Abstract
Copper accumulation and intracellular distribution in Elsholtzia splendens, a native Chinese Cu-tolerant and accumulating plant species, was investigated by transmission electron microscope (TEM) and gradient centrifugation techniques. Copper concentrations in roots, stems and leaves of E. splendens increased with increasing Cu levels in solution. After exposure to 500 μmol/L Cu for 8 d, about 1000 mg/kg Cu were accumulated in the stem and 250 mg/kg Cu in the leaf of E. splendens. At 50 µmol/L Cu, no significant toxicity was observed in the chloroplast and mitochondrion within its leaf cells, but separation appeared at the cytoplasm and the cell wall within the root cells. At >250 µmol/L Cu, both root and leaf organelles in E. splendens were damaged heavily by excessive Cu in vivo. Copper subcellular localization in the plant leaf after 8 days’ exposure to 500 µmol/L Cu using gradient centrifugation techniques was found to be decreased in the order: chloroplast>cell wall>soluble fraction>other organelles. The plant root cell wall was found to be the site of highest Cu localization. Increase of Cu exposure time from 8 d to 16 d, increased slightly Cu concentration in cell wall fraction in roots and leaves, while that in the chloroplast fraction decreased in leaves of the plants grown in both 0.25 μmol/L and 500 μmol/L Cu. TEM confirmed that much more Cu localized in cell walls of E. splendens roots and leaves, but also more Cu localized in E. splendens’ chloroplast when the plant is exposed to Cu levels>250 μmol/L, as compared to those in the plant grown in 0.25 μmol/L Cu. Copper treatment at levels>250 μmol/L caused pronounced damage in the leaf chloroplast and root organelles. Copper localization in cell walls and chloroplasts could mainly account for the high detoxification of Cu in E. splendens.
Keywords: Cell wall, Chloroplast, Cu detoxification, Elsholtzia splendens, Ultrastructural distribution
INTRODUCTION
Copper is an essential nutrient for plant growth and development, although it is highly phytotoxic at excessive levels. Mining, smelting and land applications of sewage sludge, together use of fungicides containing Cu, and other human activities, lead to widespread soil contamination with copper. Plants growing on Cu contaminated soils develop resistance to Cu in the soil and detoxify Cu inside the plant cell (Ernst et al., 1992; Macnair, 1993). E. splendens is reportedly a native Chinese Cu-tolerant and accumulating plant species based on mined areas investigation and greenhouse hydroponics and pot experiments (Yang et al., 1998; Song et al., 2004). It can survive normally in soil contaminated with more than 3000 mg/kg Cu (Tang et al., 1999), suggesting the existence of defense mechanisms in E. splendens against the harmful effects induced by copper toxicity.
Many studies had been carried out on the effect of copper on the growth, mineral nutrition and metabolism of plants. Copper excess reduces plant growth (Maksymiec et al., 1995), phytosynthetic activity (Lidon et al., 1993) and the quantum yield of PSII phytochemistry which was detected by chlorophyll fluorescence (Maksymiec and Baszynski, 1999). Copper excess may also result in membrane damage by Cu binding to the sulfhydryl groups of membrane proteins (Kennedy and Gonsalves, 1987). For most crop species, the critical level for copper toxicity in leaves is above 20−30 mg/kg dry weight (Robson and Reuter, 1981). In plant cells under normal conditions, free copper is virtually nonexistent as the cell has an overcapacity for copper sequestration (Rae et al., 1999). However, under conditions of copper overload, free copper ions can accumulate and react to generate hydroxyl radicals participating in reactions that can adversely modify proteins, lipids, and nucleic acids (Halliwell and Gutteridge, 1984). Many metal tolerant organisms are also accumulators of metals. Therefore, other mechanisms of avoiding the toxic effects of accumulated metal may induce metal sequestration in organelles or metal complexation by metal-binding molecules or metal precipitation.
In this study, the patterns of Cu localization and subcellular distribution within the root and leaf cells of E. splendens at different Cu levels were studied by TEM and different speed centriguation techniques for further elucidating Cu intracellular detoxification in E. splendens.
MATERIALS AND METHODS
E. splendens seeds, collected from adult plants growing on copper mining deposit in Zhuji County of Zhejiang Province of China, were germinated as described by Yang et al.(2002) until 3-week seedlings were observed. The uniform 3-week seedlings were transferred to a nutrient solution for 14 d pre-culture (6-leaf seedlings). High Cu (500 μmol/L) and low Cu (0.25 μmol/L) treatments were applied, added as CuSO4·5H2O, the plants were grown for 16 d in the greenhouse. Plants were harvested at day 8 and 16 of treatment for assay of Cu subcellular distribution.
For TEM assay, younger seedlings (4-leaf seedlings) were selected for Cu treatments: with 0.25, 50, 100, 250 and 500 μmol/L, added as CuSO4·5H2O, and the plants were grown for 8 d in the greenhouse.
The composition of the full strength nutrient solution was (in μmol/L): 700 K2SO4, 100 KCl, 2000 Ca(NO3)2·4H2O, 500 MgSO4·7H2O, 100 KH2PO4, 10 H3BO3, 0.5 MnSO4·H2O, 0.5 ZnSO4·7H2O, 0.2 CuSO4·5H2O, 0.01 (NH4)6Mo7O24, 100 Fe-EDTA. The experiment was randomly arranged with three replicates for each treatment. The solution was aerated and maintained at pH 5.8±0.3 adjusted daily with 0.1 mol/L NaOH or 0.1 mol/L HCl, and was renewed every 4 d during the experiment. All experiments were conducted in a greenhouse with the temperature ranging from (28±2) °C (day) to (15±2) °C (night), without supplementary artificial light.
