Abstract
To investigate inter- and intra-hemispheric electroencephalography (EEG) coherence at rest and during photic stimulation of patients with Alzheimer’s disease (AD). Thirty-five patients (12 males, 23 females; 52~64 y) and 33 sex- and age-matched controls (12 males, 21 females; 56~65 y) were recruited in the present study. EEG signals from C3-C4, P3-P4, T5-T6 and O1-O2 electrode pairs resulted from the inter-hemispheric action, and EEG signals from C3-P3, C4-P4, P3-O1, P4-O2, C3-O1, C4-O2, T5-O1 and T6-O2 electrode pairs resulted from the intra-hemispheric action. The influence of inter- and intra-hemispheric coherence on EEG activity with eyes closed was examined, using fast Fourier transformation from the 16 sampled channels. The frequencies of photic stimulation were fixed at 5, 10 and 15 Hz, respectively. The general decrease of AD patients in inter- and intra-hemispheric EEG coherence was more significant than that of the normal controls at the resting EEG, with most striking decrease observed in the alpha-1 (8.0–9.0 Hz) and alpha-2 (9.5–12.5 Hz) bands. During photic stimulation, inter- and intra-hemispheric EEG coherences of the AD patients having lower values in the alpha (9.5–10.5 Hz) band than those of the control group. It suggests that under stimulated and non-stimulated conditions, AD patients had impaired inter- and intra-hemispheric functional connections, indicating failure of brain activation in alpha-related frequency.
Keywords: Alzheimer’s disease (AD), Electroencephalography (EEG), Coherence, Photic stimulation, Cortical connectivity
INTRODUCTION
Electroencephalography (EEG) coherence obtained from spectral EEG analysis is a noninvasive technique for studying functional relationships between brain regions. It provides the sources of information about potential cortico-cortico interactions (Hogan et al., 2003). Appling coherence to the examination of function changes associated with the performance of a perceptual or cognitive task is considered to be useful for the assessment of cerebral functioning (Hogan et al., 2003; Beaumont et al., 1978; Tucker et al., 1986). Many papers have reported the deviating findings of background EEG activity in Alzheimer’s disease (AD) using, initially, data derived by visual inspection and, more recently, quantitative EEG analysis (Wada et al., 1997). However, there is little published information about inter- and intra-hemispheric EEG coherence abnormalities in AD patients.
Photic stimulation is the most common method of cerebral activation for routine EEG examinations. This technique has been validated as a useful tool to investigate neurological disorders such as epilepsy. A recent report revealed abnormal quantitative EEG during photic stimulation in schizophrenic patients (Jiang, 2002) which is easily performed, requires minimal cooperation from the patients, and has clinical use for evaluation of dementia patients (Politoff et al., 1992).
To our knowledge, there is no reported study on inter- and intra-hemispheric coherence during resting and photic stimulation. The objective of the present study is to estimate the inter- and intra-hemispheric EEG coherence in AD patients under the conditions of resting and photic stimulation, and to compare them with those of the normal controls.
METHODS
Subjects
The patient group consisted of 35 patients with DSM-IV (American Psychiatric Association, 1994) diagnosis of primary degenerative dementia, Alzheimer’s disease (23 women and 12 men; aged 52~64 y, mean 57.6±3.1 y). They were outpatients or inpatients from the Department of Psychiatry, Second Affiliated Hospital, Zhejiang University, China. The diagnosis was based on general medical, psychiatric and neuropsychological testing. Laboratory tests included neurological, serological and neuroimaging (MRI and/or CT) studies. None of the patients had received medications acting upon the central nervous system. Each patient was assessed with Functional Assessment Stages (FAST) and a Chinese version of the Mini-Mental State Examination (MMSE). According to the FAST results, 23 patients had mild (FAST 3), 12 patients moderate (FAST 4). Their MMSE scores were from 10 to 23, mean 15.2±3.4.
