Abstract
Objective: The present study was designed to test whether transplantation of human bone marrow-derived mesenchymal stem cells (hMSCs) in New Zealand rabbits with myocardial infarction can improve heart function; and whether engrafted donor cells can survive and transdifferentiated into cardiomyocytes. Methods: Twenty milliliters bone marrow was obtained from healthy men by bone biopsy. A gradient centrifugation method was used to separate bone marrow cells (BMCs) and red blood cells. BMCs were incubated for 48 h and then washed with phosphate-buffered saline (PBS). The culture medium was changed twice a week for 28 d. Finally, hematopoietic cells were washed away to leave only MSCs. Human MSCs (hMSCs) were premarked by BrdU 72 h before the transplantation. Thirty-four New Zealand rabbits were randomly divided into myocardial infarction (MI) control group and cell treated group, which received hMSCs (MI+MSCs) through intramyocardial injection, while the control group received the same volume of PBS. Myocardial infarction was induced by ligation of the left coronary artery. Cell treated rabbits were treated with 5×106 MSCs transplanted into the infarcted region after ligation of the coronary artery for 1 h, and the control group received the same volume of PBS. Cyclosporin A (oral solution; 10 mg/kg) was provided alone, 24 h before surgery and once a day after MI for 4 weeks. Echocardiography was measured in each group before the surgery and 4 weeks after the surgery to test heart function change. The hearts were harvested for HE staining and immunohistochemical studies after MI and cell transplantation for 4 weeks. Results: Our data showed that cardiac function was significantly improved by hMSC transplantation in rabbit infarcted hearts 4 weeks after MI (ejection fraction: 0.695±0.038 in the cell treated group (n=12) versus 0.554±0.065 in the control group (n=13) (P<0.05)). Surviving hMSCs were identified by BrdU positive spots in infarcted region and transdifferentiated into cardiomyocytes characterized with a positive cardiac phenotype: troponin I. Conclusion: Transplantation of hMSCs could transdifferentiate into cardiomyocytes and regenerate vascular structures, contributing to functional improvement.
Keywords: Bone marrow-derived mesenchymal stem cells, Transplantation, Myocardial infarction (MI)
INTRODUCTION
Congestive heart failure is the end stage of many cardiovascular diseases. Myocardial infarction (MI) is a life-threatening event that may cause sudden cardiac death and heart failure. Despite considerable advances in diagnosis and treatment of heart disease, cardiac dysfunction after MI is still the major worldwide cardiovascular disorder. Damaged myocardium after acute MI is gradually replaced by fibrotic noncontractile cells to form scar tissue. The developing ventricular dysfunction is primarily due to a massive loss of cardiomyocytes. Although heart transplantation is an option for severe congestive heart failure patients, organ shortage and the need for immunosuppression are problems of whole organ transplantation. Many studies in the 1990s showed that transplanted fetal smooth muscles (Li et al., 1999), skeletal myoblast cells (Taylor et al., 1998; Zibaitis et al., 1994) and cardiomyocytes (Li et al., 1996; 2000; Leor et al., 1996; Reinecke et al., 1999; Marcio et al., 1996; Sakai et al., 1999a) could limit scar expansion and prevent onset or development of heart failure, although engrafted donor cells were eventually eliminated by immune rejection (Sakai et al., 1999b; Li et al., 1997). Recent research data demonstrated that bone marrow-derived mesenchymal cells (Makino et al., 1999; Asahara et al., 1997; Kocher et al., 2001) could transdifferentiate into cardiomyocytes and improve heart function when transplanted into the myocardial infarction region. The present study tested the feasibility of human bone marrow-derived mesenchymal cells survival and differentiation into cardiomyocytes in the MI region of New Zealand rabbits.
METHODS AND MATERIALS
Preparation of hMSCs
Twenty milliliters bone marrow were obtained by bone biopsy in a healthy voluntary human subject, heparin was used for the anti-thrombosis procedure. Bone marrow was transferred to a 50 ml tube with 20 ml culture medium (DMED with 10% fetal bovine serum, penicillin G [100 U/ml] and streptomycin [100 U/ml]). The tube was centrifuged at 2000 rpm for 10 min and the cell pellet was resuspended in 40 ml culture medium. A gradient centrifugation method (Yablonka-Reuveni and Nameroff, 1987) was used to separate BMCs and red blood cells. The cell suspension was loaded on 1.073 g/ml gradient percoll. The cells were centrifuged at 900 g for 30 min. The top two thirds of the total volume were transferred into a tube and centrifuged again at 2000 rpm for 10 min and then washed with PBS to remove the percoll. This procedure was repeated and the cell pellet was then resuspended in culture medium.