At harvest, roots of intact plants were rinsed with distilled water, and then immersed in 5 mmol/L Pb(NO3)2 for 20 min to remove the putative adsorbed Cu2+ (Harrison et al., 1984), then for TEM assays, subcellular distribution of Cu, and metal analysis.
1. TEM assay: small sections of leaves and roots, 1–3 mm in length, were fixed in 4% glutaraldehyde (v/v) in 0.2 mol/L PBS (sodium phosphate buffer, pH 7.2) for 6–8 h and post-fixed in 1% OsO4 [Osmium (VIII) oxide] for 1 h, then in 0.2 mol/L PBS (pH 7.2) for 1–2 h. Dehydration was performed in a graded ethanol series (50%, 60%, 70%, 80%, 90%, 95% and 100%) followed by acetone, then samples were filtrated and embedded in Spurr’s resin. Ultra-thin sections (80 nm) were prepared and mounted on copper grids for viewing under transmission electron microscope (JEOL TEM-1200EX) at an accelerating voltage of 60.0 kV or 80.0 kV.
2. Subcellular distribution of Cu: fresh samples of plant roots and leaves of high Cu (500 µmol/L) and low Cu (0.25 μmol/L) were selected after 8 d and 16 d, weighed and ground in a quartz mortar with 10 mmol/L Tris-HCl buffer solution (pH 7.4, containing 2.5% ascorbic acid) added at the ratio of 1:5 according to the method of Brooks et al.(1981). Cells were separated into different fractions: cell wall, chloroplast, nucleus, mitochondrion, ribosome and soluble fraction by gradient centrifugation technique at 4 °C (J2-HS, BECKMAN), as shown in Fig.1. The different cell fractions obtained were oven-dried at 65 °C, ashed at 550 °C for 6 h, and dissolved in 1:1 (v:v) HNO3, then the subcellular fractions of Cu in root and leaf of E. splendens were estimated by ICP-OES (Model IRAS-AP, TJA).
Fig. 1.
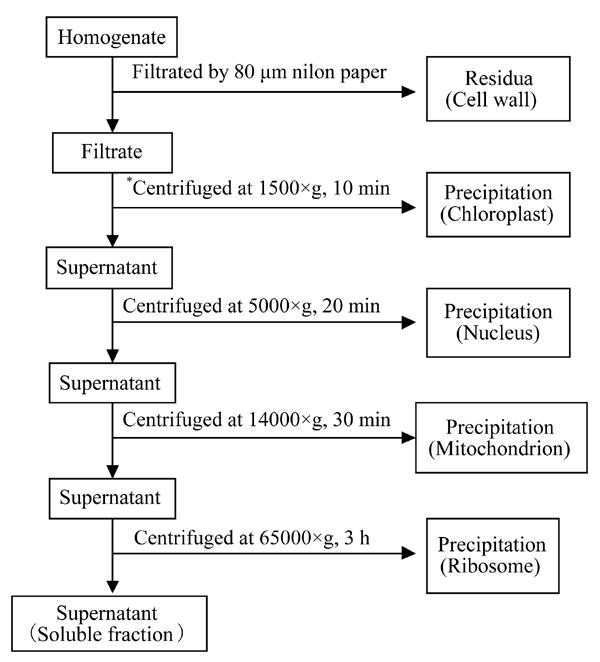
Schematic of the separation of the subcellular fractions by differential speed centrifugation (*for root: centrifuged at 2500×g for 20 min)
3. Metal analysis: roots, stems and leaves of E. splendens were separated, washed with distilled water, and oven-dried at 65 °C. The dried plant materials were ground in a stainless steel mill and passed through a 0.25-mm sieve, then ashed at 550 °C for 6 h, and dissolved in 1:1 (v:v) HNO3. Copper concentrations in the plant digests were analyzed by ICP-OES (Model IRAS-AP, TJA).
All the data were analyzed by SPSS (Version 11.0) with three replicates. One-way ANOVA was employed to evaluate whether the means were significantly different at P<0.01.