Thirty-three healthy volunteers (21 women and 12 men; aged 56~65 y, mean 59.0±3.2 y) were recruited as a control group, without personal or family history of psychiatric or neurological disease. The control group was not significantly different from the AD group in age and sex. Their MMSE score was above 25. All subjects were right-handed and agreed to participate in the study with full knowledge of the experimental nature of the research.
EEG recording and analysis
During EEG recording the subjects were in a resting state with eyes closed, sitting in a semi-darkened, electrically shielded, sound attenuated room. According to the international 10–20 systems, all EEGs were recorded by trained EEG technologists using a 16-channal electroencephalograph (EEG-NATION 918, Shanghai, China). EEG recordings were carried out with electrodes referenced to linked ear lobes with a time constant of 0.3 s and a high cut filter of 70 Hz. Impedance of electrode/skin was kept below 5000 Ω. Resting EEG was recorded for 5~10 min for each subject. Selection of segments recorded when the eyes were closed but awake was based on visual inspection of EEG and electro-oculographic (EOG) recordings. The EEG recording was repeated. Segments containing eye movements, blinks, or muscle activity were excluded from the analysis.
EEG coherence was calculated by means of the Fast Fourier Transform (FFT) method. One epoch consisted of 2 s, and 20 artifact-free epochs per subject were processed with a spectral resolution of 0.5 Hz. Coherence between two waveforms x and y was calculated as γ xy 2(f)=[G xy(f)]²/[G xx(f)G yy(f)], where G xy(f) is the mean cross-power density and G xx(f) and G yy(f) are the respective mean auto-power spectral densities. The details of the method for calculating the coherence are published in Jiang (2004). In this study, inter-hemispheric EEG coherence was measured between the following 4 homologous electrode pairs: left-right centrals (C3-C4), left-right parietals (P3-P4), left-right temporals (T5-T6) and left-right occipitals (O1-O2); intra-hemispheric EEG coherence was measured between the following 8 electrode pairs: left and right centro-parietal (C3-P3, C4-P4), left and right parieto-occipital (P3-O1, P4-O2), left and right centro-occipital (C3-O1, C4-O2), left and right temporo-occipital (T5-O1 and T6-O2). Data were banded into delta band (2.0–3.5 Hz), theta band (4.0–7.5 Hz), alpha-1 band (8.0–9.0 Hz), alpha-2 band (9.5–12.5 Hz), beta-1 band (13–19.5 Hz) and beta-2 band (20–28 Hz).
Photic stimulation
After routine EEG examination, the subjects were given photic stimulation consisting of a white flicker with flash intensity of 5020 cd/m2 at 5, 10 and 15 Hz, respectively, delivered by a photo-stimulator and a stroboscopic lamp placed 25 cm from the subjects’ eyes. Photic stimulation was given at random order for 30 s with interval of 10 s. Since artifacts occasionally contaminated the initial part of the recording, the 2 s segment after the beginning of photic stimulation was excluded from the analyses. Ten artifact-free 2 s epochs were selected. EEG coherence during photic stimulation was analyzed using the same procedure described above for the resting condition. Since photic stimulation mainly induces fundamental harmonic responses, inter- and intra-hemispheric EEG coherence was measured for the three stimulus conditions of the experiment on the frequency corresponding to each stimulus frequency of 5, 10 and 15 Hz, respectively.
Statistics
In this study, Fisher’s Z transformation was used to normalize the distribution of coherence values. Differences in inter- and intra-hemispheric EEG coherence values between the AD patients and the normal controls were analyzed on each frequency band by using two-way analyses of variance (ANOVA) with a grouping factor (patients vs controls) and a within-subject factor (electrode pairs). As in the EEG recording and analysis method, for analysis of inter-hemispheric coherence the within subject factor, i.e., electrode pair involved four levels; for analysis of intra-hemispheric coherence, electrode pair involved eight levels. Separate ANOVAs were conducted for the different frequency bands in order to test inter- and intra-hemispheric EEG coherence, respectively. The testing condition, such as resting and photic stimulation, is used as a condition variable. Then, two-tailed student’s t-test was conducted to compare the coherence value between groups. Statistical significance was defined as P<0.05.