The cells were cultured in DMED with 10% fetal bovine serum, penicillin G (100 U/ml) and streptomycin (100 U/ml). The cells were incubated for 48 h and then washed with PBS. The culture medium was changed twice a week for 28 d. Finally, almost all the hematopoietic cells were washed away after several times of medium changing.
Identification of hMSCs
After hMSCs cultured in vitro for 4 weeks, Flow CytoMeter (FCM) (Kocher et al., 2001) was used to analyze the superficial markers such as CD34, CD45, CD166 and CD29 of hMSCs.
hMSCs preparation for transplantation
Seventy-two hours before cell transplantation, hMSCs were cultured with DMED with 10 μmol/ml BrdU for 48 h in order to make the donor cells for further identification (Tomita et al., 1999). Cultured donor cells were dissociated from the culture dishes with 0.25% trypsin (Gibaco, USA), neutralized with culture medium, and collected by 2000 rpm centrifugation for 10 min at room temperature. The cells were washed twice with PBS and then suspended in PBS at final concentration of 106/ml for transplantation. 5×106 hMSCs were transplanted by local intramyocardial injection after ligation of the coronary artery for 1 h.
Myocardial infarction and hMSCs transplantation
Thirty-four 1.76–3.58 kg adult male purebred New Zealand white rabbits provided by the Zhejiang Province Academy of Medical Sciences (No. of certificate of approval: Zhejiang Experimental Animal No. D00-002, approved in 2001). After echocardiography, the animals were divided into 2 groups: the cell treated group (14 cases), consisting of MI model with MSCs injected and then transplanted into the MI region; the control group (20 cases), consisting of MI model with cells-free PBS injected into the MI region.
The New Zealand rabbits were anesthetized with intramuscular administration of sodium pentobarbital (30 mg/kg). Under general anesthesia, the heart was exposed by means of a left thoracotomy through the fourth and fifth intercostals space. The distal end (just below the third diagonal) of the left anterior descending coronary artery was ligated (Podesser et al., 1997; MacFarlane et al., 2000). After ligation for 1 h, 5×106 hMSCs were transplanted by injecting into the border area of the ischemia myocardium. In the control group, MI hearts received the same MI operation, but were injected with the same volume of PBS.
Cyclosporin A was provided alone to impress immune rejection. Starting 24 h before the operation, all experimental animals were given cyclosporin A liquid (Fujian China Kerui Medicine Co. Ltd.) at dosage of 10 mg/kg once a day for 4 weeks until the experiment stopped (Marcio et al., 1996).
Assessment of heart function and identification of transplanted hMSCs
Cardiac function was measured with echocardiography (Pennock et al., 1997; Orlic et al., 2001) in each group before and 4 weeks after surgery.
To identify transplanted cells in the host myocardium, the donor cells were labelled with 5-bromodeoxyuridine (Tomita et al., 1999) (BrdU, Sigma). Briefly, culture medium with 10 μmol/l BrdU solution was added into each culture dish at 72 h before transplantation and incubated with donor cells for 48 h. Labelling efficiency was ≈70% (Tomita et al., 1999). Labelled cells were transplanted into the MI area after the artery had been ligated for 1 h, and the samples (0.5 cm3) at the transplantation site were collected after transplantation for 4 weeks and fixed in neutralized 10% formaldehyde for immuno-histochemistry study. The heart sections were embedded and cut into 10-μm thick sections. Monoclonal antibodies against BrdU and troponin I (BIODESIGN, USA) were used to localize the transplanted hMSCs and to show whether the surviving engrafted hMSCs have transdifferentiated into cardiomyocytes (Cohen et al., 1999; Chen et al., 2001). Briefly, samples were serially rehydrated with 100%, 95% and 70% ethanol after deparaffinization with toluene. Endogenous peroxidase in the sample was blocked using 3% hydrogen peroxide for 10 min at room temperature. The sample was treated with pepsin for 5 min at 42 °C and 2 mol/L HCl for 30 min. After rinsing 3 times with PBS the prepared sample was incubated with antibodies against BrdU in a moist chamber for 16 h at room temperature. Negative control samples were incubated in PBS (without the primary antibodies) under the same conditions. The treated and control heart sections were rinsed with PBS 3 times (15 min each time) and then incubated with goat anti-rabbit immunoglobilin conjugated with peroxidase at 37 °C for 45 min. The samples were washed 3 times (15 min each time) with PBS and then immersed in diaminobenzidine H2O2 (2 mg/ml) diaminobenzidine, 0.03% H2O in 0.02 ml/L phosphate buffer for 15 min. The prepared sections were then further incubated with mouse anti-rabbit immunoglobilin conjugated with peroxidase at 37 °C for 2 h. After washing with PBS for 3 times (15 min each time), the samples were incubated with Diaminobenzidine (DAB) for 10 min, washed thrice with PBS, then was incubated with 3-amino-9-ethylcarbazole (AEC), washed thrice with PBS and finally coversliped and photographed.