RESULTS
Copper accumulation in E. splendens
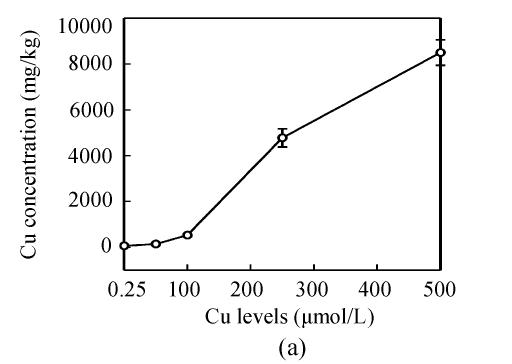
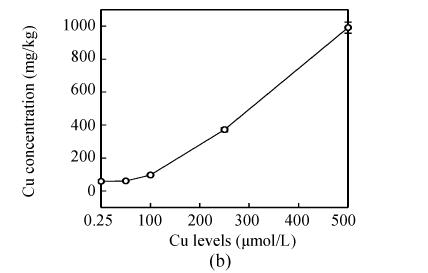
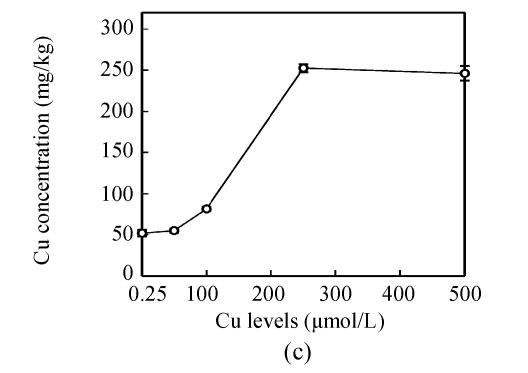
Copper levels in the roots, stems and leaves of the plants increased at increased Cu levels in the nutrient solution, and Cu distribution in the plant organs were: root>>stem>leaf (Fig.2). After Cu exposure for 8 d, slightly increased Cu in the stems and leaves of E. splendens were noted at 50 μmol/L Cu, and it significantly increased at >100 μmol/L Cu as compared to that at 0.25 μmol/L Cu. At 500 μmol/L Cu, stem Cu was about 1000 mg/kg, and leaf Cu was about 250 mg/kg.
Fig. 2.
Cu concentration in (a) roots, (b) stems and (c) leaves of E. splendens at different Cu levels for 8 d. All the data are the means of 3 replications
Transmission electron microscopy
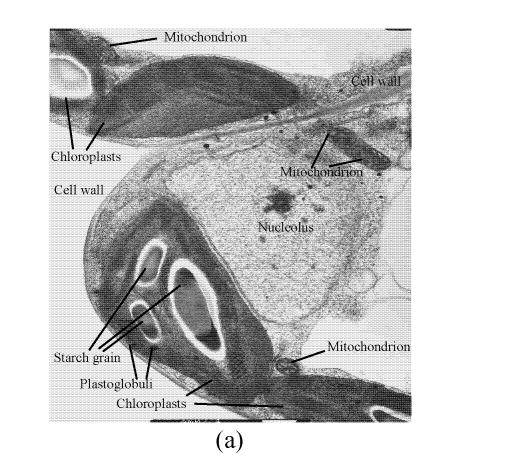
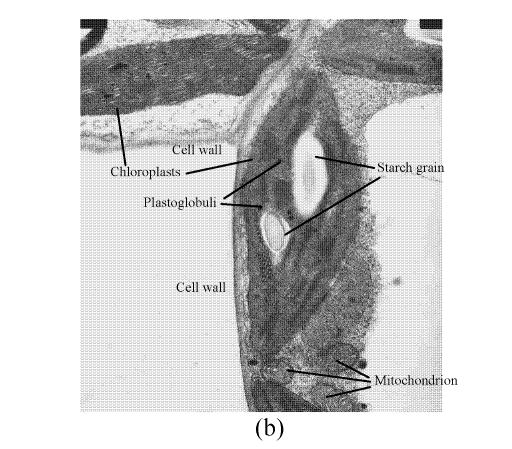
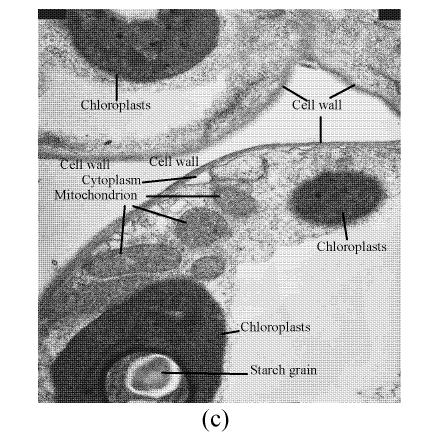
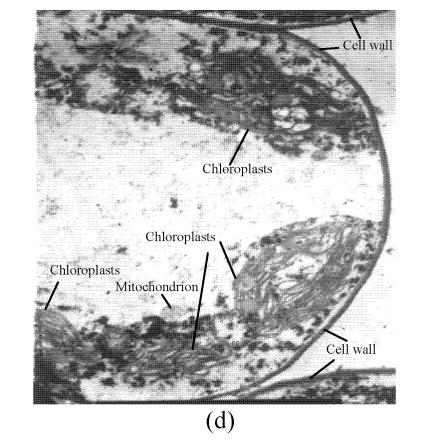
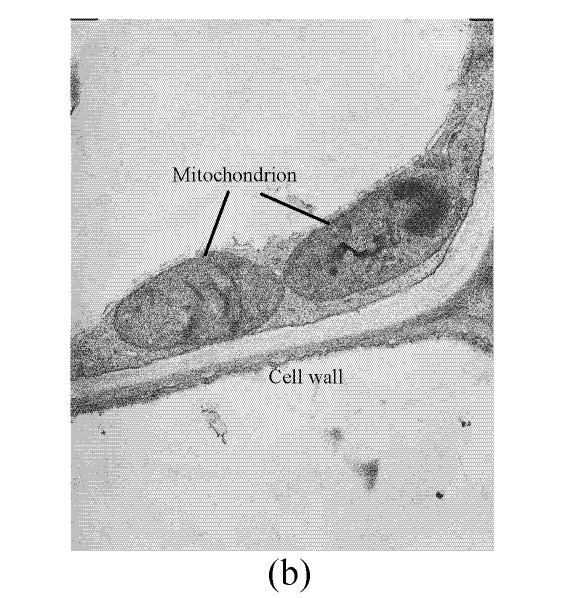
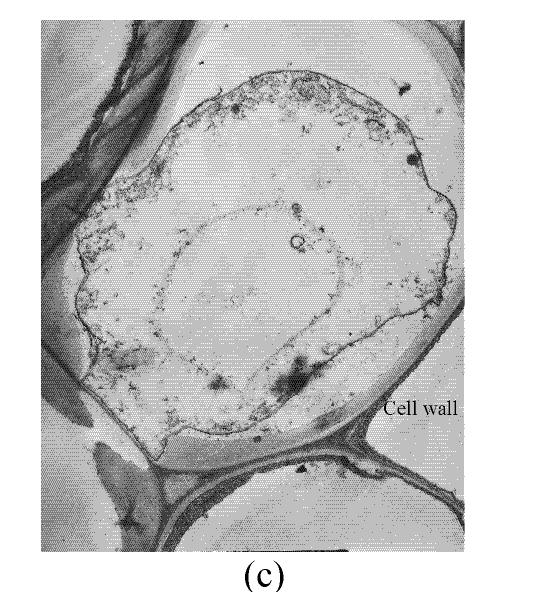
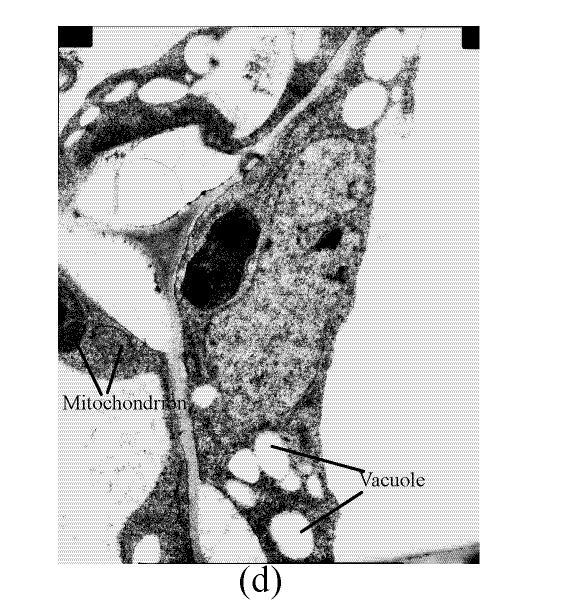
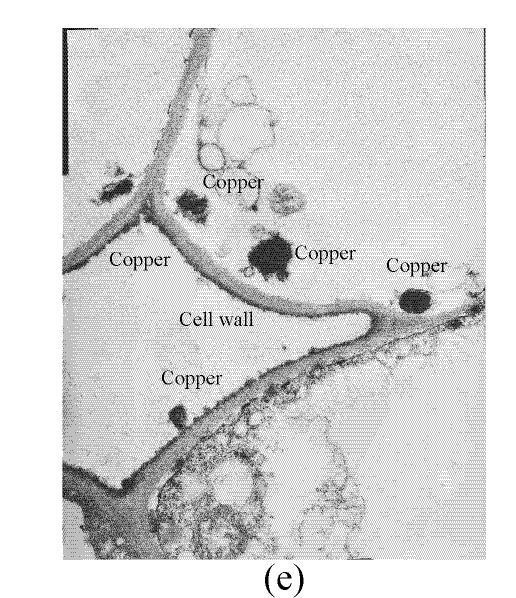
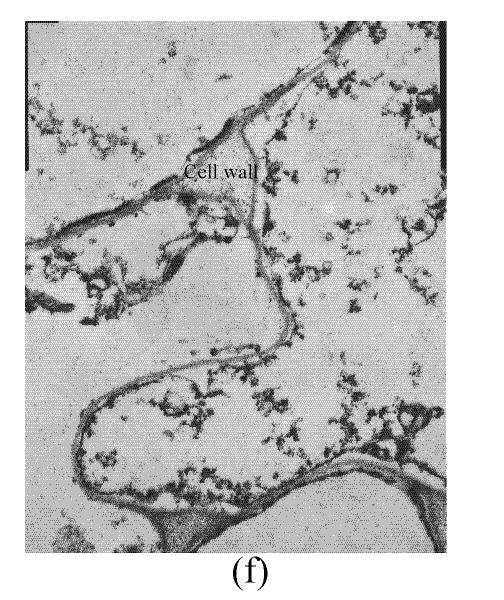