RESULTS
Inter- and intra-hemispheric EEG coherence in resting state
Tables 1 and 2 show the inter- and intra-hemispheric coherence values in the resting EEG as well as the analysis results by ANOVA for the control and AD groups on different frequency bands. In the resting condition, there were differences of inter- and intra-hemispheric EEG coherence values between the two groups.
Table 1.
Inter-hemispheric coherence values in the resting EEG (mean±SD) and analysis results by ANOVA
| Frequency bands (Hz) | C3-C4 | P3-P4 | T5-T6 | O1-O2 | ANOVA |
|||
| F | df | P | ||||||
| Delta (2.0–3.5) | AD | 0.87±0.31 | 0.95±0.31 | 0.41±0.12 | 1.00±0.32 | 4.17 | 1 | 0.04* |
| Con | 0.93±0.11 | 1.14±0.23 | 0.52±0.25 | 1.14±0.36 | ||||
| Theta (4.0–7.5) | AD | 0.74±0.16 | 0.89±0.19 | 0.34±0.08 | 0.92±0.34 | 0.13 | 1 | 0.71 |
| Con | 0.75±0.22 | 0.92±0.31 | 0.36±0.17 | 0.93±0.28 | ||||
| Alpha-1 (8.0–9.5) | AD | 0.79±0.22* | 0.82±0.27* | 0.34±0.11 | 0.89±0.28 | 13.79 | 1 | 0.004** |
| Con | 1.17±0.26 | 1.09±0.24 | 0.49±0.31 | 0.98±0.33 | ||||
| Alpha-2 (10.0–12.5) | AD | 0.78±0.28* | 0.85±0.27 | 0.31±0.18* | 0.97±0.22 | 18.91 | 1 | 0.0001** |
| Con | 1.21±0.19 | 1.13±0.41 | 0.53±0.16 | 1.21±0.43 | ||||
| Beta-1 (13.0–19.5) | AD | 0.51±0.15 | 0.64±0.18 | 0.29±0.10 | 0.75±0.29 | 0.59 | 1 | 0.44 |
| Con | 0.65±0.14 | 0.70±0.20 | 0.30±0.13 | 0.81±0.21 | ||||
| Beta-2 (20.0–29.5) | AD | 0.47±0.13 | 0.65±0.17 | 0.27±0.08 | 0.72±0.31 | 0.66 | 1 | 0.41 |
| Con | 0.57±0.15 | 0.71±0.25 | 0.36±0.11 | 0.82±0.21 | ||||
Coherence values transformed to Fisher’s Z scores, compared between two groups (two-tailed t-test). P<0.05
Coherence values transformed to Fisher’s Z scores, compared between two groups (two-tailed t-test). P<0.01
Con: the normal controls; AD: the patients of Alzheimer’s disease
Table 2.