Data Analysis
Data are expressed as mean±SE. Analysis system software SPSS 11.0 was used for all analysis. A level of P<0.05 was considered as significant difference.
RESULTS
hMSC culture and cell marker expression
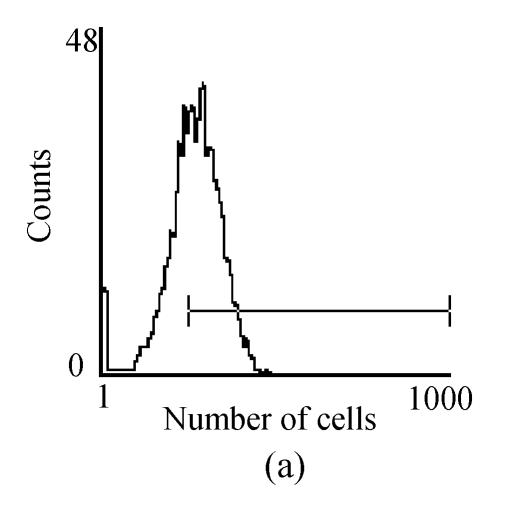
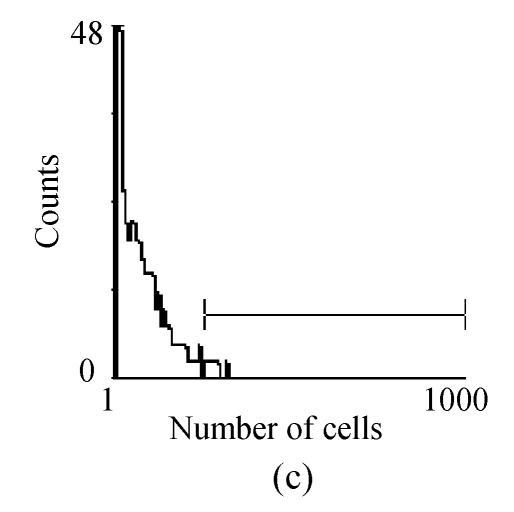
Mononuclear cells were prepared by percoll gradient centrifugation technique with two major types cells, mesenchymal stem cells and hematopoietic stem cells. The MSCs were spindle-shaped, attached to the culture dish tightly, and proliferated in the culture medium (Fig.1). Hematopoietic stem cells were round, did not attach to the culture dish, and were washed away with the culture medium changes. Human bone marrow-derived MSCs showed active proliferative capacity in vitro with primary and passage culture. After in vitro culture for 4 weeks, FCM analysis showed the result of cell marker expression (Fig.2) (CD29 expression was 98.3% and CD166 expression was 77.2%, CD34 expression was 1.7% and CD45 expression was 2.4%). All these result showed hMSCs have no obvious evidence of transdifferentiation in in vitro culture.
Fig. 1.

Human bone marrow-derived mesenchymal stem cells in in vitro culture (original magnification×100). The majority of the cultured cells were spindle-like mesenchymal stem cells after 3 d of culture and purification
Fig. 2.
FACM analysis showed the expression of cell makers in cultured hMSCs: (a) CD166 expressed 77.2%; (b) CD29 expressed 98.3%; (c) CD34 expressed 1.7%; (d) CD45 expressed 2.4%
Survival study at 4 weeks after cell transplantation
After transplantation, 2 rabbits in the cell treated group died during 4 weeks follow up (n=14), and 7 rabbits from the control group died (n=20). The mortality of the cell transplantation group was lower than that of the control group (14.3% in cell-treated group vs 35% in MI control group, P<0.05).