In a section of the E. splendens leaf (3rd leaf from the top) of the control, multiple chloroplasts were within the leaf cells, and the thylakoid array in chloroplast paralleled the long axes. Numerous plastoglobuli and starch grain were evident within the chloroplasts. Small cristates in the mitochondria arrayed closely (Fig.3a). At 50 µmol/L Cu, some thylakoid arrays in the chloroplast were tortuous. The morphology of chloroplast and mitochondrion were intact. The cytoplasm was not separated from the cell wall, the nucleus structure was intact (Fig.3b). At 250 µmol/L Cu, the chloroplasts were not spherical, and the thylakoid array structure became smaller and was damaged heavily. The mitochondrion was damaged heavily, and zigzag cytoplasms were noted (Fig.3c). At 500 µmol/L Cu, the membranes in the chloroplast, mitochondrion and the cytoplasm were damaged heavily (Fig.3d), the thylakoid arrays disassembled and chloroplasts were damaged heavily. Large amounts of copper were intensively deposited in the chloroplast membrane and the cell wall. These observations indicated that, as the Cu increased to 500 µmol/L, the chloroplast was adversely affected by excessive Cu. At 50 µmol/L Cu, no significant toxicity was observed in the chloroplast and the mitochondrion within the leaf cells of E. splendens, as compared to the control. While at 500 µmol/L Cu, the leaf organelles of E. splendens were damaged heavily by excessive Cu in vivo.