Intra-hemispheric coherence values in the resting EEG (mean±SD) and analysis results by ANOVA*
| Frequency bands (Hz) | C3-P3 | C4-P4 | P3-O1 | P4-O2 | C3-O1 | C4-O2 | T5-O1 | T6-O2 | ANOVA |
|||
| F | df | P | ||||||||||
| Delta (2.0–3.5) | AD | 1.36±0.18 | 1.40±0.14 | 1.30±0.17 | 1.25±0.22 | 0.75±0.14 | 0.73±0.13* | 1.29±0.24 | 1.16±0.16 | 10.79 | 1 | 0.001** |
| Con | 1.52±0.28 | 1.59±0.50 | 1.38±0.21 | 1.33±0.22 | 0.91±0.24 | 0.89±0.23 | 1.34±0.17 | 1.30±0.23 | ||||
| Theta (4.0–7.5) | AD | 1.36±0.13 | 1.36±0.19 | 1.20±0.16 | 1.06±0.18** | 0.70±0.16 | 0.63±0.23 | 1.04±0.27* | 1.13±0.11 | 4.58 | 1 | 0.03* |
| Con | 1.43±0.21 | 1.37±0.28 | 1.26±0.25 | 1.30±0.17 | 0.75±0.24 | 0.77±0.15 | 1.28±0.29 | 1.13±0.14 | ||||
| Alpha-1 (8.0–9.5) | AD | 1.35±0.16 | 1.32±0.26 | 1.14±0.23 | 1.23±0.33 | 0.63±0.19 | 0.69±0.27 | 1.21±0.26 | 1.13±0.22** | 8.33 | 1 | 0.004** |
| Con | 1.51±0.32 | 1.52±0.25 | 1.23±0.47 | 1.24±0.35 | 0.75±0.29 | 0.78±0.25 | 1.27±0.34 | 1.23±0.43 | ||||
| Alpha-2 (10.0–12.5) | AD | 1.34±0.28 | 1.34±0.25** | 1.16±0.23 | 1.26±0.21 | 0.62±0.26 | 0.70±0.25 | 1.24±0.22 | 1.20±0.23 | 6.88 | 1 | 0.009** |
| Con | 1.45±0.35 | 1.54±0.39 | 1.26±0.60 | 1.33±0.47 | 0.79±0.40 | 0.87±0.32 | 1.36±0.36 | 1.48±0.22 | ||||
| Beta-1 (13.0–19.5) | AD | 1.26±0.17 | 1.25±0.14 | 1.10±0.16 | 1.13±0.25 | 0.62±0.13 | 0.64±0.17 | 1.22±0.12 | 1.07±0.25 | 3.85 | 1 | 0.05 |
| Con | 1.38±0.23 | 1.33±0.22 | 1.20±0.22 | 1.18±0.19 | 0.67±0.19 | 0.70±0.14 | 1.17±0.17 | 1.15±0.19 | ||||
| Beta-2 (20.0–29.5) | AD | 1.12±0.16 | 1.17±0.15 | 1.11±0.17 | 1.12±0.14 | 0.61±0.13 | 0.67±0.10 | 1.12±0.23 | 1.04±0.27 | 0.02 | 1 | 0.91 |
| Con | 1.20±0.17 | 1.23±0.24 | 1.14±0.20 | 1.13±0.22 | 0.62±0.15 | 0.68±0.15 | 1.20±0.13 | 1.11±0.17 | ||||
Coherence values transformed to Fisher’s Z scores, compared between two groups (two-tailed t-test). P<0.05
Coherence values transformed to Fisher’s Z scores, compared between two groups (two-tailed t-test). P<0.01
Con: the normal controls; AD: the patients of Alzheimer’s disease
As shown in Table 1, in inter-hemispheric coherence, the significant group differences are in the delta [F(1,3)=4.17, P=0.04], alpha-1 [F(1,3)=13.79, P=0.004] and alpha-2 [F(1,3)=18.91, P=0.0001] bands. Post-hoc analysis by t-test indicated that the AD patients had significantly lower inter-hemispheric EEG coherence than the control subjects at C3-C4 and P3-P4 pairs for the alpha-1 band, as well as at C3-C4 and T5-T6 pairs for the alpha-2 (P<0.05). No significant group difference was found, however, in the theta [F(1,3)=0.13, P=0.71], beta-1 [F(1,3)=0.59, P=0.44] and beta-2 [F(1,3)=0.66, P=0.41] bands.
As seen in Table 2, in intra-hemispheric coherence, significant group differences are in the delta [F(1,7)=10.79, P=0.001], theta [F(1,7)=4.58, P=0.03], alpha-1 [F(1,7)=8.33, P=0.004] and alpha-2 [F(1,7)=6.88, P=0.009] bands. Post-hoc analysis revealed that the AD patients had significantly lower intra-hemispheric EEG coherence in delta band at C4-O2 pairs, in theta band at P4-O2 and T5-O1 pairs, in alpha-1 band at T6-O2 pairs, and in alpha-2 band at C4-P4 pairs (P<0.05). There was no significant group difference found, however, in beta-1 [F(1,7)=3.85, P=0.05] and beta-2 [F(1,7)=0.02, P=0.91] bands.