Cardiac functional assessment at 4 weeks after cell transplantation
Among the animals that survived for 4 weeks, the LVIDd and LVIDs of the MSCs group after operation were 1.12±0.18 cm and 0.67±0.05 cm respectively, and remarkably lower than those of the control group, which were 1.54±0.14 cm and 0.97±0.05 cm (P<0.05); the LVEF of the MSCs group after operation was remarkably higher than that of the control group, (0.66±0.04 versus 0.49±0.08, P<0.05) (Table 1).
Table 1.
LVIDd, LVIDs and LVEF of 2 groups’ animals that survived for 4 weeks*
| Control group (n=13) | MSCs group (n=12) | |
| Weight (kg) | 2.20±0.33 | 2.27±0.26 |
| LVIDd before operation (cm) | 0.95±0.12 | 0.92±0.09 |
| LVIDs before operation (cm) | 0.56±0.10 | 0.52±0.09 |
| LVEF before operation (%) | 0.76±0.06 | 0.79±0.07 |
| LVIDd after operation (cm) | 1.54±0.14 | 1.12±0.18 |
| LVIDs after operation (cm) | 0.97±0.05 | 0.67±0.05 |
| LVEF after operation (%) | 0.49±0.08 | 0.66±0.04 |
Comparison of LVIDd, LVIDs and LVEF with those of control group after operation, P<0.05
Morphological study with immunohistochemical staining
The nuclei of donor cells were labeled with BrdU 72 h before transplantation, 70% of nuclei were stained positive for BrdU. Labelled cells were transplanted into the myocardial infarction area. BrdU-labelled cells were observed at the implanted area after cell transplantation for 4 weeks. In addition, partial BrdU-stained cells were turned to muscle-like cells and stained positively for troponin I (Fig.5). Muscle-like cells formed in the MI area received the cell implantation in cell treated animals. It is a few BrdU-stained cells were found in the normal cardiomyocytes (Fig.4), but not in the control group (Fig.3). This showed that BrdU labelled hMSCs survived in the myocardial infarction region and transdifferentiated into cardiomyocytes. No lymphocyte infiltration and immunorejection were evident. Cartilage, bone, and fat did not form in the transplanted area. No tumor-like cells were found in cell-implanted area. Transplanted MSCs also stimulate angiogensis and form new vascular structure capillary walls composed of BrdU-positive endothelial cells. Below the left ventricle epicardium was a large network of capillaries, arteries, veins and blood vessel endothelium in slice shape distribution. cTn I (−) and BrdUrd (+) cells in the smooth muscle cell could be observed (Fig.6).
Fig. 5.

Immunohistochemical staining showed there were cells stained double positive for BrdU and tropnin I in the host infarcted myocardium that received hMSCs from the cell treated group (anti-BrdU and anti-tropnin I, immunoperoxidase, original magnification×800)
Fig. 4.

Immunhistochemical staining showed there were cells stained double positive for BrdU and tropnin I in the normal myocardium (anti-BrdU and anti-tropnin I, immunoperoxidase, original magnification×800)
Fig. 3.
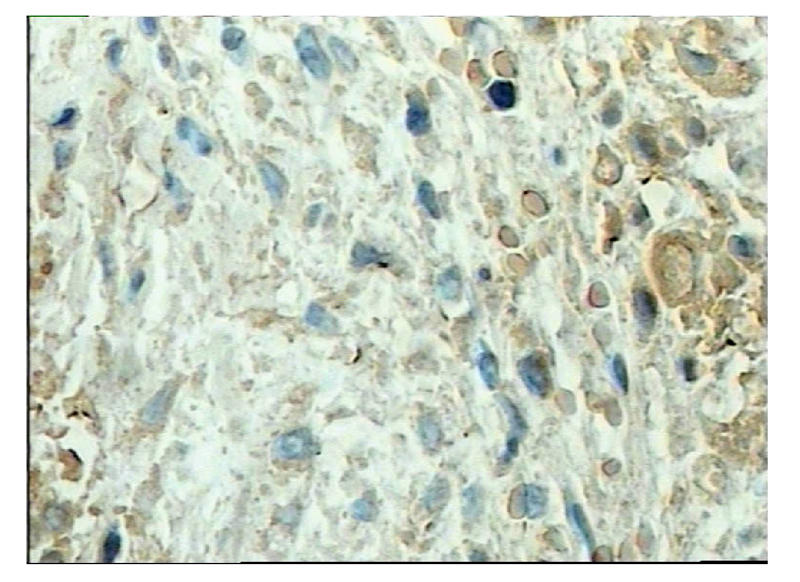
Immunhistochemical staining showed there were no cell stained double positive for BrdU and tropnin I in the control group (anti-BrdU and anti-tropnin I, immunoperoxidase, original magnification×800)
Fig. 6.