Fig. 3.
TEM photos of E. splendens leaf cell (a) Control (×10000); (b) 50 μmol/L (×15000); (c) 250 μmol/L (×12000); (d) 500 μmol/L (×8000)
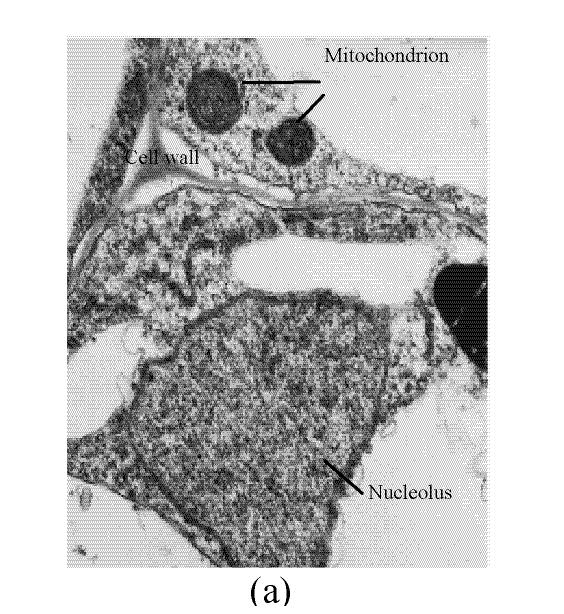
In the control, the multiple mitochondrions within the root cells were intact, most cristates in the mitochondrion double-deck membranes were evenly distributed except for small portions that were distributed unevenly (Fig.4a). At 50 µmol/L Cu, some damage was noted in the mitochondrion membranes (Fig.4b). Separation appeared between the cytoplasm and the cell wall, most of the containers were distributed unevenly, with cristates disappearing from them except for the small portions in containers that were kept intact (Fig.4c). The structure of nucleus and of nucleolus was kept intact as compared to those of the control, and many more vacuoles were within the root cell (Fig.4d). At 100–250 µmol/L Cu, the root organelles were heavily damaged (Figs.4e and 4f). Hollowed root cells appeared, big dark copper particles precipitated within the root cells and saturated at the root cell walls (Fig.4e) and at the root cell membrane (Fig.4f). Compared to the nucleus in the root cells of E. splendens, the mitochondrions are more easily destroyed by excessive Cu in vivo.
Fig. 4.
TEM photos of E. splendens root cell (a) Control (×12000); (b) 50 μmol/L (×15000); (c) 50 μmol/L (×6000); (d) 50 μmol/L (×10000); (e) 250 μmol/L (×10000); (f) 100 μmol/L (×10000)
Copper subcellular localization in the plant cell
Table 1 of Cu subcellular localization in leaf cells of E. splendens shows that Cu contents in nucleus, mitochondrion and ribosome of plants changed slightly, while that in the cell wall, chloroplast and soluble fraction increased, when the plant was exposed to 0.25 µmol/L Cu from 8 d to 16 d. At 500 µmol/L Cu, the Cu content in all organelles and the soluble fraction in the plant leaf cells was elevated as the Cu exposure time was increased from 8 d to 16 d. Furthermore, the Cu content in all organelles and the soluble fraction in the leaf cells of plants increased with the increase in Cu levels. When the Cu exposure time was increased from 8 d to 16 d, the distribution percent of Cu in the chloroplast, mitochondrion, nucleus and ribosome at 0.25 µmol/L Cu decreased, and was slightly changed in the nucleus, mitochondrion and ribosome. A decreased percent in chloroplast was noted at 500 µmol/L Cu. The Cu distribution percent in chloroplast, nucleus, mitochondrion and ribosome decreased but in the soluble fraction and cell wall in the plant leaf cells increased as increasing Cu levels. In short, the Cu subcellular localization in the leaf cells of E. splendens is uneven, and in the order of chloroplast>cell wall>soluble fraction>ribosome> mitochondrion>nucleus at 0.25 µmol/L Cu, but at 500 µmol/L Cu, the order was: cell wall>chloroplast> soluble fraction>ribosome>mitochondrion>nucleus, which may be due to the increased Cu bonded to the cell wall in the plant leaf cells under high Cu toxic condition.
Table 1.