Inter- and intra-hemispheric EEG coherence during photic stimulation
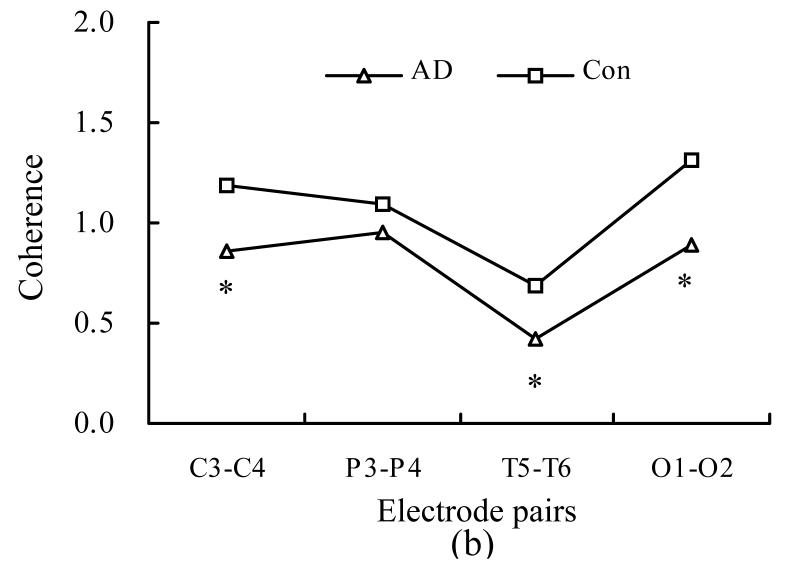
Fig.1 shows the inter-hemispheric EEG coherence during photic stimulation on different bands for the control and AD groups. Two-way ANOVA revealed significant group differences in frequencies of the alpha band (10 Hz) and beta band (15 Hz) corresponding to photic stimulation at 10 Hz [F(1,3)=17.33, P=0.001] and 15 Hz [F(1,3)=10.56, P=0.0018]. No significant group difference was found, however, in the frequency corresponding to photic stimulation at 5 Hz [F(1,3)=2.34, P=0.13]. A subsequent t-test revealed that under the condition of photic stimulation, the AD patients had significantly lower values of inter-hemispheric coherence in the C3-C4, T5-T6 and O1-O2 pairs for alpha band, P3-P4 and O1-O2 pairs for beta band.
Fig. 1.
Inter-hemispheric EEG coherence values at different electrode pairs during photic stimulation (PS) in Alzheimer’s disease (AD) patients and controls subjects (Con). (a) Theta band (5 Hz) during PS 5 Hz; (b) Alpha band (10 Hz) during PS 10 Hz; (c) Beta band (15 Hz) during PS 15 Hz (* P<0.05)
Fig.2 shows the intra-hemispheric EEG coherence during photic stimulation on different bands in the control and AD groups. The analysis results by ANOVA showed significant group differences in the frequency corresponding to photic stimulation at 5 Hz [F(1,7)=6.6, P=0.01], 10 Hz [F(1,7)=5.80, P=0.01] and 15 Hz [F(1,7)=17.63, P=0.001]. The AD patients had significantly lower values of intra-hemispheric coherence in C4-P4 and C3-O1 pairs for theta band, C3-P3, C3-O1 and T6-O2 pairs for alpha band, P3-O1, P4-O2, C3-O1, C4-O2 and T6-O2 pairs for beta band.
Fig. 2.