Immunohistochemical staining showed there were cells which were nuclear stained positive for BrdU, and formed partial neovasulalization in the hMSCs transplanted group (anti-BrdU and anti-tropnin I, immunoperoxidase, original magnification×800)
DISCUSSION
Cell transplantation may be an alternative treatment for heart failure in the future. It is necessary to understand what type of cell is the potential donor cell for cell transplantation. Allogenic cells have been successfully transplanted by several groups. Engrafted cells survived in normal myocardial and scar tissue with the use of cyclosporin A and improved heart function. Unfortunately, a long-term study showed that allogeneic cells were rejected 24 weeks after transplantation despite cyclosporin A therapy (Li et al., 1997). Because of the disadvantages of immunorejection, autologous cell transplantation would be an ideal technique. However, cardiac and skeletal muscle biopsies do not yield sufficient cell numbers to repair the damaged myocardium. Bone marrow is routinely aspirated clinically and contains multipotential progenitor cells such as bone marrow mesenchymal stem cells (MSCs). MSCs have significant capability for regeneration and multi-potential differentiation (Kocher et al., 2001).
Many researchers have found that cultured MSCs could be induced to differentiate into myogenic cells (Tomita et al., 1999; Kocher et al., 2001), neurons, skin cells, etc. Adding 5-aza (Kocher et al., 2001) into culture medium could facilitiate MSCs differentiation into myogenic cells, which may change the superficial type of the MSCs. In the present study we did not use any chemicals to induce human MSCs to differentiate into myogenic cells in in vitro culture. After in vitro culture for 4 weeks, FCM analysis indicated that hMSCs (positive for CD29 and CD166, but negative for CD34 and CD45) did not differentiate before transplantation. BrdU labelled cells survived, transdifferentiated into cardiomyocytes and induced angiogenesis at 4 weeks after cell transplantation. It had been shown that cardiac milieu (Wang et al., 2000) had important effect on myogenesis and angiogenesis. The ischemia myocardium may produce some myogenic factors, such as TNF, IL6 and CRP. Released myogenic factors are important in inducing immature MSCs to transdifferentate into cardiomyocyte-like cells. However, the precise mechanism of how MSCs could differentiate into cardiomyocytes-like cells is still unknown.
Our data showed that implanted hMSCs had transdifferented into cardiomyocytes reflected by positive staining for BrdU and troponin I, and also induced angiogenesis in the cell treated group. Engrafted hMSCs may have the function similar to that of endothelial progenitor cells that could contribute to neovascularization in the MI area. Cardiomyocytes generated from implanted hMSCs should limit scar expansion and prevent onset or development of heart failure. The neovascularization should improve the contractile function by improving blood flow to the hibernating cardiomyocytes. Thereafter, myogenesis and angiogenesis derived from engrafted hMSCs should contribute synergistically to cardiac functional improvement reflected by enhancement of ejection fraction after MI and cell transplantation for 4 weeks.
Our research showed that human bone marrow-derived mesenchymal stem cell can survive and transdifferentate into cardiomyocyte-like cells and induce regeneration of vascular structures, and further improve cardiac function in the MI region of the New Zealand rabbits’ hearts. Our results indicated that bone marrow-derived mesenchymal stem cell transplantation can improve heart function in infarcted hearts through myogenesis and neovascularization.
CONCLUSION
The hMSCs should be considered as an alternative transplant cell source to repair damaged myocardium after MI. When prepared donor cells were transplanted into the myocardial infarction region in the New Zealand rabbits MI model the hMSCs could transdifferentiate into cardiomyocytes and induced regeneration of vascular structures, then improve cardiac function at 4 weeks after MI and cell transplantation.