Copper subcellular distribution in the leaf cell of plants exposued to 0.25 and 500 µmol/L Cu (mg/kg FW)
| Fractions | Cu subcellular localization |
|||
| 0.25 µmol/L Cu (control) |
500 µmol/L Cu |
|||
| 8 d | 16 d | 8 d | 16 d | |
| Cell wall | 8.6 (29)* | 11.9 (31) | 45.6 (37) | 58.4 (38) |
| Chloroplast | 13.9 (46) | 16.6 (43) | 47.5 (38) | 51.4 (34) |
| Nucleus | 1.2 (4.0) | 1.3 (3.4) | 2.8 (2.3) | 3.7 (2.4) |
| Mitochondrion | 1.5 (5.0) | 1.6 (4.2) | 4.6 (3.7) | 5.15 (3.4) |
| Ribosome | 2.3 (7.7) | 2.7 (7.0) | 5.23 (4.2) | 6.13 (4.0) |
| Soluble fraction | 2.5 (8.3) | 4.2 (11) | 18.2 (15) | 27.3 (18) |
| Total Cu | 35.5 | 43.8 | 137 | 163 |
| Percentage recovery (%)# | 85 | 87 | 90 | 93 |
Values in the bracket represent distribution percent (%)
Percentage recovery (%)=(Cell wall+Chloroplast+Nucleus+Mitochondrion+Ribosome+Soluble fraction)/Total
The subcellular localization of Cu in the root cells of E. splendens is shown in Table 2. There was no notable change in Cu absolute contents in nucleus, mitochondrion, ribosome and soluble fractions in plant root cells, when Cu contents in the cell wall fractions increased after the plant was exposed to 0.25 µmol/L Cu for 8 d to 16 d. While at 500 µmol/L Cu, the Cu contents in all organelles rose considerably as the Cu exposure time increased from 8 d to 16 d. Elevated Cu levels increased Cu contents in all organelles in the root cells of the plants. When Cu exposure time increased from 8 d to 16 d, the Cu distribution percent in nucleus, mitochondrion, ribosome and soluble fraction decreased at both 0.25 µmol/L and 500 µmol/L Cu, that in cell wall fraction increased and in plastid fraction changed slightly at 500 µmol/L Cu. In short, the order of Cu subcellular localization in root cells of E. splendens was: cell wall>plastid>soluble fraction>ribosome>mitochondrion=nucleus at both 0.25 µmol/L and 500 µmol/L Cu.
Table 2.
Copper subcellular distribution in the root cell of plants exposued to 0.25 and 500 µmol/L Cu (mg/kg FW)
| Fractions | Cu subcellular localization |
|||
| 0.25 µmol/L Cu (control) |
500 µmol/L Cu |
|||
| 8 d | 16 d | 8 d | 16 d | |
| Cell wall | 7.82 (47)* | 8.75 (45) | 1038 (56) | 2380 (61) |
| Plastid | 4.17 (25) | 5.69 (29) | 265 (14) | 558 (14) |
| Nucleus | 0.91 (5.6) | 1.05 (5.3) | 90.3 (4.9) | 162 (4.2) |
| Mitochondrion | 0.96 (5.7) | 1.02 (5.2) | 84.7 (4.6) | 126 (3.2) |
| Ribosome | 1.12 (6.7) | 1.27 (6.5) | 83.2 (4.5) | 103 (2.6) |
| Soluble fraction | 1.85 (11) | 1.91 (9.7) | 295 (16) | 573 (15) |
| Total Cu | 19.6 | 23.5 | 2024 | 3804 |
| Percentage recovery (%)# | 86% | 84% | 92% | 103% |
Values in the bracket represent distribution percent (%)
Percentage recovery (%)=(Cell wall+Plastid+Nucleus+Mitochondrion+Ribosome+Soluble fraction)/Total
DISCUSSION AND CONCLUSION
Much more Cu was located at the roots than shoots of E. splendens (Fig.2) after Cu exposure for 8 d. Fifty μmol/L Cu slightly increased Cu in the stems and leaves of E. splendens, while it was significantly increased at Cu levels>100 μmol/L. At 500 μmol/L Cu, stem Cu was around 1000 mg/kg, and leaf Cu was approximately 250 mg/kg. TEM observations confirmed that chloroplasts was damaged more heavily than other organelles in leaf cell of E. splendens when Cu levels increased up to 500 µmol/L in nutrient solution. At 50 µmol/L Cu, no significant toxicity was observed in the chloroplast and the mitochondrion within the leaf cells of E. splendens, as compared to the control. Whereas at 250 µmol/L Cu, chloroplasts deviated considerably from spherical shape and thylakoid arrays decreased significantly, plasmalemma exhibited zigzag pattern. At Cu levels up to 500 µmol/L, the organelles in leaf cells of E. splendens were damaged heavily by excessive Cu in vivo. Large amounts of electron dense bodies (copper particles) were deposited near the inner side of cell wall, at the outer side of the chloroplast membrane and within the chloroplast (Fig.3). TEM photos of E. splendens root cells showed that some damage occurred in the mitochondrion membranes, separation of the cytoplasm from the cell wall was noted at 50 µmol/L Cu (Fig.4b). At 100–250 µmol/L Cu, the root organelles were heavily damaged, and big dark copper particles precipitated within the root cell and saturated at the outer side of the root cell walls (Fig.4e) and at the root cell membrane (Fig.4f). But the structure of the nucleus and of the nucleolus was intact. Compared to the nucleus in the root cells of E. splendens, the mitochondrions can be more easily destroyed by excessive Cu in vivo.