Intra-hemispheric EEG coherence values at different electrode pairs during photic stimulation (PS) in Alzheimer’s disease (AD) patients and controls subjects (Con). (a) Theta band (5 Hz) during PS 5 Hz; (b) Alpha band (10 Hz) during PS 10 Hz; (c) Beta band (15 Hz) during PS 15 Hz (* P<0.05, ** P<0.01)
DISCUSSION
The present study is focused on the inter- and intra-hemispheric EEG coherence during rest and photic stimulation of the AD patients. The experimental results in Tables 1 and 2 indicated that AD patients had smaller coherence values during resting than those of the sex- and age-matched normal controls. Abnormal changes of inter- and intra-hemispheric coherence EEG suggest that the decrease of coherence may be interpreted as due to AD causing diffuse and widespread cerebral degeneration, which result in fewer neuronal connections. Moreover the lower coherence values may also reflect impairment of inter- and intra-hemispheric functional connections.
As shown in Figs.1b and 2b, significant group differences are found in alpha band under the photic stimulation. The AD patients show significantly lower inter- and intra-hemispheric EEG coherence than that of the normal controls. This experimental result provided evidence that AD patients show lower coherence during photic stimulation, suggesting failure of stimulation-related brain activation in AD. In this study, 23 of the 35 AD patients were diagnosed as having mild dementia, suggesting that the lower inter- and intra-hemispheric EEG coherence during rest and photic stimulation may appear even in the early stage of AD.
The natural EEG frequency as expressed in the spontaneous alpha activity is responsible for the generation of the alpha frequency component of evoked potentials. Some EEG studies provided evidences that the alpha band is involved in the process of information in brain. Moreover, recent studies (Basar et al., 1997; Schurmann and Basar, 2001) suggested that when an external sensory stimulation is applied to the brain, alpha rhythms may have functional correlation with primary sensory processing and preparatory processes. This study showed lower EEG coherence in the alpha band not only during rest, but also during photic stimulation of AD patients. Therefore, our experimental result reflects that alpha band oscillation involving the process of information may be responsible for the less expressed alpha coherence during photic stimulation of AD patients. Moreover, the observed lower coherence values in both conditions suggest that the lower alpha coherence in AD patients at rest may be responsible for the lower coherence under the photic stimulation.
AD patients had been found to have atrophy of corpus callosum as compared with age-matched control subjects by using neuroimaging techniques and postmortern brains (Vermersch et al., 1994). Moreover, Koeda et al.(1995) showed a decrease in inter-hemispheric coherence values in patients with agenesis of the corpus callosum. Inter-hemispheric EEG coherence is assumed to index functional coupling between the brain regions under the electrodes, therefore, it is considered that the corpus callosum plays an essential role on is critically involved in inter-hemispheric EEG synchronization. The present findings suggest that AD patients had a defective interaction between hemispheres, and reflect an impairment of inter-hemispheric function connectivity across the corpus callosum.
It is well known that cortical cholinergic dysfunction and loss of cholinergic neurons in the nucleus basalis of Meynert, a main source of the cholinergic projection to the cerebral cortex in patients with AD. Sloan et al.(1992) showed that the anticholinergic drug, scopolamine, can reduce inter-hemispheric EEG coherence in the alpha and beta bands when administered to normal subjects. In addition, Kikuchi et al.(2000) reported that scopolamine significantly reduced inter-hemispheric EEG coherence in the delta and beta-1 bands in the resting state, caused significant increase during photic stimulation, and provided evidence that central cholinergic dysfunction can alter inter-hemispheric functional connectivity. Our results suggest that AD patients had a defective interaction between hemispheres as well as in the hemispheres.
CONCLUSION
The AD patients had a general significant decrease in inter- and intra-hemispheric EEG coherence than that of normal controls at rest and during photic stimulation, with most striking decrease observed in the alpha bands. It was revealed that under the stimulation and non-stimulation conditions, AD patients have impairment of inter- and intra-hemispheric functional connections, a failure of brain activation in alpha-related frequency.
Acknowledgments
The author sincerely thanks the members of Clinic EEG Laboratory, Second Affiliated Hospital, School of Medicine, Zhejiang University, for their help in this study.