Footnotes
Project (No. 301549) supported by the Natural Science Foundation of Zhejiang, China
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MV, Yang XM, Downey JM. Smaller infarct after preconditioning does not predict extent of early functional improvement of reperfused heart. Am J Physiol. 1999;277:1754–1761. doi: 10.1152/ajpheart.1999.277.5.H1754. [DOI] [PubMed] [Google Scholar]
- 4.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocytes apoptosis reduces remolding and improve cardiac function. Nature medicine. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 5.Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardial of rat, a potential method for repair of infarcted myocardium? Circulation. 1996;94(supplII):332–336. [PubMed] [Google Scholar]
- 6.Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, Rao V, Ivanov J. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62(3):654–660. doi: 10.1016/s0003-4975(96)00389-x. [DOI] [PubMed] [Google Scholar]
- 7.Li RK, Mickle DA, Weisel RD, Mohabeer MK, Zhang J, Rao V, Li G, Merante F, Jia ZQ. Natural history of fetal rat cardiomyocytes transplantation into adult rat myocardial scar tissue. Circulation. 1997;96(9suppl):II179–II186. [PubMed] [Google Scholar]
- 8.Li RK, Jia ZQ, Weisel RD, Merante F, Mickle DA. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol. 1999;31(3):513–522. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- 9.Li RK, Weisel RD, Mickle DA, Jia ZQ, Kim EJ, Sakai T, Tomita S, Schwaetz L, Iwanochko M, Husain M, et al. Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J Thorac Cardiovasc Surg. 2000;119(1):62–68. doi: 10.1016/s0022-5223(00)70218-2. [DOI] [PubMed] [Google Scholar]
- 10.MacFarlane NG, Darnley GM, Smith GL. Cellular basis for contractile dysfunction in the diaphragm from a rabbit infarct model of heart failure. Am J Physiol Cell Physiol. 2000;278:C739–C746. doi: 10.1152/ajpcell.2000.278.4.C739. [DOI] [PubMed] [Google Scholar]
- 11.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcio S, Marotte F, Sabri A, LeDref O, Demirag M, Samuel JL, Rappaport L, Menasche P. Can grafted cardiomyocytes colonize Peri-Infarct myocardial areas. Circulation. 1996;94(9suppl):II337–II340. [PubMed] [Google Scholar]
- 13.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, David M. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci. 2001;98(18):10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennock GD, Yun DD, Agarwal PG, Spooner PH, Goldman S. Echocardiographic changes after myocardial infarction in a model of left ventricular diastolic dysfunction. Am J physiol. 1997;273:2018–2029. doi: 10.1152/ajpheart.1997.273.4.H2018. [DOI] [PubMed] [Google Scholar]
- 15.Podesser B, Wollenek G, Seitelberger R, Siegel H, Olner E, Firbas W. Epicardial branches of the coronary arteries and their distribution in the rabbit heart: The rabbit heart as a model of regional ischemia. The Anatomical Record. 1997;247:521–527. doi: 10.1002/(SICI)1097-0185(199704)247:4<521::AID-AR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Sakai T, Li RK, Weisel RD, Mickle DA, Jia ZQ, Tomita S, Kim EJ, Yau. TM. Fetal cell transplantation: a comparison of three cell types. J Thorac Cardiovasc Surg. 1999;118(4):715–724. doi: 10.1016/S0022-5223(99)70018-8. [DOI] [PubMed] [Google Scholar]
- 18.Sakai T, Li RK, Weisel RD, Mickle DA, Kim EJ, Tomita S, Jia ZQ, Yau TM. Autologous heart cell transplantation improves heart function after myocardial injury. Ann Thorac Surg. 1999;68:2074–2080. doi: 10.1016/s0003-4975(99)01148-0. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4(8):929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 20.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(19suppl):II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 21.Wang JS, Shum-Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorcac Cardiovasc Surg. 2000;120:999–1005. doi: 10.1067/mtc.2000.110250. [DOI] [PubMed] [Google Scholar]
- 22.Yablonka-Reuveni Z, Nameroff M. Skeletal muscle cell populations separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry. 1987;87(1):27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zibaitis A, Greentree D, Ma F, Marelli D, Duong M, Chiu RC. Myocardial regeneration with satellite cell implantation. Transplantation Proc. 1994;26:3294. [PubMed] [Google Scholar]