being treated with LITT (Matthewson et al., 1987). These phenomena demonstrated that hepatic blood inflow could reduce the effect of LITT, and that other factors might adversely affect cancer cell sensitive to thermotherapy, liver cancer cells short of oxygen, the low pH at liver location is reduced by interrupting hepatic blood inflow and then is effectively improved by LITT.
The combination of LITT and hepatic blood inflow occlusion, especially occlusion of the portal vein or all hepatic blood inflow interruption considerably increases the efficacy of LITT in the treatment of liver cancer. We suggest that less invasive techniques will be a good choice for liver blood inflow interruption, it can improve the efficacy of LITT in the treatment of liver cancer and protect liver function by keeping enough liver blood inflow.
Acknowledgments
We thank Dr. Welch from Loma Linda University, USA for suggesting improvements to the manuscript.
Footnotes
Project supported by the National Basic Research and Development Program (973) (No. 863-410-2001-5) of China and Science Foundation of Zhejiang Province (No. 2004C33016), China
References
- 1.Erce C, Parks RW. Interstitial ablative techniques for hepatic tumors. Br J Surg. 2003;90(3):272–289. doi: 10.1002/bjs.4091. [DOI] [PubMed] [Google Scholar]
- 2.Germer CT, Albrecht D, Roggan A, Isbert C, Buhr HJ. An experimental study of laparoscopic laser-induced thermotherapy treatment for liver tumor. Br J Surg. 1997;84(3):317–324. [PubMed] [Google Scholar]
- 3.Germer CT, Isbert C, Albrecht D, Roggan A, Pelz J, Ritz J, Muller G, Buhr HJ. Laser-induced thermotherapy combined with hepatic arterial embolization in the treatment of liver tumors in a rat tumor model. Ann Surg. 1999;230(1):55–62. doi: 10.1097/00000658-199907000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heisterkamp J, van Hillegersberg R, Mulder PGH, Sinofsky EL, Ijzermans JNM. The importance of eliminating portal flow in producing large intra-hepatic lesions with interstitial laser coagulation. Br J Surg. 1997;84(9):1245–1249. [PubMed] [Google Scholar]
- 5.Heisterkamp J, van Hillegersberg R, Ijzermans JNM. Interstitial laser coagulation for hepatic tumors. Br J Surg. 1999;86(9):293–304. doi: 10.1046/j.1365-2168.1999.01059.x. [DOI] [PubMed] [Google Scholar]
- 6.Li DJ, Hu ZS. Tumor Thermotherapy. Henan, China: Henan Medical University Press; 1995. pp. 186–187. (in Chinese) [Google Scholar]
- 7.Matthewson K, Coleridge-smith P, O’Sullivan JP, North-field TC, Bown SG. Biological effects of intrahepatic neodymium: yttrium-aluminum-garnet laser photocoagulation in rats. Gastroenterology. 1987;93(3):550–557. doi: 10.1016/0016-5085(87)90918-8. [DOI] [PubMed] [Google Scholar]
- 8.Nikfarjam M, Christophi C. Interstitial laser thermotherapy for liver tumor. Br J Surg. 2003;90(9):1033–1047. doi: 10.1002/bjs.4326. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe KK, Curley SA, Dodd GD, Siperstein AE, Goldberg SN. Radiofrequency ablation: the experts weigh in. Cancer. 2004;100(3):641–650. doi: 10.1002/cncr.11919. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZX, Jiang DZ. Laser-Tissue Interactions: Foundaments and Application. Xi'an, China: Xi’an Jiaotong University Press; 1999. pp. 64–65. (in Chinese) [Google Scholar]