Footnotes
Project supported by the Foundation from the Health Bureau of Zhejiang Province (2004-2005) and the Science & Technology project of Zhejiang Province (2004-2005), China
References
- 1.Basar E, Yordanova J, Kolev V, Basar-Eroglu C. Is the alpha rhythm a control parameter for brain responses? Boil Cybern. 1997;76:471–480. doi: 10.1007/s004220050360. [DOI] [PubMed] [Google Scholar]
- 2.Beaumont JG, Mayes AR, Rugg MD. Asymmetry in EEG alpha coherence and power: effects of task and sex. Electroencephalogy Clin Neurophysiol. 1978;45:393–401. doi: 10.1016/0013-4694(78)90190-6. [DOI] [PubMed] [Google Scholar]
- 3.Hogan MJ, Swanwick GRJ, Kaiser J, Rowan M, Lawlor B. Memory-related EEG power and coherence reduction in mild Alzheimer’s disease. Int J Psychophysiol. 2003;49:147–163. doi: 10.1016/s0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 4.Jiang ZY. A study of EEG Photic driving in schizophrenia. Chinese Journal of Nervous and Mental Disease. 2002;28(3):198–200. (in Chinese) [Google Scholar]
- 5.Jiang ZY. The research of diagnosis of Alzheimer’s disease based on the coherence analysis of EEG signal. Chinese Journal of Sensor and Actuator. 2004;17(3):363–366. (in Chinese) [Google Scholar]
- 6.Kikuchi M, Wada Y, Koshino Y, Nanbu Y, Hashino T. Effects of scopolamine on inter-hemispheric EEG coherence in healthy subjects: analysis during rest and photic stimulation. Electroencephalogr Clin Neurophysiol. 2000;31:109–115. doi: 10.1177/155005940003100210. [DOI] [PubMed] [Google Scholar]
- 7.Koeda T, Knyazeva M, Njiokiktjien C, Jonkman EJ, De Sonneville L, Vildavsky V. The EEG in acallosal children. Coherence values in the resting state: left hemisphere compensatory mechanism? Electroencephalogr Clin Neurophysiol. 1995;95:397–407. doi: 10.1016/0013-4694(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 8.Politoff AL, Monson N, Hass P, Stadter R. Decreased alpha bandwidth responsiveness to photic driving in Alzheimer disease. Electroencephalogr Clin Neurophysiol. 1992;82:45–52. doi: 10.1016/0013-4694(92)90181-g. [DOI] [PubMed] [Google Scholar]
- 9.Schurmann M, Basar E. Functional aspects of alpha oscillations in the EEG. Int J Psychophysiol. 2001;39:151–158. doi: 10.1016/s0167-8760(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 10.Sloan EP, Fenton GW, Standage KP. Anticholinergic drug effects on quantitative electroencephalogram, visualevoked potential, and verbal memory. Biol Psychiatry. 1992;31:600–606. doi: 10.1016/0006-3223(92)90246-v. [DOI] [PubMed] [Google Scholar]
- 11.Tucker DM, Roth DL, Bair TB. Function connections among cortical regions: topography of EEG coherence. Electroencephalogr Clin Neurophysiol. 1986;63:242–250. doi: 10.1016/0013-4694(86)90092-1. [DOI] [PubMed] [Google Scholar]
- 12.Vermersch P, Scheltens P, Barkhof F, Steinling M, Leys D. Evidence for atrophy of the corpus callosum in Alzheimer’s disease. Eur Neurol. 1994;34(2):83–86. doi: 10.1159/000117014. [DOI] [PubMed] [Google Scholar]
- 13.Wada Y, Nanbu Y, Jiang ZY, Koshino Y, Yamaguchi N, Hashimoto T. Electroencephalographic abnormalities in patients with presenile dementia of the Alzheimer type: quantitative analysis at rest and during photic stimulation. Biol Psychiatry. 1997;41:217–225. doi: 10.1016/0006-3223(95)00651-6. [DOI] [PubMed] [Google Scholar]