Copper subcellular localization in the plant’s leaf cell after 8 days’ exposure of the plant to 500 µmol/L Cu decreased in the order: chloroplast>cell wall>soluble fraction>other organelles. Whereas for the plant root cell, cell wall was the highest Cu localization site, followed by the plastid and the soluble fraction, and the lowest in the other organelles. The chloroplasts, nucleis, mitochondrions and ribosomes are the key cell organs in the plant cell, for the major cell life activities (Carroll, 1989; Westerhoff, 1985). Copper has strong affinity to the functional groups like sulfhydryl in the organelle membrane. At proper levels, Cu can keep the structure steady in the organelle membrane (Henriques, 1989), while at excessive levels, it can damage the integrity of the membrane structure within the plant cell (De Vos et al., 1989; 1991). At 500 μmol/L Cu, the Cu distribution percent in organelles in the root and leaf cells decreased, but that in the cell wall and soluble fraction increased, as compared to that at 0.25 μmol/L Cu. The decrease in Cu distribution percent observed at exposure concentrations that affect the structure of many organelles may just be due to effects on transport systems. For the same Cu exposure level and exposure time, Cu is mainly deposited in the cell wall, then chloroplast and the soluble fraction in the plant leaf cells. At 0.25 µmol/L Cu, chloroplast is the main Cu localization site in plant leaf cells for the normal requirements for plant growth (Lastra et al., 1987). After increasing Cu to 500 µmol/L, the distribution percent of Cu in the cell wall and chloroplast was even at 8 d, but after the plant’s exposure to Cu for 16 d, increased distribution percentage in the cell wall and decreased distribution percentage in the chloroplast were noted in the plant leaf cells (Table 1).
The uneven increase of distribution percent and Cu absolute content in cell wall of plant root cells were observed as the Cu in solution increased (Table 2). Root cell wall is the important localization site of heavy metals in plants due to its quantities of cation ligand (Hayens, 1980; Leita et al., 1996). In this study, 60%–70% root Cu localized at the root cell wall, which accorded with reports that over 50% root Cu is bonded to the root cell wall of plants (Cathala and Salsac, 1975; Iwasaki et al., 1990). Nishizono et al.(1989) reported that about 70%–90% Cd, Cu and Zn in the root cell of Athyrium yokosense were bonded to the root cell wall. The plastid can be the main storage organelle in the plant root because of its role as the precursor of chloroplast, but its function cannot be equivalent to that of the chloroplast (Carroll, 1989). The Cu distribution percent in the plastid significantly decreased at 500 µmol/L Cu when Cu exposure time was extended from 8 d to 16 d. Moreover, at the high Cu level, the soluble fraction that increased markedly, resulted mainly from the enhanced syntheses of Cu-complex of low molecular weight in the protoplast of plant (Krotz et al., 1989; Wagner and Krotz, 1989).
Plant cell wall is the main composition of apoplasts, which are the “dead” tissues in the plants with lower physiological metabolism activity. The plant cell wall contains protein and polyoses such as cellulose, hemicellulose, and lignin, mucilage glue, and so on, which have a number of potential ligands such as hydroxyl, carboxyl, amino group, aldehyde group, phosphate, thiol, etc. (Hayens, 1980) that can participate in a variety of reactions including ion exchange, adsorption, complexation, precipitation and crystallization, leading to metal sequestration under metal toxicity (Mullen et al., 1992). When exposed to higher levels of metals, the plant cell can actively secrete calluses which have the ability of chelation to the apoplast parts (Wissenmeier et al., 1987). So, the plant cell wall is the chief site for detoxification of heavy metals in plant (Hayens, 1980; Allan and Jarrell, 1989). When plants were exposed to nonlethal levels of Ni, 70% Ni in the Ni-hyperaccumulator Thlaspi goesingense was combined with cell wall substances (Krämer et al., 2000). In this study, at 500 μmol/L Cu, the plant cell wall is the main Cu location site both in the leaf and root cell of E. splendens. While in the leaf cell, chloroplast was the other important Cu location site. At exposure to 500 μmol/L Cu or with the longer Cu exposure time, Cu location in the cell wall increased considerably, and that in the chloroplast decreased markedly. The Cu localization in the cell walls and chloroplasts could mainly account for the high detoxification of Cu in E. splendens.
Acknowledgments
The authors thank Dr. J.Y. Li from the Center of Analysis & Measurement of Zhejiang University for her kind help in the transmission electronic microscope analyses of the plant samples.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 20307008) and the National Basic Research Program (973) (No. 2002CB410804) of China
References
- 1.Allan DL, Jarrell WM. Proton and copper adsorption to maize and soybean root cell wall. Plant Physiology. 1989;89:823–832. doi: 10.1104/pp.89.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks RR, Shaw S, Marfil AA. The chemical form and physiological function of nickel in some Iberian Alyssum species. Physiologial Plantarum. 1981;51:167–170. [Google Scholar]
- 3.Carroll M. Organelles. London: The Guiford Press; 1989. [Google Scholar]
- 4.Cathala N, Salsac L. Absorption of copper by roots of corn (Zea mays) and sunflower (Helianthus annuus) Plant and Soil. 1975;42:65–83. [Google Scholar]
- 5.De Vos CHR, Schat H, Vooijs R, Ernst WHO. Copper-induced damage to the permeability barrier in roots of Silene cucubalus . Journal of plant physiology. 1989;135:165–169. [Google Scholar]
- 6.De Vos CHR, Schat H, De Waal MAM, Vooijs R, Ernst WHO. Increased resistance to copper-induced damage of the root cell plasmalemma in copper-tolerant Silene cucubalus . Physiologia Plantarum. 1991;82:523–528. [Google Scholar]
- 7.Ernst WHO, Verkleij JAC, Schat H. Metal tolerance in plants. Acta Botanica Neerlandica. 1992;41:229–248. [Google Scholar]
- 8.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemistry Journal. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison SJ, Lepp NW, Phipps DA. Uptake of copper by excised roots. II. Copper desorption from the free space. Z Pflanzenernähr Bodenkd. 1984;94:27–34. [Google Scholar]
- 10.Hayens RJ. Ion exchange properties of roots and ionic interactions within the root POPLsm: Their role in ion accumulation by plants. Botany Review. 1980;46:75–99. [Google Scholar]
- 11.Henriques FS. Effects of copper deficiency on the photosynthetic apparatus of sugar beet (Beta vulgaris L.) Journal of plant physiology. 1989;135:453–458. [Google Scholar]
- 12.Iwasaki K, Sakurai K, Takahashi E. Copper binding by the root cell walls of Italian ryegrass and red clover. Soil Science and Plant Nutrition. 1990;36(3):431–439. [Google Scholar]
- 13.Kennedy CD, Gonsalves FAN. The action of divalent zinc, cadmium, mercury, copper and lead on the trans-root potential and H+ efflux of excised roots. Journal of Experimental Botany. 1987;38:800–817. [Google Scholar]
- 14.Krämer U, Pickering IJ, Prince RC, Raskin I, Salt DE. Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species . Plant Physiology. 2000;122:1343–1353. doi: 10.1104/pp.122.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krotz RM, Evangelou BP, Wagner G. Relationships between Cadmium, Zinc, Cd-peptide, and organic acid in tobacco suspension cells. Plant Physiology. 1989;91:780–787. doi: 10.1104/pp.91.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lastra O, Chueca A, González C, Lachica M, Gorge JL. El cobre como nutriente de la planta. Anales Edafol Agrobiol. 1987;46:1005–1020. [Google Scholar]
- 17.Leita L, De Nobili M, Cesco S. Analysis of intercellular cadmium forms in roots and leaves of bush bean. Journal of Plant Nutrition. 1996;19(3-4):527–533. [Google Scholar]
- 18.Lidon FC, Ramalho JC, Henriques FS. Copper inhibition of rice photosynthesis. Journal of Plant Physiology. 1993;142:12–17. [Google Scholar]
- 19.Macnair MR. The genetics of metal tolerance in vascular plants. New Phytologist. 1993;124:541–559. doi: 10.1111/j.1469-8137.1993.tb03846.x. [DOI] [PubMed] [Google Scholar]
- 20.Maksymiec W, Baszynski T. The role of Ca2+ ions in modulating changes induced in bean plants by an excess of Cu2+ ions. Chlorophyll fluorescence measurements, Physiologial Plantarum. 1999;105:562–568. [Google Scholar]
- 21.Maksymiec W, Bednara J, Baszynski T. Responses of runner plants to excess copper as a function of plant growth stages: effects on morphology and structure of primary leaves and their chloroplast ultrastructure. Photosynthetica. 1995;31:427–435. [Google Scholar]
- 22.Mullen MD, Wolf DC, Beveridge TJ, Bailey GW. Sorption of heavy metala by soil fungi Aspergillus niger and Mucor ruoxii. Soil Biology and Biochemistry. 1992;24:129–135. [Google Scholar]
- 23.Nishizono H, Watanabe T, Orii T, Suzuki S. Suppression of inhibitory effects of copper on enzymatic activities by copper-binding substances from Athyrium yokoscene assayed in vitro. Plant Cell Physiology. 1989;30(4):565–569. [Google Scholar]
- 24.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 25.Robson AD, Reuter DJ. Diagnosis of Copper Deficiency and Toxicity. In: Loneragan JF, Robson AD, Graham RD, editors. Copper in Soils and Plants. London: Academic Press; 1981. pp. 287–312. [Google Scholar]
- 26.Song J, Zhao FJ, Luo YM, McGrath SP, Zhang H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environmental Pollution. 2004;128:307–315. doi: 10.1016/j.envpol.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Tang SR, Wilke BM, Huang CY. The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People’s Republic of China. Plant and Soil. 1999;209:225–232. [Google Scholar]
- 28.Wagner GJ, Krotz RM. Perspectives on Cd and Zn Accumulation, Accomodation and Tolerance in Plant Cells: The Role of Cd-binding Peptide versus Other Mechanisms. In: Alan R, editor. Molecular Biology and Chemistry (Metal ion homeostasis) Liss, Inc.; 1989. pp. 325–336. [Google Scholar]
- 29.Westerhoff HV. Organization in cell soup. Nature. 1985;318:106–108. [Google Scholar]
- 30.Wissenmeier AH, Klotz F, Horst WJ. Aluminum induced callose synthesis in roots of soybean (Glycine max L.) Journal of Plant Physiology. 1987;129:487–492. [Google Scholar]
- 31.Yang XE, Shi WY, Fu CX, et al. Sustainable Agriculture for Food, Energy and Industry. James & James (Science Publishers) Ltd; 1998. Copper-hyperaccumulators of Chinese Native Plants Characteristics and Possible Use for Phytoremediation. [Google Scholar]
- 32.Yang MJ, Yang XE, Roemheld V. Growth and nutrient composition of Elsholtzia splendens nakai under copper toxicity. Journal of Plant Nutrition. 2002;25(7):1359–1375. [Google Scholar]